Abstract
Cultured epidermal autografts have been used worldwide since 1981 for patients with extensive third-degree burn wounds and limited skin donor sites. Despite significant progress in techniques toward improving clinical outcome of skin grafts, the long in vitro preparation time of cultured autografts has remained a major factor limiting its widespread use. Here, we show that pharmacological inhibition of TGF-β signaling promotes the expansion of human epidermal keratinocytes (HEKs) with high proliferative potential in co-cultures with both murine 3T3-J2 cells and human feeder cells, including dermal fibroblasts and preadipocytes. In contrast, TGF-β signaling inhibition does not enhance the growth of HEKs in a serum- and feeder-free condition, an alternative approach to propagate HEKs for subsequent autograft production. Our results have important implications for the use of TGF-β signaling inhibition as a viable therapeutic strategy for improving Green’s methodology and for more efficient production of customized skin autografts with human feeder cells.
Keywords: Autograft, Burn wound, Epidermal cell, p63, Proliferative potential, TGF-β signaling
INTRODUCTION
Cutaneous burns are one of the most common and devastating forms of trauma. Patients with extensive third-degree burn wounds require immediate treatment to minimize morbidity and mortality, for which many synthetic and allogeneic skin substitutes have been developed and successfully used as a temporary coverage of the wounds.1–3 However, to achieve permanent wound closure, autologous keratinocytes with high proliferative potential must be used. Since 1981, cultured epidermal autografts (CEA) developed by Green and colleagues have been used worldwide for patients with massive burn wounds and limited skin donor sites, saving their lives.4
Green’s method to expand human epidermal keratinocytes (HEKs) utilizes 3T3-J2 cells, a murine mesenchymal cell line, as a feeder layer.4 A modified Green’s method has implemented the use of a fibrin matrix underneath the 3T3-J2 cell layer to improve the handling of the grafts at the surgery, which has successfully increased the number of grafts to be transplanted at a time.1,4–7 However, the long in vitro expansion time to prepare autografts from a small skin biopsy has remained one of the major factors limiting widespread use of CEA. It has been shown that a feeder-free culture can reduce the time required for HEK expansion.8,9 However, although keratinocyte autografts prepared in a feeder-free culture have been used in treating relatively small wounds, such as chronic venous leg ulcers,10 few burn units implement feeder-free autografts for patients with severe burn wounds.2 An improved methodology to more rapidly expand keratinocytes with high proliferative potential in 3T3-J2 co-culture would reduce the number of surgical procedures and decrease hospitalization time for patients with massive burn injuries who undergo replacement by Green’s method of skin covering nearly the entire body surface area.
We have shown recently that 3T3-J2 cells express Dact1, an antagonist of β-catenin signaling that suppresses keratinocyte-mediated upregulation of TGF-β in 3T3-J2 cells.11 As TGF-β suppresses keratinocyte proliferation,12 the relative inability of 3T3-J2 cells to upregulate this inhibitory cytokine may contribute to their support of long-term proliferation of HEKs. However, although predominantly latent, TGF-β is also found in serum, a key component of 3T3-J2 co-culture, and is activated by a number of cellular mechanisms,13 which in turn restricts potential growth of HEKs in 3T3-J2 co-culture.
The specific aim of this study was, first, to investigate whether pharmacological inhibition of TGF-β signaling promotes the expansion of HEKs with high proliferative potential in 3T3-J2 co-culture. Second, we aimed at investigating if TGF-β signaling inhibition would also promote the expansion of HEKs in co-culture with human dermal fibroblasts and preadipocytes, two major human cell types utilized as alternatives to 3T3-J2 cells,14,15 with a long-term goal of efficiently generating customized skin autografts.
METHODS
Cell culture
HEKs (gift from J. Seykora, University of Pennsylvania School of Medicine) were grown in CnT-PR media (Cellntec) or chemically-defined SFM media supplemented with growth factors (Invitrogen) in a humidified chamber at 37°C with 5% CO2. To induce differentiation of keratinocytes, the cells were harvested from 3T3-J2 co-cultures and keratinocytes were selectively expanded in CnT-PR media, followed by stimulation with 1.3 mM CaCl2 for 6 days. 3T3-J2 cells (gift from H. Green, Harvard Medical School) and human dermal fibroblasts (hDF) (Genlatis) were grown in DMEM containing 10% calf serum (Hyclone), 10 U/ml penicillin and 100 μg/ml streptomycin in a humidified chamber at 37°C with 10% CO2. Human preadipocytes (hPA) (gift from M. Reilly, University of Pennsylvania School of Medicine) were grown in PM4 medium as described.16 3T3-J2 co-culture was performed according to the Green method.17 To visualize epidermal cell clones, the culture plates were stained with 1% Rhodamine B (Sigma-Aldrich). RepSox, LY364947, and SB525334 were purchased from Selleck Chemicals.
Antibodies
The following antibodies were used in this study: mouse anti-p63 (Santa Cruz), rabbit anti-cytokeratin 5 (Abcam), rabbit anti-integrin β1 (Abcam), rabbit anti-loricrin (Covance), goat anti-involucrin (Santa Cruz), mouse anti-tubulin α (Developmental Studies Hybridoma Bank), mouse anti-BrdU (Roche), rabbit anti-Smad2/3 (Cell Signaling), Alexa 488-goat anti-mouse IgG, Alexa 488-goat anti-rabbit IgG, Alexa 594-goat anti-rabbit IgG, R-phycoerythrin-goat anti-mouse IgG (Molecular Probes), horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (KPL), HRP-conjugated rabbit anti-goat IgG (Invitrogen), and HRP-conjugated goat anti-rabbit IgG (Cell Signaling).
Protein analysis
Immunofluorescence and Western blot were performed as previously described.11
BrdU labeling
BrdU labeling and detection were performed as previously described.11 CK5+BrdU+ cell populations were analyzed on a FACSCanto II (BD Biosciences).
Statistical analysis
Values are reported as mean±standard error of the mean (SEM). Student’s t-tests were performed where P<0.05 was considered statistically significant.
RESULTS
To determine whether TGF-β signaling is active in proliferating HEKs in 3T3-J2 co-culture, we investigated the nuclear localization of Smad2/3, downstream effectors of activated TGF-β signaling.18 Our data show that a substantial fraction of Smad2/3 was found in the nucleus of HEKs and that treatment with RepSox, a small molecule inhibitor of the TGF-β type 1 receptor (TGFβRI/ALK5),19 significantly reduced the nuclear localization of Smad2/3 (Figure 1a and 1b). These data indicate that TGF-β signaling is active in proliferating HEKs in 3T3-J2 co-culture.
Figure 1. Inhibition of TGF-β signaling promotes the expansion of HEKs with high proliferative potential in co-culture with 3T3-J2 cells.
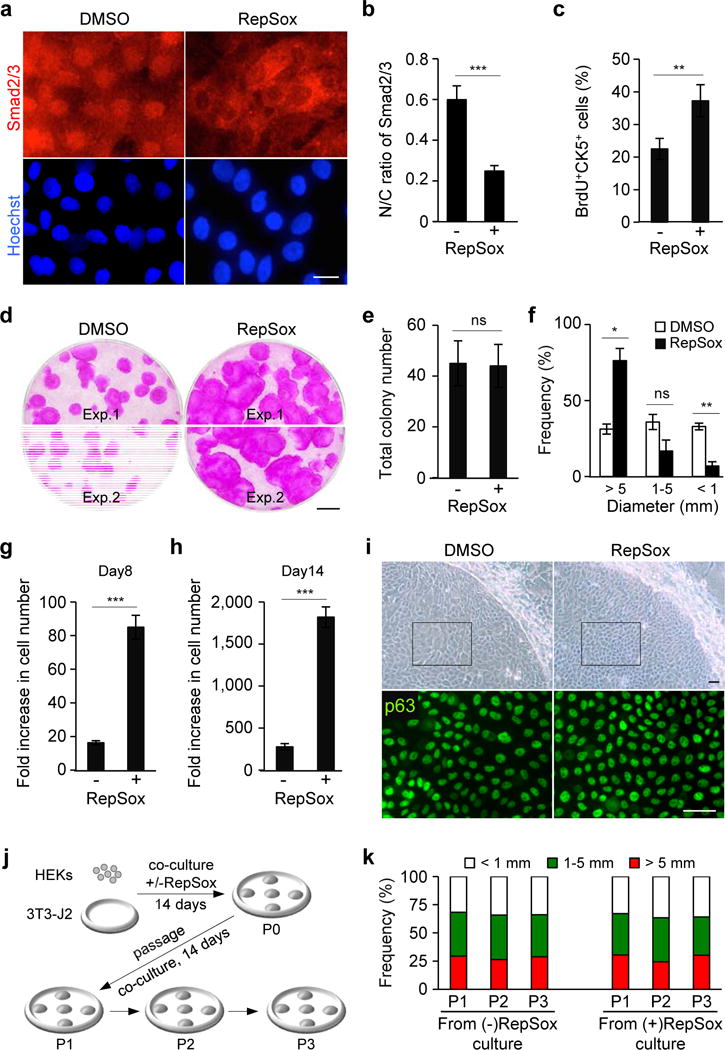
(a) Subcellular localization of Smad2/3 in HEKs grown in 3T3-J2 co-culture treated with dimethyl sulfoxide (DMSO), as a control, or 1 μM RepSox for 2 hrs (top panels). Lower panels represent corresponding nuclear counterstaining with Hoechst 33342. Bar=10 μm.
(b) Nuclear-to-cytoplasmic ratios of Smad2/3 in HEKs as determined by fluorescence intensity using NIH ImageJ. Data shown are mean±SEM (n=6). ***P<0.001.
(c) Proliferation of CK5+ epidermal cells in 3T3-J2 co-culture at day 5 as determined by a short-pulse labeling with BrdU, followed by quantification by flowcytometric analysis. Data shown are mean±SEM (n=3). **P<0.01.
(d) Rhodamine B staining of epidermal cell clones grown in the absence (left) or presence (right) of 0.1 μM RepSox for 14 days. Equal numbers of HEKs (1×103 cells) were plated in each well. Two representative data are shown. Bar=5 mm.
(e, f) Total number (e) and size distribution (f) of epidermal clones in 3T3-J2 co-culture at day 14, expressed as mean±SEM (n=3). *P<0.05; **P<0.01; ns, not significant.
(g, h) Fold increase in cell number of CK5+ epidermal cells grown in 3T3-J2 co-culture in the presence or absence of 0.1 μM RepSox for 8 days (g) and 14 days (h) as determined by flowcytometric analysis. Five thousand cells were seeded in each Φ60 mm dish. Data shown are mean±SEM (n=3). ***P<0.001.
(i) Bright field images of representative epidermal clones (upper panels) and corresponding p63 immunofluorescence of the boxed areas (lower panels) in 3T3-J2 co-culture treated or untreated with 0.1 μM RepSox for 14 days. Bars=25 μm.
(j, k) Experimental scheme (j) and size distribution of epidermal clones in serial 3T3-J2 co-cultures (k). Epidermal cells were harvested from 3T3-J2 co-culture treated or untreated with 0.1 μM RepSox (P0). Equal numbers of epidermal cells were then serially cultivated in 3T3-J2 co-cultures with two-week intervals in the absence of RepSox (P1-P3). Data shown in (k) are representative of three independent experiments with similar results. P, passage number.
Our data show that proliferation of HEKs was significantly enhanced by the treatment with RepSox, as determined by a short pulse labeling with 5-bromo-2′-deoxyuridine (BrdU) (Figure 1c). Accordingly, RepSox treatment enhanced the clonal expansion of HEKs, yielding a >2.4-fold increase in the percentage of the largest epidermal clones (>5 mm in diameter) compared to untreated controls (76.2±8.1% versus 31.2±3.3%, P<0.05) at day 14 (Figure 1d–1f). Treatment with LY364947 and SB525334, two other TGFβRI/ALK5 inhibitors20,21, also enhanced the growth of HEKs in 3T3-J2 co-culture (Supplementary Figure S1). Specificity of the TGF-β signaling inhibitors used in this study is summarized in Supplementary Table S1. Flowcytometric analysis with an anti-cytokeratin 5 (CK5) antibody, a marker of basal epidermal cells, shows that RepSox-treated cultures produced 5.3-fold more CK5+ keratinocytes than did untreated control cultures at day 8 (Figure 1g). At day 14, when RepSox-treated cultures approached confluence, they produced 6.5-fold more CK5+ keratinocytes than did untreated control cultures (Figure 1h). Notably, we found that RepSox treatment did not enhance the growth of HEKs in a serum- and feeder-free condition (Supplementary Figure S2). These data indicate that inhibition of TGF-β signaling by small molecule inhibitors promotes the expansion of basal keratinocytes in feeder cell co-culture with 3T3-J2.
Because the use of keratinocytes with high proliferative capacity is essential for wound healing success, we determined the clone-forming efficiency of RepSox-treated HEKs in serial 3T3-J2 co-cultures.17 Immunofluorescence staining shows that, at the end of the initial two-week culture, keratinocytes within the largest epidermal clones in both RepSox-treated and -untreated cultures expressed similar levels of p63, a marker for epidermal progenitor cells22 (Figure 1i). We plated equal numbers of epidermal cells harvested from both cultures and assessed the distribution of clone sizes in multiple passages with two-week intervals in the absence of RepSox (Figure 1j). Our data show that epidermal cells expanded by RepSox treatment produced large clones (>5 mm in diameter) at similar frequencies to those of untreated HEKs derived from standard 3T3-J2 co-cultures in multiple passages (Figure 1k). These data indicate that RepSox treatment does not impair high proliferative capacity of epidermal progenitor cells.
To determine the ability of RepSox-treated keratinocytes to differentiate, we harvested epidermal cells from RepSox-treated and -untreated 3T3-J2 co-cultures and allowed them to differentiate in response to Ca2+ stimulation in CnT-PR keratinocyte basal media. Western blot analysis shows that upon stimulation with Ca2+, expressions of both p63 and a basal cell marker integrin β1 decreased in keratinocytes harvested from RepSox-treated cultures to similar extents to those in control cultures (Supplementary Figure S3). In addition, expressions of both loricrin and involucrin, markers for terminal differentiation of epidermal cells, increased in keratinocytes harvested from RepSox-treated cultures to similar levels to those in control cultures (Supplementary Figure S3). These data indicate that HEKs treated with RepSox are capable of differentiation in response to Ca2+ stimulation.
Finally, we investigated if TGF-β signaling inhibition promotes the expansion of HEKs with high proliferative potential in co-cultures with human feeder cells. Our data show that RepSox treatment drastically enhanced the expansion of HEKs in co-culture with both dermal fibroblasts14 and preadipocytes15 in a dose-dependent manner (Figure 2a–2c). In addition, RepSox-treated HEKs harvested from human feeder cell co-cultures maintained high proliferative capacity in subsequent 3T3-J2 co-cultures in the absence of RepSox to the extent similar to that of untreated HEKs in standard 3T3-J2 co-cultures (Figure 2d and 2e). These data indicate that inhibition of TGF-β signaling promotes the expansion of keratinocytes with high proliferative potential in co-culture with human feeder cells.
Figure 2. Inhibition of TGF-β signaling promotes the expansion of HEKs with high proliferative potential in co-culture with human feeder cells.
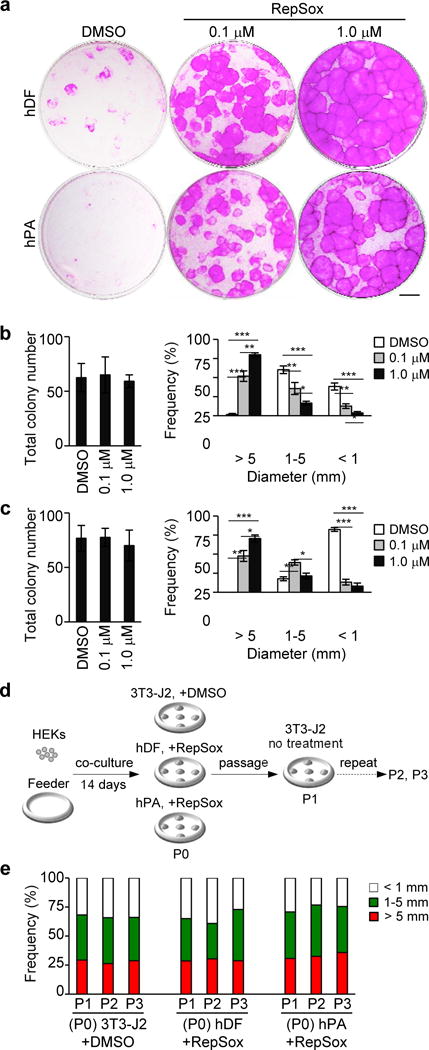
(a) Rhodamine B staining of epidermal cell clones grown for 14 days in co-culture with human dermal fibroblasts (hDF) or human preadipocytes (hPA) in the absence or presence of 0.1–1.0 μM RepSox as indicated. Equal numbers of HEKs (1×103 cells) were plated in each well. Data shown are representative of three independent experiments with similar results. Bar=5 mm.
(b, c) Total number (left) and size distribution (right) of epidermal clones in co-culture with hDF (b) and hPA (c). Data shown are mean±SEM (n=3). *P<0.05; **P<0.01; ***P<0.001.
(d, e) Experimental scheme (d) and size distribution of epidermal clones in serial 3T3-J2 co-cultures (e). Epidermal cells were harvested from a standard 3T3-J2 co-culture (+DMSO) and 1 μM RepSox-treated co-cultures with hDF or hPA feeder cells (P0), followed by serial cultivation of equal numbers of epidermal cells in 3T3-J2 co-cultures with two-week intervals in the absence of RepSox (P1-P3). Data shown in (e) are representative of three independent experiments with similar results. P, passage number.
DISCUSSION
We have shown that pharmacological inhibition of TGF-β signaling promotes the expansion of HEKs with high proliferative potential in co-cultures with both murine 3T3-J2 cells (Figure 1) and human feeder cells (Figure 2). In contrast, RepSox treatment did not enhance the growth of HEKs in a serum- and feeder-free condition (Supplementary Figure S2). Serum-free monoculture can expand HEKs8,9 and has recently been implemented in therapies for small wounds.10 Co-culture of HEKs on feeder cells, however, is still widely used for therapies involving major burn wounds that require much larger autografts.1,2,4 Thus, our results have important implications for more rapid generation of CEA in Green’s methodology and for the use of TGF-β signaling inhibition as a viable therapeutic strategy for efficient production of customized skin autografts with human feeder cells.
There are limitations to our study. First, while we have shown that RepSox-treated HEKs are capable of both proliferation in serial 3T3-J2 co-cultures and differentiation in response to Ca2+ stimulation, further analysis would be necessary to assure long-term capabilities of self-renewal and differentiation, while avoiding immortality, in transplantation models in vivo. Second, we utilized fetal bovine serum (FBS) in feeder cell co-cultures based on the Green protocol.17 Although the use of FBS for therapeutic expansion of HEKs in 3T3-J2 co-culture has been approved by the US Food and Drug Administration,4 human serum can potentially replace FBS and contribute to the development of xenobiotic-free epithelial cell cultures.15 Third, it is important to determine if the presence of supportive matrices (e.g. a fibrin matrix) interferes with biological activities of TGF-β signaling inhibitors during preparation of CEA. Finally, we have not investigated in the present study if alterations of feeder cells mediated by TGF-β signaling inhibition would impact keratinocyte proliferation.
We have shown recently that RepSox treatment could rescue HEK-mediated decrease of Igf2, a growth-promoting factor of keratinocytes, in 3T3-J2 cells.11 This can potentially contribute to the TGF-β signaling inhibition-mediated increase of HEK growth in 3T3-J2 co-culture. However, other effects mediated by TGF-β signaling inhibition in feeder cells need to be fully investigated to explore if such information can be utilized to further improve rapid production of CEA in future studies. While our results are provocative, additional anti-TGF-β compounds currently in clinical trials for different diseases23 should also be investigated to see if they help to promote therapeutic expansion of keratinocytes suitable for clinical use.
Supplementary Material
Acknowledgments
We thank Jeff Holcombe and Leslie King for proof reading of the manuscript.
Source of Funding
This study was supported by an R01AR066755 grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health to M.S.
Abbreviations
- BrdU
5-bromo-2′-deoxyuridine
- CEA
cultured epidermal autograft
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- HEK
human epidermal keratinocyte
- RepSox
2-[3-(6-methyl-2-pyridinyl)-1H-pyrazol-4-yl]-1,5-naphthyridine
- TGF-β
transforming growth factor-β
Footnotes
Conflict of Interest
The authors have no conflicts of interest related to this study.
References
- 1.Chua AW, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ. Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma. 2016;4:3. doi: 10.1186/s41038-016-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mcheik JN, Barrault C, Levard G, Morel F, Bernard FX, Lecron JC. Epidermal healing in burns: autologous keratinocyte transplantation as a standard procedure: update and perspective. Plast Reconstr Surg Glob Open. 2014;2:e218. doi: 10.1097/GOX.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–5. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 4.Green H. The birth of therapy with cultured cells. Bioessays. 2008;30:897–903. doi: 10.1002/bies.20797. [DOI] [PubMed] [Google Scholar]
- 5.Ronfard V, Broly H, Mitchell V, Galizia JP, Hochart D, Chambon E, Pellerin P, Huart JJ. Use of human keratinocytes cultured on fibrin glue in the treatment of burn wounds. Burns. 1991;17:181–4. doi: 10.1016/0305-4179(91)90099-3. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini G, Ranno R, Stracuzzi G, Bondanza S, Guerra L, Zambruno G, Micali G, De Luca M. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–79. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 7.Ronfard V, Rives JM, Neveux Y, Carsin H, Barrandon Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70:1588–98. doi: 10.1097/00007890-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 9.Wille JJ, Pittelkow MR, Shipley GD, Scott RE. Integrated control of growth and differentiation of normal human prokeratinocytes cultured in serum-free medium: clonal analyses, growth kinetics, and cell cycle studies. J Cell Physiol. 1984;121:31–44. doi: 10.1002/jcp.1041210106. [DOI] [PubMed] [Google Scholar]
- 10.Wille JJ, Burdge JJ, Pittelkow MR. Rapid healing of chronic venous stasis leg ulcers treated by the application of a novel serum-fee cultured autologous epidermis. Wound Rep Reg. 2011;19:464–74. doi: 10.1111/j.1524-475X.2011.00702.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki D, Senoo M. Dact1 regulates the ability of 3T3-J2 cells to support proliferation of human epidermal keratinocytes. J Invest Dermatol. 2015;135:2894–97. doi: 10.1038/jid.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shipley GD, Pittelkow MR, Wille JJ, Jr, Scott RE, Moses HL. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986;46:2068–71. [PubMed] [Google Scholar]
- 13.Robertson IB, Rifkin DB. Unchaining the beast; insights from structural and evolutionary studies on TGFβ secretion, sequestration, and activation. Cytokine Growth Factor Rev. 2013;24:355–72. doi: 10.1016/j.cytogfr.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullock AJ, Higham MC, MacNeil S. Use of human fibroblasts in the development of a xenobiotic-free culture and delivery system for human keratinocytes. Tissue Eng. 2006;12:245–55. doi: 10.1089/ten.2006.12.245. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama H, Maeda K, Yamato M, Hayashi R, Soma T, Hayashida Y, Yang J, Shirakabe M, Matsuyama A, Kikuchi A, Sawa Y, Okano T, Tano Y, Nishida K. Human adipose tissue-derived mesenchymal stem cells as a novel feeder layer for epithelial cells. J Tissue Eng Regen Med. 2008;2:445–9. doi: 10.1002/term.111. [DOI] [PubMed] [Google Scholar]
- 16.Skurk T, Ecklebe S, Hauner H. A novel technique to propagate primary human preadipocytes without loss of differentiation capacity. Obesity. 2007;15:2925–31. doi: 10.1038/oby.2007.349. [DOI] [PubMed] [Google Scholar]
- 17.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–6. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Transforming growth factor-β signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25:2017–24. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Gellibert F, Woolven J, Fouchet MH, Mathews N, Goodland H, Lovegrove V, Laroze A, Nguyen VL, Sautet S, Wang R, Janson C, Smith W, Krysa G, Boullay V, De Gouville AC, Huet S, Hartley D. Identification of 1,5-naphthyridine derivatives as a novel series of potent and selective TGF-β type I receptor inhibitors. J Med Chem. 2004;47:4494–506. doi: 10.1021/jm0400247. [DOI] [PubMed] [Google Scholar]
- 20.Grygielko ET, Martin WM, Tweed C, Thornton P, Harling J, Brooks DP, Laping NJ. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-β type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther. 2005;313:943–51. doi: 10.1124/jpet.104.082099. [DOI] [PubMed] [Google Scholar]
- 21.Li HY, Wang Y, Heap CR, King CH, Mundla SR, Voss M, Clawson DK, Yan L, Campbell RM, Anderson BD, Wagner JR, Britt K, Lu KX, McMillen WT, Yingling JM. Dihydropyrrolopyrazole transforming growth factor-β type I receptor kinase domain inhibitors: A novel benzimidazole series with selectivity versus transforming growth factor-β type II receptor kinase and mixed lineage kinase-7. J Med Chem. 2006;49:2138–42. doi: 10.1021/jm058209g. [DOI] [PubMed] [Google Scholar]
- 22.Senoo M, Pinto F, Crum CP, McKeon F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 23.Buijs JT, Stayrook KR, Guise TA. The role of TGF-β in bone metastasis: novel therapeutic perspectives. Bonekey Rep. 2012;1:96. doi: 10.1038/bonekey.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


