Abstract
The level of assistance patients with Alzheimer’s disease (AD) require in their care may be an important predictor of resource use, costs of care and quality of life. The Dependence Scale (DS), a measure of care-assistance required, was used to estimate costs of care and quality of life of patients with AD categorized into six dependence stages based upon the summated item scores of the DS. Data was derived from a three-year, non-interventional study of 132 patients with probable AD (ages 50 to 85) and caregiver dyads. We investigated the association between DS scores and healthcare costs, health-related quality of life (HRQoL), caregiver burden and estimated annual costs and HRQoL for six dependence stages in adjusted models. DS scores were significant predictors of healthcare costs, HRQoL, and caregiver burden. The estimated annual healthcare costs and a measure of HRQoL (EuroQoL-5D) ranged from $11,418 and 1.00 for those at very mild dependence stage to $101,715 and 0.26 for those at very severe dependence stage. DS scores classified into six dependence stages provides a useful method to estimate unique levels of care-associated costs and health utilities for pharmacoeconomic evaluations of new treatments for AD.
Keywords: Alzheimer’s disease, dependence scale, costs of care, quality of life, pharmacoeconomic evaluation, disease stage
1. BACKGROUND
Alzheimer’s disease (AD) will place a considerable economic burden on society as our population ages. A number of AD clinical trials are being conducted to intervene early in the disease process to modify disease progression for more effective treatment of AD. These studies involve expensive diagnostic and treatment approaches. The economic impact of disease modifying therapies on long term costs associated with AD is unknown. Economic evaluations in AD have involved decision analytic models of total societal costs of care and quality-adjusted life years (QALYs) using discrete stages of AD as measured by clinical scales.1 A variety of scales have been used in the clinical and cost-effectiveness literature to measure the severity of AD symptoms and the staging of disease severity.2 Among them, the most commonly used scales have been the Mini-Mental State Examination (MMSE)3 and the Clinical Dementia Rating (CDR) scale,4 but both have limitations in modeling pharmacoecnomic outcomes in AD.1,2,5
It is not adequate to evaluate the changes in healthcare costs and health-related quality of life (HRQoL) for new AD interventions simply by one or two symptom domains.6–8 The MMSE is a general and incomplete measure of cognition. The CDR incorporates aspects of cognition and function into a discrete stage of dementia, but is time consuming to administer, requires a trained clinician to complete, and categorizes dementia severity into relatively broad categories (i.e. mild, moderate, severe dementia). Therefore, dependence, as measured by the Dependence Scale (DS), has been proposed as a better measure of AD progression that incorporates changes in the individual domains of cognition, function, and behavior.9
The DS, developed by Stern and colleagues, was designed to capture the caregiver’s report of the level of assistance and care needed by AD patients rather than specific task performance ability.10 Several validation studies have shown that the DS measure of dependence is related to, but provides information distinct from, cognition, physical functioning, and behavior.10–14 Previous research has shown that dependence directly impacts costs of care and is associated with HRQoL and caregiver burden.7,9,12,15 Studies have also suggested that the DS is a better candidate than the MMSE or CDR for modeling AD progression and in explaining variation in quality of life, health status and medical costs of AD patients, as well as caregiver time and associated resource use.5,9,16 These studies suggest that the DS would be useful in models evaluating the economic impact of interventions in AD.5,12
Previous studies have described the relationship between costs of care and HRQoL in patients with AD based upon levels of dependence as measured by the DS.7,17–19 These studies have used either a summated score of the DS (i.e. 0–15)18,19 or divided the DS into quartiles7 to describe these relationships. However, these two approaches are not ideal for pharmacoeconomic modeling, since they are not easily used in decision analytic models in a clinically meaningful way. The original DS paper proposed a method to use DS item scores to derive five levels of dependence.10 We examined the use of these five levels of dependence in our dataset to explain pharmacoeconomic outcomes, but found several limitations. For example, scoring for some levels was complicated (e.g. level 2 is defined as two of items A, B or C=1, or A or B =2, or D-1), and there were inconsistent differences in important clinical scales (e.g. CDR) between some levels (e.g. clinical scales were very different between levels 3 and 4 and very similar between levels 4 and 5). These limitations were the motivation for us to try to derive a new approach to using the DS to stage dementia severity.
We sought to use summated DS scores to provide an estimate of the stage of dependence for pharmacoeconomic modeling. The CDR has been the standard for dementia staging in clinical research, but has limitations for pharmacoeconomic modeling as described previously. Six stages of dementia were proposed in the Global Deterioration Scale (GDS) for the assessment of primary degenerative dementia,20,21 and were operationalized based upon function in the Functional Assessment Staging (FAST) scale.22 We used an empirically-driven approach to develop six stages of dependence using summated DS scores. The objectives of this study were: (1) to examine the relationships between dependence stages, healthcare costs, HRQoL, and caregiver-perceived burden in our dataset (2) to estimate the healthcare costs and HRQoL at six dependence stages derived from summated DS scores.
2. MATERIALS AND METHODS
2.1 Subjects
The subjects included in the present study were from a three-year longitudinal, non-interventional study. Patients ages 50–85 years and their caregivers were recruited from 9 clinical practice sites (6 Neurology, 3 Geriatric Medicine) in Michigan, USA from 2000 to 2004. Details of the original study have been documented elsewhere.23,24 Patients with probable AD diagnosis based on the NINCDS-ADRDA criteria25 were included. Eligible subjects were interviewed, tested cognitively and examined neurologically at baseline, and caregivers were interviewed in person at baseline. Follow-up telephone interviews were made with the caregiver one year and two years after the baseline visit. One subject was lost to follow-up during the follow-up period and not included in the analysis.
2.2 Measurement
2.2.1 Dependence
Dependence was measured using the dependence scale (DS), a 13-item questionnaire with summated score ranges of 0–15. The higher the value, the more severe the dependence. We used an empirically-driven approach to developing six stages of dependence based upon our dataset, by examining groupings of the summated DS scores that reflected important changes in costs of care and/or HRQoL that were anchored in clinically meaningful and defined stages in the CDR scale. The naming of our dependence stages corresponds to the dementia severity terminology used in the CDR for mild, moderate and severe stages. Scoring of dependence stages is simply a specified range of summated DS scores. Specifically, we defined DS summated scores of 1–3 as very mild, 4–5 as mild, 6–8 as mild to moderate, 9–10 as moderate, 11–12 as severe, and 13–15 as very severe. A comparison of dependence stages, CDR scores and terminology, and estimated FAST scores can be seen in Table 1.
Table 1.
Comparison of Dependence Stage, CDR Scores and Terminology, and FAST Scores
| Dependence Stage | DS scores (summated) | Description of Dependence | CDR means† | CDR Terminology | FAST Score‡ |
|---|---|---|---|---|---|
| No Dependence | 0 | No dependence | 0 = No Dementia | 1 & 2 | |
| Very Mild | 1–3 | Infrequent reminders and/or minor assistance with a few IADL | 0.8 | 0.5 = Questionable Dementia | 3 |
| Mild | 4–5 | Needs frequent reminders and/or assistance with more IADL | 1.0 | 1.0 = Mild Dementia | 4 |
| Mild to Moderate | 6–7 | Needs household chores done for them and/or needs supervision | 1.5 | 5 | |
| Moderate | 8–9 | Needs to be escorted when outside and/or accompanied when bathing or eating | 2.0 | 2.0 = Moderate Dementia | 5 & 6 |
| Severe | 10–12 | Needs significant help with some or all BADL (e.g. Needs to be dressed, washed, groomed, taken to toilet) | 2.8 | 3.0 = Severe Dementia | 6 & 7 |
| Very Severe | 13–15 | Needs significant help with all BADL, and needs to be moved and transferred, incontinent, needs to be fed or tube fed | 3.7 | 4.0 = Profound Dementia | 7 |
CDR means obtained in this study for each dependence stage.
FAST score was estimated post hoc based upon descriptions of functional limitations at each FAST score and descriptions of dependence scale item scores that would most commonly be represented at each dependence stage.
Abbreviations: DS, Dependence Scale; CDR, Clinical Dementia Rating; FAST, Functional Assessment Staging; IADL, Instrumental Activities of Daily Living; BADL, Basic Activities of Daily Living.
2.2.2 Healthcare costs
Healthcare costs were estimated from the healthcare utilization of AD patients in the 12 months before the survey interview, in the first year after baseline and in second year after baseline for three time points (i.e. baseline, year 1 follow-up, year 2 follow-up). Costs included direct costs (formal and informal) of care for AD patients across the disease severity spectrum. To estimate direct costs, caregiver-reported utilization counts were summed for each type of care and then multiplied by a unit cost for each type of care. The types of care included in this estimation included physician visits, medications, hospitalizations, paid home care, adult daycare, and long-term care services (nursing home, assisted living, and foster home care). The paid home care services were separated into professional (e.g. nurse, physical therapist, speech therapist) and non-professional categories. The informal direct costs included unpaid caregiving estimated by the approach of replacement wages. Caregiver time was estimated using the Caregiver Time Survey. The details of our cost computations, unit costs, and caregiver time estimates have been described previously23,24 Our cost estimates did not include emergency room visits without an overnight stay or outpatient surgical procedures in the cost estimates. All the cost estimates were inflated to 2015 US dollars using the medical component of the Consumer Price Index.
2.2.3 Health-related quality of life
HRQoL was measured by the US-weighted version of EuroQoL five dimension, three-level, questionnaire (EQ5D-US),26 and the Health Utility Index Mark 2 (HUI:2).27 Both are preference weighted HRQoL scales that can be used to calculate QALYs, and each range from 0 to 1, with 0 as death and 1 as perfect health. Questionnaire responses were proxy ratings by the caregiver for the subject with dementia. Scores represented current HRQoL and were measured at baseline, and during year 1, and year 2 follow-up interviews. Previous research in patients with AD have shown that proxy ratings of the HUI:2 or HUI:3 correlate better with changes in dementia severity than patient ratings.28,29
2.2.4 Unpaid caregiver perceived burden
Unpaid caregiver burden was measured by the Screen for Caregiver Burden (SCB), developed by Vitaliano and colleagues to identify distress experienced specifically for spouse caregivers of AD patients.30 This 25-question tool was created to assess the prevalence of caregiver experiences (objective burden), and the distress experienced by spouse caregivers of AD patients (subjective burden). The total score of objective burden (range, 0–25) represents the number of experiences that have occurred to caregivers with 0 as did not occur and 1 as occurrence. Subjective burden was measured on a 5-point scale: 0 (no occurrence of the experience), 1 (occurrence of the experience, but not distress), 2 (occurrence with mild distress), 3 (occurrence with moderate distress), and 4 (occurrence with severe distress), with a range of 0–100. The higher scores denote greater burden for both objective and subjective measures.
In addition, the Neuropsychiatric Inventory caregiver distress (NPI-D) scale31 (an abridged version of the Relative Stress Scale) was also used to quantify caregiver burden related to care recipients’ neuropsychiatric symptoms (10 domains) on a 6-point scale: 0 (not at all distressing, 1 (minimally distressing), 2 (mildly distressing), 3 (moderately distressing), 4 (severely distressing), and 5 (very severely distressing), with a range of 0–50.
Measures of caregiver perceived burden were measured during the baseline interview only, to help limit the duration of the caregiver follow-up interviews because of concerns of subject burden and risk of losing subjects during follow-up.
2.2.5 Other Baseline Measures
Demographic information including current age, age at onset of AD symptoms, sex, education level, marital status, race, living situation, and caregivers’ age, sex, and relationship to patient was collected at baseline. Cognitive function was measured with the MMSE (range, 0–30).3 Behavioral symptoms were measured with the Neuropsychiatric Inventory (NPI), which measures frequency and severity of behavioral symptoms in 12 domains to derive the total NPI score (range, 0–144).32 The presence and severity of signs of parkinsonism were measured using a modified Columbia University Parkinson’s Disease Rating Scale that was developed to measures extrapyramidal signs (EPS) in patients with dementia.33 Dementia severity was measured by the CDR scale which categorizes dementia severity into 0.5 (questionable dementia), 1 (mild dementia), 2 (moderate dementia), 3 (severe dementia), and 4 (profound dementia) using a semi-structured interview of caregivers followed by an interview with the dementia patient. Six domains are scored and a scoring algorithm determines the total CDR score.4 Alternatively the scores on the six domains can be added for a more continuous measure (i.e. CDR sum of boxes scoring (CDR-SB). A measure of the number and severity of co-morbid medical conditions were measured by Cumulative Illness Rating Scale (CIRS).34
2.3 Statistical analysis
Baseline demographic and clinical data by disease severity (from the very mild to the very severe dependence stage) were compared using a non-parametric Kruskal-Wallis test for continuous covariates and Chi-square test or Fisher’s exact test (given the potential small number in certain cells) for categorical covariates.
Due to the three, time-point measurements, repeated measures models were used to examine the relationship between the dependence stages and total healthcare costs and HRQoL. The analyses were performing using generalized estimating equations (GEE) models with the Gamma distribution and the log link was used to predict point estimates of costs of care, whereas GEE models with Gaussian distribution and the identity link was applied to the estimation of EQ5D-US and HUI:2. The Gamma distribution has been advocated by health economists to model skewed outcomes such as health cost data with a log link.35 All the other covariates were controlled in the multivariable models.
Furthermore, ordinary least square (OLS) regression analyses was conducted to examine the relationship between baseline characteristics and baseline caregiver-perceived burden (as measured by objective burden, subjective burden, and NPI-Q) and other covariates mentioned previously.
All analyses were conducted using Stata version 14 (StataCorp, College Station, TX). The P values were considered significant at α < 0.05 (2-tailed tests). Race and ethnicity were not included in multivariable statistical models because the sample was predominantly white.
3. RESULTS
3.1 Descriptive Statistics
Overall, 132 patients with probable AD diagnosis were included in the present study. Table 2 presents their baseline characteristics by the dependence stage. At baseline, there were 8 subjects were very mild stage, 22 were mild, 47 were mild to moderate, 24 were moderate, 25 were severe, and 6 were very severe. The mean age was 76.5 years, the mean age at onset of AD was 71.5 years, 54 % were female, 59% had education < 12 years, 64% were married, 97% were white, and 73% lived with others at home. The mean age of the caregivers was 63.9 years, 68% of them were female, and 59% of them were patients’ spouses. Significant differences were observed among the dependence stages in terms of the following: marital status, living arrangement, MMSE, CDR, CDR-SB, total NPI score, Parkinson’s disease rating scale, health care costs in baseline year, HUI:2, EQ5D-US, caregiver’s relation to patients, and caregiver burden measures. Subject were less likely to be married and more likely to be widowed in the higher dependence stages. As expected, greater dependence was associated with greater cognitive impairment, dementia severity, neuropsychiatric symptoms, parkinsonism, costs of care and caregiver burden, and lower health related quality of life scores. (Table 2).
Table 2.
Baseline characteristics of study sample by dependence stage.
| Dependence Stage based upon sum of DS scores
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Covariates,‡ | Very Mild n=8 |
Mild n=22 |
Mild to Moderate n=47 |
Moderate n=24 |
Severe n=25 |
Very severe n=6 |
Total n=132 |
P-value |
| Patients characteristics | ||||||||
| Age at baseline, mean (SD) | 73.3(8.8) | 73.0(9.4) | 77.4(8.1) | 76.8(9.0) | 78.0(9.3) | 81.0(7.9) | 76.5(8.8) | 0.152 |
| Age at onset, mean (SD) | 70.8(8.3) | 69.2(9.7) | 73.2(8.5) | 71.5(9.7) | 71.0(9.9) | 71.7(8.2) | 71.5(9.1) | 0.674 |
| Female, % | 75.0 | 31.8 | 46.8 | 58.3 | 68.0 | 83.3 | 54.1 | 0.066 |
| Education (year), % | 0.254 | |||||||
| <=12 | 50.0 | 63.6 | 51.1 | 58.3 | 72.0 | 66.7 | 58.6 | |
| 13–16 (high school- college) | 50.0 | 22.7 | 38.3 | 25.0 | 16.0 | 16.7 | 28.6 | |
| >=17 (graduate school) | 0 | 13.6 | 10.6 | 16.7 | 12.0 | 16.7 | 12.8 | |
| Marital status, % | 0.021 | |||||||
| Married | 87.5 | 90.9 | 57.4 | 62.5 | 60.0 | 0.0 | 63.9 | |
| Windowed | 0.0 | 4.5 | 34 | 33.3 | 36.0 | 83.3 | 29.3 | |
| Divorced/Separated/Never married | 12.5 | 4.5 | 8.5 | 4.2 | 4.0 | 16.7 | 6.8 | |
| Race, % | 0.853 | |||||||
| White | 100 | 100 | 100 | 91.7 | 92.0 | 100 | 97.0 | |
| African American | 0 | 0 | 0 | 4.2 | 4 | 0 | 1.5 | |
| Native/Others | 0 | 0 | 0 | 4.2 | 4 | 0 | 1.5 | |
| Living arrangement, % | <0.001 | |||||||
| Living alone at home | 12.5 | 4.5 | 8.5 | 0 | 0 | 0 | 4.5 | |
| Living with others at home | 87.5 | 95.5 | 72.3 | 75 | 60.0 | 16.7 | 72.9 | |
| Assisted living/Fostercare home | 0 | 0 | 19.1 | 20.8 | 24.0 | 16.7 | 15.8 | |
| Nursing home | 0 | 0 | 0 | 4.2 | 16.0 | 66.7 | 6.8 | |
| Household income (patient and caregiver), mean (SD) | $33,088 ($17,072) | $43,922 ($32,000) | $35,529 ($26,637) | $33,477 ($22,761) | $37,973 ($37,372) | $22,757 ($25,786) | $36,693 ($28,940) | 0.279 |
| APOE ε4 carrier status, % | 25 | 59.1 | 44.7 | 50 | 52 | 16.7 | 47.4 | 0.231 |
| Clinical measurement | ||||||||
| MMSE, mean (SD) | 23.0(2.3) | 20.0(4.7) | 18.0(6.2) | 13.5(8.3) | 5.9(6.0) | 2.2(4.0) | 14.9(8.5) | <0.001 |
| CDR, mean (SD) | 0.8(0.3) | 1.0(0.4) | 1.5(0.6) | 2.0(0.9) | 2.8(0.5) | 3.7(0.5) | 1.8(1.0) | <0.001 |
| CDR-SB, mean (SD) | 4.6(0.9) | 5.4(1.9) | 8.4(3.2) | 12.0(4.7) | 15.7(2.5) | 21.3(4.3) | 10.3(5.4) | <0.001 |
| Total NPI score, mean (SD) | 1.5(1.9) | 9.3(10.8) | 16.8(13.1) | 28.6(17.3) | 22.6(16.1) | 26.7(11.0) | 18.2(15.5) | <0.001 |
| CIRS, mean (SD) | 18.6(4.3) | 21.1(4.0) | 20.3(3.3) | 21.0(4.0) | 21.3(4.3) | 24.5(5.5) | 20.8(4.0) | 0.212 |
| Parkinson’s disease rating scale | 2.1(3.0) | 2.1(1.7) | 3.1(3.1) | 2.5(2.0) | 5.2(3.1) | 13.3(6.4) | 3.6(3.8) | <0.001 |
| Healthcare costs in the baseline year, mean (SD) | $4,903 ($2,360) | $15,576 ($13,065) | $39,125 ($30,840) | $58,515 ($21,584) | $60,442 ($26,349) | $68,246 ($27,395) | $41,758 ($30,844) | <0.001 |
| Health-related quality of life | ||||||||
| HUI:2 | 0.84(0.12) | 0.78(0.14) | 0.65(0.15) | 0.48(0.16) | 0.38(0.13) | 0.20(0.04) | 0.58(0.22) | <0.001 |
| EQ5D-US | 0.92(0.09) | 0.83(0.05) | 0.77(0.12) | 0.62(0.18) | 0.44(0.16) | 0.30(0.14) | 0.68(0.21) | <0.001 |
| Caregiver characteristics | ||||||||
| Age, mean (SD) | 71.5(11.9) | 68.50(10.54) | 63.00(13.04) | 62.83(15.81) | 61.80(12.64) | 56.67(12.14) | 63.9(13.2) | 0.202 |
| Female, % | 37.5 | 68.2 | 72.3 | 62.5 | 72 | 83.3 | 67.7 | 0.314 |
| Relationship to patients, % | 0.008 | |||||||
| Spouse | 87.5 | 86.4 | 55.3 | 54.2 | 52 | 0 | 59.4 | |
| Children | 12.5 | 4.5 | 42.6 | 41.7 | 48 | 83.3 | 36.8 | |
| Siblings/relatives/friends | 0 | 9.1 | 2.1 | 4.2 | 0 | 16.7 | 3.8 | |
| Caregiver objective burden, mean (SD) | 2.6(3.2) | 5.7(2.8) | 6.9(3.2) | 10.5(3.5) | 9.8(3.5) | 8.2(5.2) | 7.7(4.0) | <0.001 |
| Caregiver subjective burden, mean (SD) | 3.9(4.8) | 13.0(8.1) | 17.3(11.2) | 26.5(10.6) | 23.3(10.5) | 19.7(14.2) | 18.6(11.8) | <0.001 |
| Caregiver NPI distress score, mean (SD) | 1.1(1.3) | 5.4(6.3) | 8.8(6.9) | 12.6(7.9) | 9.2(5.9) | 9.50(5.3) | 8.5(7.1) | <0.001 |
Total number of probable AD was 132. Missing data was very low, except for APOE ε4 carrier status where 34 subjects having missing data.
Text in bold represents a statistical significance at α=0.05.
Abbreviation: DS, Dependence Scale; SD, standard deviation; MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating sum of boxes scoring; NPI, Neuropsychiatric Inventory; CIRS, Cumulative Illness Rating Scale; HUI:2, Health Utilities Index Mark 2; EQ5D-US, EuroQoL 5 Dimensions with US population scoring
3.2 Dependence stage and healthcare costs
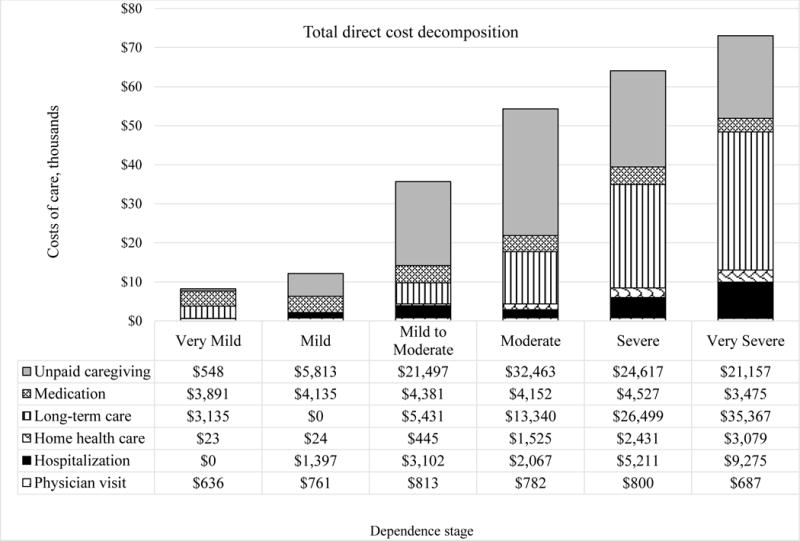
Descriptive results showed that the dependence stage (defined by DS scores) and total healthcare costs had a positive correlation, as AD patients in the very severe stage had the highest total healthcare costs (Figure 1; see Appendix Table S1 for standard deviations for each unadjusted value and Table S2 for annual resource use by dependence stage and cost category). When examining the cost composition of care, unpaid caregiving was the component with the highest average costs of $21,671 per patient, but varied by the dependence stage. For example, this service was the highest cost component for patients in the mild, mild to moderate, and moderate stages; however, the formal long-term care was the highest cost component for patients in the severe and very severe stages (Figure 1). For the cohort at all time points, the annual average direct cost of formal care per patient was $24,405 and $21,671 for informal care.
Figure 1.
Unadjusted, annual direct costs (formal and informal) by dependence stage for all three time points.
Table 3 summarizes the results of GEE models examining the relationship between important covariates and total, direct health care costs in this cohort. Dependence and co-morbid medical conditions, as measured by CIRS, were significantly associated with total costs in multivariable models. Other covariates, including age, sex, education, marital status, neuropsychiatric symptoms, parkinsonism, caregiver characteristics and caregiver burden were not significantly associated with total, direct health care costs. Table 4 presents the covariate-adjusted mean, annual total healthcare costs with estimated standard deviations by dependence stages (see Appendix Table S3 for the cost estimates excluding unpaid caregiving).
Table 3.
Summary of GEE models results for the relationship between outcomes of interests (healthcare costs and HRQoL) and other covariates.
| Healthcare costs‡ | HRQoL§
|
|||||
|---|---|---|---|---|---|---|
| HUI:2 | EQ5D-US | |||||
|
|
||||||
| Covariates† | Model 1¶ | Model 2¶ | Model 1¶ | Model 2¶ | Model 1¶ | Model 2¶ |
| Patients characteristics | ||||||
| Age at baseline | −0.002 | 0.001 | 0.003** | 0.004* | 0.003*** | 0.004** |
| Sex (female=0) | −0.040 | −0.092 | −0.005 | 0.003 | 0.022 | 0.023 |
| Education (≤12 years =0) | ||||||
| >12 years | 0.048 | 0.040 | 0.011 | 0.013 | 0.019 | 0.021 |
| Marital status (currently married=0) | ||||||
| Not currently married | −0.057 | −0.069 | −0.043* | −0.038 | 0.008 | −0.002 |
| Clinical measurement | ||||||
| Total NPI score | 0.004 | 0.002 | −0.002*** | −0.002 | −0.002** | −0.002** |
| CIRS | 0.031** | 0.030** | −0.004 | −0.003 | −0.007** | −0.007** |
| Summated dependence scale | 0.173*** | 0.172*** | −0.050*** | −0.050*** | −0.058*** | −0.060*** |
| Parkinson’s disease rating scale | 0.006 | 0.007 | −0.007*** | −0.007*** | −0.008*** | −0.008*** |
| Caregiver characteristic | ||||||
| Age at baseline | −0.004 | −0.001 | −0.001 | |||
| Sex (female=0) | −0.064 | 0.014 | 0.015 | |||
| Relationship to patients (spouse=0) | ||||||
| Non-spouse | −0.108 | −0.016 | −0.006 | |||
| Caregiver subjective burden | 0.00004 | 0.00002 | 0.002 | |||
| Caregiver NPI distress score | 0.005 | −0.002 | 0.00001 | |||
P < 0.05;
P < 0.01;
P < 0.001.
CDR, CDR-SB, MMSE, HUI:2 and EQ5D-US were removed from the models due to collinearity with dependence scale (r = 0.77, 0.81, −0.70, −0.78, and −0.78, respectively); Age at onset was removed from the model due to collinearity with age at baseline (r = 0.93); We kept NPI in the GEE models because an independent association between NPI scores and pharmacoeconomic outcomes, over and above dependence scale scores, was found in our previous study.23
Numbers presented in Table are coefficients from GEE models.
GEE models with Gamma distribution and log link. The estimate of costs of healthcare was applied by y=exp(xβ).
GEE models with Gaussian distribution and identity link. The estimate of HRQoL was applied by y=xβ.
The difference between Model 1 and Model 2 was whether caregiver characteristics were included in the model or not.
Abbreviation: HQRoL, health-related quality of life; GEE, generalized estimating equation; MMSE, Mini–Mental State Examination; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating sum of boxes scoring; NPI, Neuropsychiatric Inventory; CIRS, Cumulative Illness Rating Scale; HUI:2, Health Utilities Index Mark 2; EQ5D-US, EuroQoL 5 Dimensions with US population scoring.
Table 4.
Estimated total, annual healthcare costs and health-related quality of life, by dependence stage. †
| Total Costs§($) | HUI:2 | EQ5D-US | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Dependence stage‡ | Mean | SD | Mean | SD | Mean | SD |
| Very Mild | $11,418 | $1,990 | 0.93 | 0.06 | 1.00 | 0.07 |
| Mild | $19,245 | $3,196 | 0.78 | 0.04 | 0.85 | 0.05 |
| Mild to Moderate | $28,670 | $6,188 | 0.65 | 0.06 | 0.72 | 0.07 |
| Moderate | $48,856 | $8,185 | 0.49 | 0.06 | 0.54 | 0.06 |
| Severe | $69,493 | $13,961 | 0.39 | 0.06 | 0.42 | 0.07 |
| Very Severe | $101,715 | $18,283 | 0.25 | 0.06 | 0.26 | 0.06 |
The values presented here were estimated by Model 2 in Table 2 using equation: y=exp(xβ) for healthcare costs and y=xb for HUI:2 and EQ5D-US. y is the outcome of interest; x is the covariate; β is the coefficient.
Dependence stage was derived from summated dependence scale scores, as described in the text.
Total costs included costs of physician visits, hospitalizations, home health care, long-term care (nursing home, assisted living, and adult daycare), medications, and unpaid caregiving.
Abbreviation: SD, standard deviation.
3.3 Dependence stage and health-related quality of life
Table 3 also reports the results of GEE models examining the relationship between important covariates and HRQoL, as measured by the HUI:2 and EQ5D-US. For EQ5D-US, age, neuropsychiatric symptoms, co-morbid medical conditions, dependence, and parkinsonism were significant, independent predictors of HRQoL, while sex, education, marital status, caregiver characteristics and caregiver burden were not significant, independent predictors of EQ5D-US scores. Similar results were found for the HUI:2 scores, except co-morbid medical conditions did not significantly predict HUI:2 scores, nor did neuropsychiatric symptoms in models that included caregiver characteristics and caregiver burden. In addition, in HUI:2 models that did not include caregiver characteristics or burden, marital status did significantly predict HUI:2 scores.
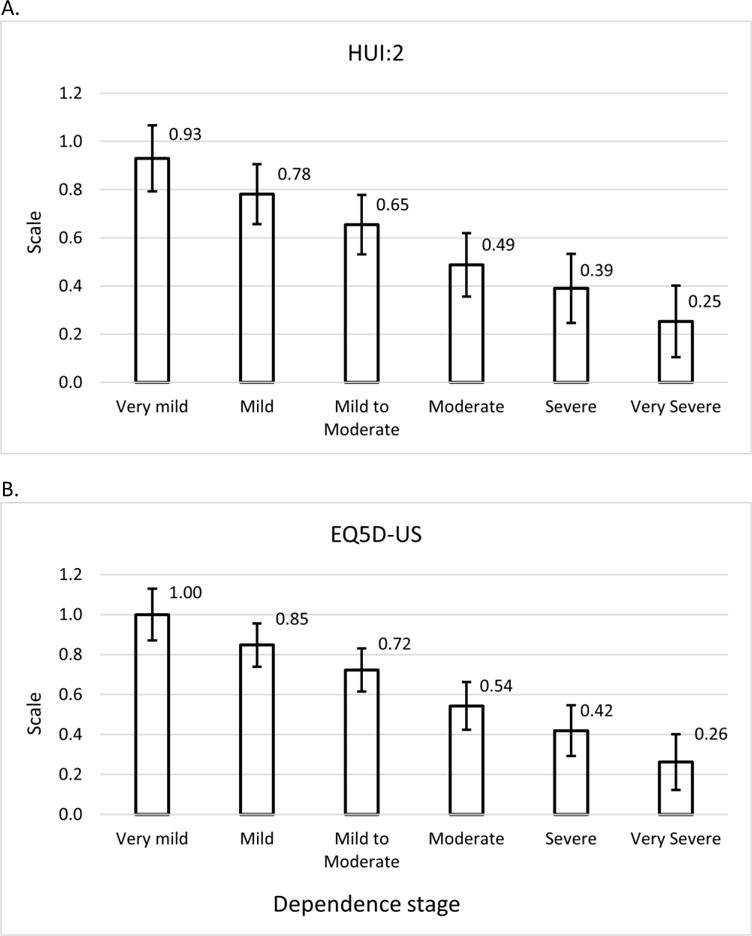
Figure 2 shows that the covariate-adjusted HRQoL measures decreased as dependence severity increased, after controlling other covariates. The estimates of HUI:2 were slightly lower than those for EQ5D-US regardless of dependence stages. The covariate-adjusted, estimated mean HUI:2 and EQ5D-US scores and associated standard deviations for the six dependence stages are shown in Table 4.
Figure 2.
The estimated HRQoL by dependence stage (defined by DS scores). (A) HUI:2; (B) EQ5D-US. Numbers indicate mean values; error bar indicates 95% confidence interval.
3.4 Unpaid caregiver-perceived burden
Table 5 presents the relationship between baseline, unpaid caregiver-perceived burden and other covariates. In general, OLS results indicated that the worsening dependence level was associated with higher objective and subjective burden. Higher neuropsychiatric symptoms (NPI scores) and dependence scores of patients, and younger age of caregivers were associated with a higher caregiver objective and subjective burden scores. In addition, not currently married patients were less likely to cause caregiver objective burden but not subjective burden than their married counterparts. When using NPI distress score as an outcome measure, caregivers who were not spouses of the AD patients were less likely have distress than caregivers who were spouses of the AD patients. In addition, there was a high correlation between the total NPI score of AD patients and the NPI distress score of their caregivers (Spearman rank correlation coefficient ρ=0.8, p < 0.01, not shown).
Table 5.
Ordinary least square regression analysis of the relationship between baseline unpaid caregiver-perceived burden (measured by objective burden, subjective burden, and NPI distress score) and other covariates.
| Caregiver perceived burden
|
|||
|---|---|---|---|
| Covariates† | Objective burden | Subject burden | NPI distress score |
| Patient characteristics | |||
| Age at baseline | 0.020 | 0.059 | 0.029 |
| Sex (female=0) | 1.731** | 3.840 | 0.078 |
| Education (≤12 years =0) | |||
| >12 years | 0.706 | 1.620 | 0.066 |
| Marital status (currently married=0) | |||
| Not currently married | −2.068* | −3.803 | 3.179 |
| Clinical measurement | |||
| Total NPI score | 0.095*** | 0.321*** | 0.337*** |
| CIRS | 0.077 | 0.158 | 0.221* |
| Summated dependence scale | 0.537*** | 1.256*** | 0.035 |
| Parkinson’s disease rating scale | −0.106 | −0.265 | −0.110 |
| Caregiver characteristic | |||
| Sex (female=0) | −0.062* | −0.256* | −0.123* |
| Age at baseline | 0.107 | −1.233 | −0.753 |
| Relationship to patients (spouse=0) | |||
| Non-spouse | 0.545 | −2.593 | −6.099** |
P < 0.05;
P < 0.01;
P < 0.001.
Numbers presented in Table are coefficients from ordinary least square analyses.
4. DISCUSSION
In this study, we found that the increasing levels of dependence, as measured by the DS, were associated with significant increases in total healthcare costs, unpaid caregiving costs, and caregiver burden (both objective and subjective), and with decreases in HRQoL (EQ5D-US, HUI:2) after controlling for other covariates. DS scores classified into six dependence stages identified unique and clinically meaningful levels of care-associated costs and health utilities that could be used in pharmacoeconomic evaluations of new treatments for AD patients.
Our results are in line with previous studies that have shown that increased dependence is associated with higher healthcare costs for dementia patients.17,24,36–38 Moreover, our results confirmed the impact of comorbidities on costs of care, which is consistent with other AD cost studies that have shown increased healthcare costs in patients with dementia that have comorbid conditions, because of increased the risk of hospital care and increasing length of stay.38–40 This suggests that effective management of common general medical comorbidities could decrease dementia care costs by preventing hospitalizations or prolonged lengths of stay.41 In addition, it is possible that unpaid caregiving time and costs reached a peak for patients in the moderate stage and then were substituted by formal long-term care services and costs in advanced dependence stages as suggested in our study, corroborating previous studies shown that informal care costs made the highest contribution to the total costs for community-dwelling AD patients,17 and are considered a major component of total healthcare costs due to dementia.17,42,43
Our results were consistent with previous studies demonstrating that behavioral disturbances measured by NPI questionnaires are associated with increased caregiver burden.31,38,44,45 Subjective feelings of caregivers have been associated directly with perceived caregiver burden, and a caregiver’s reaction to problematic behaviors is one of the strongest predictors of nursing home placement.31 Accordingly, screening, assessment, and monitoring of the degree of burden associated with caregiving is crucial. A potential economic gain from providing support to caregivers may be expected because studies have shown that caregiver interventions that decrease their distress can reduce the risk of nursing home placement for AD patients.46,47 This in turn may reduce the total costs of care.
Our finding that HRQoL, measured by two different scales, decreases as dependence increases in patients with AD is consistent with previous studies that have examined this association.5,7 Andersen and colleagues48 concluded that the estimated health utility (proxy-rated EQ-5D) decrement due to the loss of independence was 0.30, in which the dependence level was simply categorized into two groups- either dependent (0.34) or independent (0.64). In the present study, we estimated a EQ5D-US value of 1.00 for the very mild dependence stage compared with 0.26 for completely dependent patients, which yields a difference between dependence stages of 0.74. Thus, our analysis encompassed a wider range of dependence levels and provides estimates of HRQoL for six dependence stages instead of Anderson’s dichotomous classification. In addition, even though research has indicated that caregiver factors such as burden and depression may influence the HRQoL ratings provided by a proxy,49–51 we found that dependence remained a significant independent predictor of HRQoL in multivariable models that accounted for caregiver burden and caregiver distress in the analyses.
In this study, we proposed a new classification of the DS into six stages of dependence with the goal of defining clinically-meaningful stages for use in economic evaluations of new AD interventions. This and previous studies have demonstrated the advantages of the DS in predicting important pharmacoeconomic outcomes. In our analyses, each of the six dependence stages provided unique information about outcomes important for economic evaluations, such as costs of care and HRQoL. An advantage of the DS and our classification is the ease of administration and classification into dependence stages.
Strengths of this study include our comprehensive dataset, which not only includes covariates on cognition, function, and behavior, but also includes covariates on comorbidities and severity of signs of Parkinsonism. In addition, other than recent studies that have used arbitrary grouping methods of DS scores (e.g., quartiles),7,38 we classified patients by stages of dependence severity. Our classification approach may provide more clinically meaningful interpretation and be more valuable in pharmacoeconomic evaluations of new interventions for patients with AD. Our approach also better characterizes temporal disease progression compared to CDR or MMSE for which several years may elapse before progression to the next stage occurs. Finally, our results provide estimates of costs of care and HRQoL stratified by dependence levels, which are critically important in pharmacoeconomic evaluations of new AD interventions.
Several limitations should be considered when interpreting results of this study. First, the study population was predominantly white residents of Michigan, so results may not generalize to patients across the U.S. However, our sample included patients from across the dementia severity spectrum and with a variety of co-morbid conditions in contrast to most AD clinical trials that include only patients with mild to moderate dementia that do not have comorbid conditions. Second, we were not able to examine other physical function measurements such as the Disability Assessment for Dementia, to examine the relationship between functional limitations, dependence, costs, and quality of life. However, the DS served as a composite measure of cognition, function, and behavioral symptoms of dementia patients, and has been validated by several previous studies.10–14 Fourth, we had limited sample size (n=132) in the present study. The estimates of costs of care and HRQoL may suffer from larger variances. Further research with larger sample sizes may be of value to provide more accurate point estimates for pharmacoeconomic evaluation. Finally, we derived costs of care by multiplying units of health care utilization by a unit cost for each type of service. The accuracy of our estimates could be compromised by error in recall of services, inaccurate estimates of unit costs or changes in unit costs over time or by region. Despite these potential limitations, this approach to estimating costs of care has been used commonly in previous economic evaluations for AD treatments.2
Summated DS scores classified into six dependence stages provide a useful method to estimate unique levels of care-associated costs and health utilities that can be used in economic evaluations of new treatments for AD. Moreover, dependence levels predict the amount of paid and unpaid care required, and can be used to monitor caregiver burden and patient symptoms likely to be associated with transitions from home based care to institutional, long-term care facilities. These dependence levels can therefore be used to design and implement targeted patient and caregiver interventions and support.1
Supplementary Material
Acknowledgments
Source of support: The National Institute on Aging supported this work through grants K08-AG00864 for Dr. Murman and P50-AG08671 for the Michigan AD Research Center for data collection. Support was received from Janssen Alzheimer Immunotherapy, LLC for data analysis and reporting.
References
- 1.Cohen JT, Neumann PJ. Decision analytic models for Alzheimer’s disease: state of the art and future directions. Alzheimer’s & Dementia. 2008;4(3):212–222. doi: 10.1016/j.jalz.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Green C, Picot J, Loveman E, Takeda A, Kirby J, Clegg A. Modelling the cost effectiveness of cholinesterase inhibitors in the management of mild to moderately severe Alzheimer’s disease. Pharmacoeconomics. 2005;23(12):1271–1282. doi: 10.2165/00019053-200523120-00010. [DOI] [PubMed] [Google Scholar]
- 3.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Pesearch. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin T, Buxton M, Mittendorf T, et al. Assessment of potential measures in models of progression in Alzheimer disease. Neurology. 2010;75(14):1256–1262. doi: 10.1212/WNL.0b013e3181f6133d. [DOI] [PubMed] [Google Scholar]
- 6.Jönsson L, Wimo A. The cost of dementia in Europe. Pharmacoeconomics. 2009;27(5):391–403. doi: 10.2165/00019053-200927050-00004. [DOI] [PubMed] [Google Scholar]
- 7.Jones RW, Romeo R, Trigg R, et al. Dependence in Alzheimer’s disease and service use costs, quality of life, and caregiver burden: The DADE study. Alzheimer’s & Dementia. 2015;11(3):280–290. doi: 10.1016/j.jalz.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Green C, Shearer J, Ritchie CW, Zajicek JP. Model-based economic evaluation in Alzheimer’s disease: a review of the methods available to model Alzheimer’s disease progression. Value in Health. 2011;14(5):621–630. doi: 10.1016/j.jval.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin T, Feldman H, Fillit H, et al. Dependence as a unifying construct in defining Alzheimer’s disease severity. Alzheimer’s & Dementia. 2010;6(6):482–493. doi: 10.1016/j.jalz.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenderking WR, Wyrwich KW, Stolar M, et al. Reliability, validity, and interpretation of the dependence scale in mild to moderately severe Alzheimer’s Disease. American Journal of Alzheimer’s Disease and Other Dementias. 2013;28(8):738–749. doi: 10.1177/1533317513504609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern Y, Albert SM, Sano M, et al. Assessing patient dependence in Alzheimer’s disease. Journal of Gerontology. 1994;49(5):M216–M222. doi: 10.1093/geronj/49.5.m216. [DOI] [PubMed] [Google Scholar]
- 12.Spackman DE, Kadiyala S, Neumann PJ, Veenstra DL, Sullivan SD. The validity of dependence as a health outcome measure in Alzheimer’s disease. American Journal of Alzheimer’s Disease and Other Dementias. 2013;28(3):245–252. doi: 10.1177/1533317513481092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyrwich KW, Auguste P, Buchanan J, et al. Psychometric Properties of the Dependence Scale in Large Randomized Clinical Trials of Patients With Mild and Moderate Alzheimer’s Disease. American Journal of Alzheimer’s Disease and Other Dementias. 2014;29(7):620–629. doi: 10.1177/1533317514527336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garre-Olmo J, Vilalta-Franch J, Calvó-Perxas L, Monserrat-Vila S, López-Pousa S. Dependence Scale for Alzheimer’s Disease Relationship With Other Clinical Indicators and Psychometric Properties. Journal of Geriatric Psychiatry and Neurology. 2015;28(2):117–125. doi: 10.1177/0891988714554711. [DOI] [PubMed] [Google Scholar]
- 15.Lacey LA, McLaughlin TP, Mucha L, Grundman M, Black R. Relationship between patient dependence on others and caregiver burden in Alzheimer’s Disease (AD) Alzheimer’s & Dementia. 2009;5(4):P229–P230. [Google Scholar]
- 16.Kahle-Wrobleski K, Andrews J, Belger M, et al. Clinical and Economic Characteristics of Milestones along the Continuum of Alzheimer’s Disease: Transforming Functional Scores into Levels of Dependence. The Journal of Prevention of Alzheimer’s Disease. 2015;2(2):115–120. doi: 10.14283/jpad.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacey LA, Niecko T, Leibman C, Liu E, Grundman M. Association between illness progression measures and total cost in Alzheimer’s disease. The Journal of Nutrition, Health & Aging. 2013;17(9):745–750. doi: 10.1007/s12603-013-0368-1. [DOI] [PubMed] [Google Scholar]
- 18.Murman DL, Charlton M, High R, Leibman C, McLaughlin T. Predicting costs of care for unique dependence levels in patients with Alzheimer’s disease. Alzheimer’s & Dementia. 2009;5(4):P408. [Google Scholar]
- 19.Murman DL, Charlton M, High R, Leibman C, McLaughlin T. Estimating health-related quality of life for unique dependence levels in patients with Alzheimer’s disease. Alzheimer’s & Dementia. 2009;5(4):P236–P237. [Google Scholar]
- 20.Reisberg B, Ferris SH, De Leon M, et al. The stage specific temporal course of Alzheimer’s disease: functional and behavioral concomitants based upon cross-sectional and longitudinal observation. Progress in Clinical and Biological Research. 1988;317:23–41. [PubMed] [Google Scholar]
- 21.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. The American Journal of Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 22.Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. International Psychogeriatrics. 1992;4(03):55–69. doi: 10.1017/s1041610292001157. [DOI] [PubMed] [Google Scholar]
- 23.Murman D, Chen Q, Powell M, Kuo S, Bradley C, Colenda C. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59(11):1721–1729. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]
- 24.Murman DL, Von Eye A, Sherwood PR, Liang J, Colenda CC. Evaluated need, costs of care, and payer perspective in degenerative dementia patients cared for in the United States. Alzheimer Disease & Associated Disorders. 2007;21(1):39–48. doi: 10.1097/WAD.0b013e31802f2426. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Medical Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Torrance GW, Feeny DH, Furlong WJ, Barr RD, Zhang Y, Wang Q. Multiattribute utility function for a comprehensive health status classification system: Health Utilities Index Mark 2. Medical Care. 1996;34(7):702–722. doi: 10.1097/00005650-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Karlawish JH, Zbrozek A, Kinosian B, Gregory A, Ferguson A, Glick HA. Preference-based quality of life in patients with Alzheimer’s disease. Alzheimer’s & Dementia. 2008;4(3):193–202. doi: 10.1016/j.jalz.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Karlawish JH, Zbrozek A, Kinosian B, et al. Caregivers’ assessments of preference-based quality of life in Alzheimer’s disease. Alzheimer’s & Dementia. 2008;4(3):203–211. doi: 10.1016/j.jalz.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Vitaliano PP, Russo J, Young HM, Becker J, Maiuro RD. The screen for caregiver burden. The Gerontologist. 1991;31(1):76–83. doi: 10.1093/geront/31.1.76. [DOI] [PubMed] [Google Scholar]
- 31.Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. Journal of the American Geriatrics Society. 1998;46(2):210–215. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 32.Cummings JL. The Neuropsychiatric Inventory Assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):10S–16S. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 33.Richards M, Marder K, Bell K, Dooneief G, Mayeux R, Stern Y. Interrater reliability of extrapyramidal signs in a group assessed for dementia. Archives of Neurology. 1991;48(11):1147–1149. doi: 10.1001/archneur.1991.00530230055021. [DOI] [PubMed] [Google Scholar]
- 34.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Research. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 35.Azuero A, Pisu M, McNees P, Burkhardt J, Benz R, Meneses K. An application of longitudinal analysis with skewed outcomes. Nursing Research. 2010;59(4):301. doi: 10.1097/NNR.0b013e3181e507f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gillespie P, O’shea E, Cullinan J, Lacey L, Gallagher D, Ni Mhaolain A. The effects of dependence and function on costs of care for Alzheimer’s disease and mild cognitive impairment in Ireland. International Journal of Geriatric Psychiatry. 2013;28(3):256–264. doi: 10.1002/gps.3819. [DOI] [PubMed] [Google Scholar]
- 37.Zhu CW, Leibman C, McLaughlin T, et al. The effects of patient function and dependence on costs of care in Alzheimer’s disease. Journal of the American Geriatrics Society. 2008;56(8):1497–1503. doi: 10.1111/j.1532-5415.2008.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akerborg O, Lang A, Wimo A, et al. Cost of Dementia and Its Correlation With Dependence. Journal of Aging and Health. 2016 doi: 10.1177/0898264315624899. [DOI] [PubMed] [Google Scholar]
- 39.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. Journal of the American Geriatrics Society. 2004;52(2):187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 40.Hill J, Futterman R, Duttagupta S, Mastey V, Lloyd J, Fillit H. Alzheimer’s disease and related dementias increase costs of comorbidities in managed Medicare. Neurology. 2002;58(1):62–70. doi: 10.1212/wnl.58.1.62. [DOI] [PubMed] [Google Scholar]
- 41.Lyketsos CG. Prevention of unnecessary hospitalization for patients with dementia: The role of ambulatory care. JAMA. 2012;307(2):197–198. doi: 10.1001/jama.2011.2005. [DOI] [PubMed] [Google Scholar]
- 42.Gustavsson A, Cattelin F, Jönsson L. Costs of care in a mild-to-moderate Alzheimer clinical trial sample: key resources and their determinants. Alzheimer’s & Dementia. 2011;7(4):466–473. doi: 10.1016/j.jalz.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Rapp T, Andrieu S, Molinier L, et al. Exploring the relationship between Alzheimer’s disease severity and longitudinal costs. Value in Health. 2012;15(3):412–419. doi: 10.1016/j.jval.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Schnaider Beeri M, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. International Journal of Geriatric Psychiatry. 2002;17(5):403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 45.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 46.Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 47.Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B. A family intervention to delay nursing home placement of patients with Alzheimer disease: a randomized controlled trial. JAMA. 1996;276(21):1725–1731. [PubMed] [Google Scholar]
- 48.Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sørensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health and Quality of Life Outcomes. 2004;2(1):1. doi: 10.1186/1477-7525-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conde‐Sala JL, Garre‐Olmo J, Turró‐Garriga O, López‐Pousa S, Vilalta‐Franch J. Factors related to perceived quality of life in patients with Alzheimer’s disease: the patient’s perception compared with that of caregivers. International Journal of Geriatric Psychiatry. 2009;24(6):585–594. doi: 10.1002/gps.2161. [DOI] [PubMed] [Google Scholar]
- 50.Schiffczyk C, Romero B, Jonas C, Lahmeyer C, Müller F, Riepe MW. Generic quality of life assessment in dementia patients: a prospective cohort study. BMC Neurology. 2010;10(1):48. doi: 10.1186/1471-2377-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trigg R, Jones RW, Knapp M, King D, Lacey LA. The relationship between changes in quality of life outcomes and progression of Alzheimer’s disease: results from the Dependence in AD in England 2 longitudinal study. International Journal of Geriatric Psychiatry. 2015;30(4):400–408. doi: 10.1002/gps.4150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


