Abstract
Hospitalization is known to occur frequently in the first 6 months following liver transplantation (LT). Using a novel data linkage between the Scientific Registry of Transplant Recipients and Centers for Medicare and Medicaid Services, our study has two objectives: (i) determine risk factors for “early” hospitalization (i.e., within 6 months of LT) (ii) quantify the importance of hospitalization history in the first 6 months with respect to subsequent patient survival (i.e., survival, conditional on surviving 6 months post-LT).
Methods
The study population consisted of patients aged ≥18 years who underwent deceased donor LT between January 1, 2003, and December 31, 2010, with Medicare as primary or secondary insurance and were discharged alive from the index LT hospitalization (n = 7,220).
Results
The early hospitalization rate was 2.76 per patient-year and was significantly associated with many recipient factors (e.g., recipient age, hepatitis C, diabetes, poor renal function including dialysis and recipient of TIPSS procedure before LT), as well as donor race and donation after cardiac death (DCD). Conditional on surviving 6 months post-LT, the covariate-adjusted death rate increased by 22% for each additional hospitalization occurring in the first 6 months (HR=1.22; p<0.001).
Conclusions
Several LT recipient factors are significantly associated with early hospitalization. Moreover, a patient’s hospitalization profile during follow-up months 0–6 is a very strong predictor of survival thereafter. Efforts and resources should be devoted towards identifying LT recipients at risk for early hospitalization and modifying the actionable risk factors such as hepatitis C, diabetes and BMI to improve resource utilization and overall outcomes.
Keywords: Model for End Stage Liver Disease, Hepatitis C, Renal Risk Index, Resource utilization, Readmission
Introduction
Hospitalization after a surgical procedure or discharge following a medical condition such as pneumonia or congestive heart failure adds significantly to morbidity and mortality.(1) Consequently, reduction of hospital readmission has become a new target for quality improvement.(2) As part of the Affordable Care Act (ACA), the Centers for Medicare and Medicaid Services (CMS) are directed to push hospitals to reduce 30-day readmission rates via reduction in payments to hospitals for acute care readmission within 30-days of discharge as opposed to longer time periods.(2) Transplant procedures are not included in the ACA mandate since transplant procedures are completely different and more complex than any other surgical procedures or medical conditions. Furthermore, hospitalizations within 6 months of index transplantation (“early” hospitalization) are common and may directly or indirectly affect patient outcomes, quality of care and healthcare costs.
The estimated per-patient cost for deceased donor LT is more than $500,000 for the first year, amounting to greater than $3 billion in total annual costs.(3) Post-LT discharges and hospitalization within 180 days contribute significantly to such cost.(3) Rates of post-LT hospitalization are not accurately known. Most of the research pertaining to hospitalization per se has focused on hard outcomes such as in-patient mortality or 30-day mortality. The majority of published data on post-LT hospitalization incidence and associated risk factors are from single center studies and, hence, lack generalizability and precision. (4–6)
Systematic examination of the association of recipient, donor and transplant factors with early hospitalization is important, in order to understand the primary drivers of early hospitalization so that evidence-based point of care interventions can be developed; such interventions would be expected to improve outcomes and quality. We aimed to estimate the incidence rates of early hospitalization and to determine the risk factors associated with early post-LT hospitalization rates. To carry out our objectives, we linked data from the Scientific Registry for Transplant Recipients (SRTR) and Centers from Medicare and Medicaid Services (CMS). (7) Furthermore, we examined the impact of early hospitalization rates on patient survival conditional upon surviving the first six months post-LT. The novelty in our study chiefly derives from (a) the study cohort; a linkage of two widely known national databases that are commonly used, but not often combined (b) determination of risk factors for early hospitalization among LT patients (c) explicit use of early hospitalization history as a predictor of subsequent survival.
Methods
Patient Data and Source
Clinical, demographic and claims information for adult patients who received LT between 2003 and 2010 was obtained from the SRTR and linked with CMS claims data. To allow for appropriate longitudinal follow-up, the population was limited to those enrolled in Medicare at LT and discharge from the index LT hospitalization.
This study used data from the SRTR. The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The SRTR database has a uniform structure based on transplant candidate registration information provided by each transplant center at the time of placement on the wait-list; transplant recipient registration information provided by the transplant center at the time of LT; and transplant follow-up provided by the transplant center at six months, one year, and annually thereafter. The SRTR supplements information on vital status with data on deaths from the Social Security Death Master Files and CMS, and for data on ESRD from CMS.(8)
CMS hospital claims files contain enrollment and utilization data for each beneficiary. It also has a beneficiary summary file, as well as outpatient and inpatient claims data. The MedPAR File contains inpatient hospital and skilled nursing facility (SNF) final action stay records. Each MedPAR record represents a stay in an inpatient hospital or SNF. Each MedPAR record may represent one claim or multiple claims, depending on the length of a beneficiary’s stay and the amount of services used throughout the stay. The MedPAR file includes the diagnosis (ICD-9 diagnosis), procedure (CPT procedure code), diagnosis related group, dates of admission, dates of discharge, reimbursement amount, hospital provider and beneficiary demographic information
Data Linkage
A list of adult deceased donor LT recipients from 2003 – 2010 was sent from SRTR to CMS-Contractor Buccaneer to link the SRTR records with the CMS data. The linkage was performed based on: social security number, first and last name, sex, and date of birth. Buccaneer produced a crosswalk file that allowed us to match records in SRTR and CMS data using de-identified patient identifiers as described previously.(7)
This study was approved by the University of Michigan Institutional Review Board.
Cohort Determination
The study included adult deceased donor recipients ≥18 years of age who underwent LT between January 2003 and December 2010 in the United States and were discharged alive without re-LT from the index LT hospitalization (n=7,220). We excluded recipients of living donor LT or multi-organ transplant including simultaneous liver and kidney transplant recipients, as well as patients with non-Medicare insurance.
Analytic Approach
Descriptive statistics for continuous variables were expressed as median (interquartile range) and categorical variables were expressed as counts and percentages. Unadjusted rates of post-LT hospitalization were expressed as admissions per-patient year. Patients were followed from the time of discharge from the index hospitalization (during which LT occurred) to death or loss to follow-up. Covariate missingness for the SRTR data varied from 0–9%. The exception was serum sodium (21% missingness), which was not consistently available in the SRTR prior to 10/31/2004; hence, this covariate was not included in the models. We tested missingness as a 0/1 indicator variable for each covariate, with non-significant missingness indicators then dropped from the final model. Note that results of a sensitivity analysis using complete case analysis (i.e., including patients with no missingness for any covariate) were consistent with the main results reported here.
Modeling of Early Hospitalization Rate
We focused on early hospitalizations (defined as hospitalizations within the first six months of LT) due to their relatively high frequency of occurrence, and their potential association with recipient, donor, and transplant factors. We used a proportional rates model to examine associations between recipient, donor and transplant characteristics and the rate of early hospitalization.(9) The proportional rates model is essentially an extension of the Cox model that accommodates recurrent events (i.e., events that can occur repeatedly for a patient; e.g., hospitalizations). Like the Cox model, the proportional rates model is quite flexible; the shape of the baseline rate (over follow-up time) is not specified, nor is the nature of the dependence structure of events within-patient. Note that hospitalizations for a given patient are not assumed to be independent; standard errors for the rate ratios are based on a robust (sandwich) variance estimator that accounts correlation among events within-subject, without assuming a particular structure for said correlation.
The following recipient factors were examined: age, gender, race/ethnicity, body mass index (BMI), diagnosis, on life support, hospitalization/ICU status, diabetes, ascites, albumin, creatinine, bilirubin, international normalized ratio (INR) of prothrombin time, dialysis, status 1, portal vein thrombosis and history of transjugular intrahepatic portosystemic shunt (TIPSS). The following donor and transplant factors were included: donor age, donor gender, donor race/ethnicity, height, donation after cardiac death (DCD), shared organ, cold ischemia time, donor cause of death and split liver. We also calculated the donor risk index (DRI) for descriptive purposes as described previously.(9, 10) Transplant center was adjusted for using stratification.
Three separate models of hospitalization stratified by transplant center were used to examine associations between recipient factors at LT and early post-LT hospitalizations, adjusting for donor and transplant related factors. The first model was adjusted for recipient and donor factors; the second model replaced the recipient factors with the MELD score; and the third model replaced the recipient factors with renal risk index (RRI). The RRI was calculated using the equation from Sharma et al.(11)(https://rri.med.umich.edu(12)).
Conditional Survival Modeling
Next, we examined the effect of hospitalization on post-LT mortality using Cox regression. To be specific, the Cox model being fitted here evaluates the effect of the various risk factors on survival beyond 6 months, conditional on survival to the 6-month post-LT mark. The focus in this model was the impact of the early (i.e., first 6 months following LT) hospitalization on subsequent conditional survival (i.e., given survival of the patient through the “early” post-LT period). These models all included the individual recipient, donor and transplant factors mentioned above. This model was adjusted for recipient, donor and transplant factors, as well as, the number of hospitalizations within the first 6 months after discharge from the LT hospitalization and stratified by transplant center, in order to flexibly adjust for center effects.
All statistical analyses were carried out using SAS (v9.4; SAS Institute: Cary, NC). Results with a two-sided p-value <0.05 were considered statistically significant.
Results
Cohort description
There were 38,041 adult recipients of deceased donor liver only transplants in the United States during the study period. Of these, 9,753 recipients had Medicare coverage for their transplant and at the time of discharge from the index transplant hospitalization. We excluded 136 subjects who received a previous transplant, 740 for death or graft failure during index LT hospitalization, and 1,657 without a transplant hospitalization record bracketing the date of the transplant. The final study group consisted of 7,220 recipients.
Characteristics of recipients at the time of LT are summarized in Table 1. The median age at LT was 59 years (Q1: 52; Q3: 66), 66% were males, 74% were Caucasians, 36% had hepatitis C, and 28% had history of diabetes. The median donor risk index (DRI) was 1.45 (Q1:1.22; Q3: 1.75).
Table 1.
Characteristics of the cohort at LT
| Characteristic at LT | Median (IQR) or n (%) (n=7,220) |
|---|---|
| Age | 59 (52–66) |
|
| |
| Female | 2,428 (34%) |
| Male | 4,792 (66%) |
|
| |
| White | 5,332 (74%) |
| Black | 550 (8%) |
| Asian | 276 (4%) |
| Hispanic/Latino | 985 (14%) |
| Multi-racial/other | 77 (1%) |
|
| |
| Status 1 at transplant | 81 (1%) |
|
| |
| Body mass index (BMI) | 27.8 (24.6–32.0) |
|
| |
| Hepatitis C | 2,574 (36%) |
| Cholestatic liver disease | 526 (7%) |
| Non-cholestatic liver disease | 2,288 (32%) |
| Hepatocellular carcinoma | 1,228 (17%) |
| Other liver disease | 604 (8%) |
|
| |
| Lab MELD at transplant | 17 (13–24) |
|
| |
| Albumin at transplant (g/dl) | 2.9 (2.5–3.4) |
|
| |
| Diabetes | 2,057 (28%) |
|
| |
| Dialysis | 316 (4%) |
|
| |
| No ascites | 1,346 (19%) |
| Slight ascites | 4,010 (56%) |
| Moderate ascites | 1,864 (26%) |
|
| |
| Portal vein thrombosis at transplant | 546 (8%) |
|
| |
| History of TIPSS | 768 (11%) |
|
| |
| In intensive care unit (ICU) at LT | 504 (7%) |
| Hospitalized, not in ICU | 970 (13%) |
| Not hospitalized | 5,746 (80%) |
|
| |
| Renal risk index (RRI) | 1.60 (0.99–2.84) |
|
| |
| Donor risk index (DRI) | 1.45 (1.22–1.75) |
Hospitalization rates by post-LT follow-up time
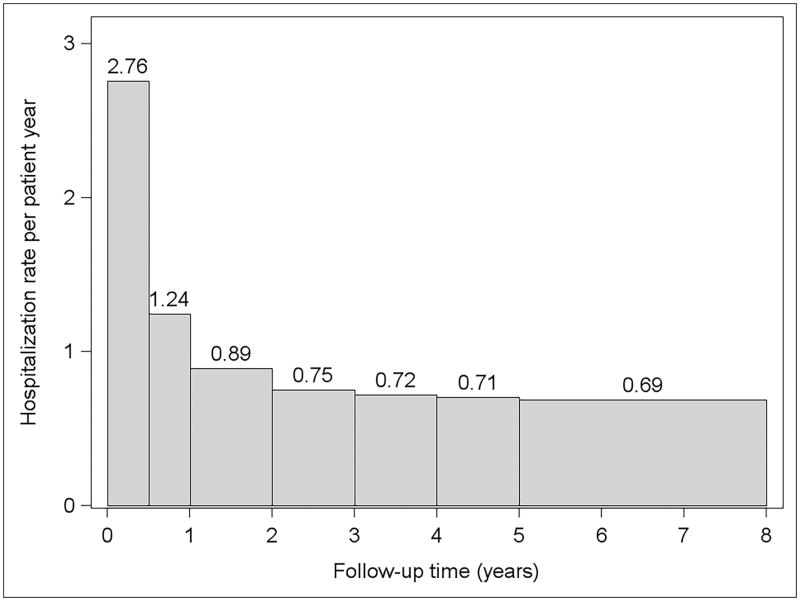
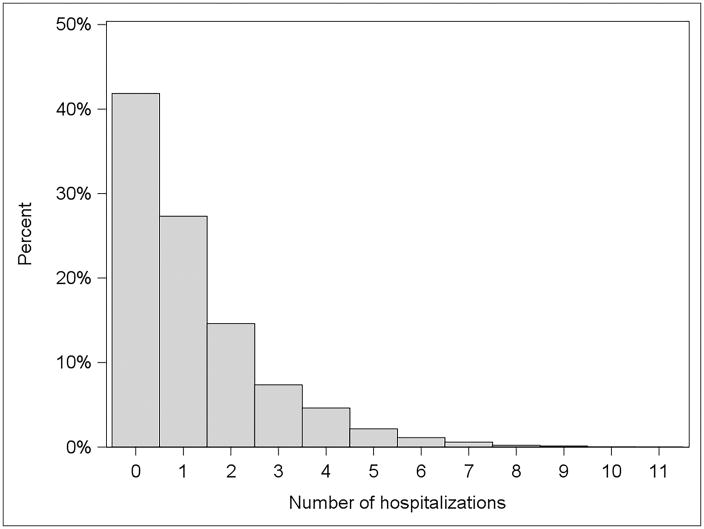
Figure 1 shows the hospitalization rates by follow up time. The hospitalization rate was highest in the first six months after LT (2.76 hospitalizations per patient-year) and decreased quickly over time to less than one hospitalization per patient-year beyond the first post-LT year. In the first six months after discharge from the LT hospitalization, 3,021 (42%) of patients had no hospitalization, 1,972 (27%) had one hospitalization, 1,055 (15%) had two hospitalization, and 1,172 (16%) had three or more hospitalizations (Figure 2).
Figure 1.
Post-liver transplantation hospitalization rate by follow up time.
Figure 2.
Proportion of hospitalizations in the first 6 months after discharge from the liver transplantation hospitalization.
The primary reasons recorded for early hospitalizations were allograft-liver related (28%) followed by infections (14%), renal complications (11%), gastrointestinal complications (9%), cardiovascular complications (5%), and other medical complications (32%).
Risk factors for early hospitalization
Table 2 shows the results of the adjusted model using recipient, donor and transplant factors as predictors of early hospitalization. Hepatitis C, diabetes, poor renal function including dialysis, and recipient of TIPSS procedure before LT independently predicted higher early hospitalization rates after adjusting for donor and transplant factors (Table 2).
Table 2.
Recipient, donor and transplant factors: Multivariable model of early hospitalization
| Factors | Rate ratio (95% confidence interval) | p-value |
|---|---|---|
| Recipient factors | ||
|
| ||
| Age (years) (ref. 18–39) | 0.01* | |
| 40–49 | 0.91 (0.77, 1.07) | 0.25 |
| 50–54 | 0.89 (0.75, 1.05) | 0.15 |
| 55–59 | 0.81 (0.68, 0.95) | 0.01 |
| 60–64 | 0.90 (0.76, 1.07) | 0.25 |
| ≥ 65 | 0.81 (0.69, 0.95) | 0.01 |
|
| ||
| Female | 1.16 (1.08, 1.23) | <0.001 |
|
| ||
| Race | 0.049* | |
| African American | 1.02 (0.91, 1.14) | 0.75 |
| Asian | 0.80 (0.67, 0.96) | 0.02 |
| Hispanic/Latino | 0.97 (0.88, 1.07) | 0.56 |
| Other race | 0.74 (0.55, 1.00) | 0.05 |
|
| ||
| BMI | 1.00 (0.99, 1.00) | 0.05 |
|
| ||
| Diagnosis | 0.04* | |
| Hepatitis C | 1.12 (1.03, 1.21) | 0.006 |
| Cholestatic liver disease | 1.04 (0.91, 1.19) | 0.54 |
| Hepatocellular carcinoma | 1.05 (0.95, 1.16) | 0.35 |
| Other liver disease | 0.96 (0.84, 1.10) | 0.55 |
|
| ||
| On life support at LT | 1.03 (0.84, 1.25) | 0.79 |
|
| ||
| Medical Condition (ref. not hospitalized) | 0.10 | |
| In ICU | 0.98 (0.83, 1.15) | 0.81 |
| Hospitalized (not in ICU) | 1.10 (1.00, 1.21) | 0.05 |
|
| ||
| ESRD at baseline | 1.24 (1.05, 1.47) | 0.01 |
|
| ||
| Diabetes | 1.18 (1.11, 1.26) | <0.001 |
|
| ||
| On dialysis | 1.29 (1.10, 1.52) | 0.002 |
|
| ||
| Ascites (ref. none) | 0.11* | |
| Slight | 1.09 (1.01, 1.19) | 0.04 |
| Moderate | 1.07 (0.97, 1.18) | 0.20 |
|
| ||
| Loge(creatinine) | 1.22 (1.13, 1.31) | <0.001 |
|
| ||
| Loge(bilirubin) | 0.96 (0.92, 1.00) | 0.06 |
|
| ||
| Loge(INR) | 1.07 (0.96, 1.20) | 0.24 |
|
| ||
| Loge(albumin) | 0.83 (0.72, 0.95) | 0.008 |
|
| ||
| Status 1 | 1.21 (0.90, 1.64) | 0.21 |
|
| ||
| Portal vein thrombosis | 0.96 (0.85, 1.08) | 0.49 |
|
| ||
| TIPSS | 1.10 (1.00, 1.21) | 0.05 |
|
| ||
| Donor and transplant factors | ||
|
| ||
| Age (years) (ref. 18–39) | 0.19* | |
|
| ||
| Under 18 | 1.01 (0.88, 1.14) | 0.93 |
|
| ||
| 40–49 | 1.06 (0.97, 1.16) | 0.19 |
|
| ||
| 50–59 | 1.11 (1.01, 1.21) | 0.03 |
|
| ||
| 60–69 | 1.07 (0.96, 1.19) | 0.19 |
|
| ||
| 70 or older | 0.97 (0.84, 1.12) | 0.67 |
|
| ||
| Female | 0.95 (0.87, 1.03) | 0.20 |
|
| ||
| Race (ref. Caucasian) | <0.001* | |
|
| ||
| African American | 1.11 (1.02, 1.21) | 0.01 |
|
| ||
| Asian | 1.55 (1.27, 1.89) | <0.001 |
|
| ||
| Hispanic/Latino | 0.94 (0.86, 1.04) | 0.26 |
|
| ||
| Other race | 0.73 (0.48, 1.11) | 0.14 |
|
| ||
| Height (cm) | 1.00 (0.99, 1.00) | 0.18 |
|
| ||
| Donation after cardiac death | 1.21 (1.05, 1.38) | 0.007 |
|
| ||
| Cause of death (ref. all others) | 0.27* | |
|
| ||
| Anoxia | 1.02 (0.94, 1.12) | 0.60 |
|
| ||
| Cardiovascular accident | 1.07 (0.99, 1.15) | 0.11 |
|
| ||
| Split liver | 1.07 (0.83, 1.39) | 0.58 |
|
| ||
| Donor location (ref. local) | 0.44* | |
|
| ||
| Regional share | 1.00 (0.92, 1.09) | 0.99 |
|
| ||
| National share | 1.09 (0.95, 1.24) | 0.21 |
|
| ||
| Cold ischemia time (hours) | 1.00 (0.99, 1.01) | 0.68 |
p-value from overall test of significance for all levels of the factor.
MELD score and early hospitalization
MELD score was significantly associated with the rate of early hospitalization when it replaced the individual recipient factors in the model described above. Recipients transplanted at MELD scores 23–29 and 30–40 had 15% (rate ratio [RR]=1.15; p=0.005) and 23% (RR=1.23; p<0.001) higher rates of early hospitalization, respectively, compared to those transplanted at MELD scores 16–18 at LT. Of the three MELD components, only serum creatinine was significantly associated with the rate of early hospitalization (RR=1.27; p<0. 001) when separately included in the model (Table 2) (RR=1.22; p<0. 001).
RRI score and early hospitalization
Higher RRI was associated with a higher rate of early hospitalization (RR=1.03; p<0.001) after adjusting for donor and transplant factors. Among RRI components, diabetes (RR=1.18; p<0.001), renal function at LT (loge(Creatinine): RR=1.22; p<0.001 and dialysis: RR=1.29; p=0.002), loge(albumin) (RR=0.83, p=0.008), and history of TIPSS procedure (RR=1.10; p=0.05) were each associated with higher rates of early hospitalization.
Results based on conditional survival
Table 3 shows the independent predictors of mortality conditional upon survival at 6 months after discharge from LT hospitalization. The adjusted relative risk of mortality increased by 22% with every additional hospitalization (HR=1.22; p<0.001). Being in the hospital at the 6 month post-LT follow-up point (compared to not) was associated with 2.3-fold higher risk of death. Additional factors significantly affecting mortality (conditional on 6-month survival) include race (African-Americans being at 38% higher death risk: HR=1.38, and Hispanic/Latino being 34% lower risk: HR=0.66), BMI, Hepatitis C (HR=1.59), Hepatocellular carcinoma (HR=1.69), recipient on life support (HR=1.72), presence of ESRD at 6 months (HR=1.85), INR, and albumin. With respect to donor factors, increasing age, death due to cerebrovascular accident and regional share each significantly increased the death rate conditional on 6-month post-LT survival.
Table 3.
Predictors of post-LT mortality conditional upon 6 months survival after LT
| Factor | Hazard ratio (95% confidence interval) | p-value |
|---|---|---|
| Number of early hospitalizations | 1.22 (1.18, 1.27) | <0.001 |
|
| ||
| In hospital at six months | 2.32 (1.81, 2.97) | <0.001 |
|
| ||
| Recipient Age (years) (ref. 18–39) | 0.16* | |
| 40–49 | 0.85 (0.58, 1.26) | 0.43 |
| 50–54 | 0.98 (0.67, 1.44) | 0.92 |
| 55–59 | 1.00 (0.68, 1.47) | 0.99 |
| 60–64 | 0.99 (0.66, 1.47) | 0.96 |
| 65 or older | 1.13 (0.78, 1.65) | 0.51 |
|
| ||
| Female recipient | 0.95 (0.83, 1.09) | 0.46 |
|
| ||
| Recipient Race (ref. Caucasian) | <0.001* | |
| African American | 1.38 (1.11, 1.71) | 0.004 |
| Asian | 1.11 (0.81, 1.52) | 0.51 |
| Hispanic/Latino | 0.66 (0.53, 0.82) | <0.001 |
| Other race | 0.97 (0.49, 1.92) | 0.94 |
|
| ||
| Recipient BMI | 0.99 (0.98, 1.00) | 0.01 |
|
| ||
| Recipient diagnosis (ref. non-cholestatic liver disease) | <0.001* | |
| Hepatitis C | 1.59 (1.36, 1.86) | <0.001 |
| Cholestatic liver disease | 0.75 (0.56, 1.01) | 0.06 |
| Hepatocellular carcinoma | 1.69 (1.37, 2.07) | <0.001 |
| Other liver disease | 0.85 (0.65, 1.10) | 0.22 |
|
| ||
| Recipient on life support at LT | 1.72 (1.07, 2.77) | 0.02 |
|
| ||
| Recipient medical condition (ref. not hospitalized) | 0.20* | |
| In ICU | 0.77 (0.52, 1.12) | 0.17 |
| Hospitalized (not in ICU) | 1.09 (0.89, 1.33) | 0.42 |
|
| ||
| Diabetes | 1.06 (0.92, 1.21) | 0.41 |
|
| ||
| ESRD at six months | 1.85 (1.40, 2.46) | <0.001 |
|
| ||
| On dialysis at LT | 1.01 (0.70, 1.45) | 0.97 |
|
| ||
| Ascites (ref. none) | 0.95* | |
| Slight | 0.97 (0.82, 1.16) | 0.77 |
| Moderate | 0.99 (0.80, 1.22) | 0.92 |
|
| ||
| Loge(creatinine) | 1.16 (1.00, 1.35) | 0.06 |
|
| ||
| Loge(bilirubin) | 0.99 (0.91, 1.07) | 0.82 |
|
| ||
| Loge(INR) | 0.62 (0.48, 0.79) | <0.001 |
|
| ||
| Loge(albumin) | 0.62 (0.47, 0.82) | <0.001 |
|
| ||
| Status 1 | 1.16 (0.60, 2.23) | 0.66 |
|
| ||
| Portal vein thrombosis | 0.94 (0.73, 1.22) | 0.65 |
|
| ||
| TIPSS | 1.14 (0.94, 1.39) | 0.19 |
|
| ||
| Donor age (years) (ref. 18–39) | <0.001* | |
| Under 18 | 0.98 (0.74, 1.28) | 0.87 |
| 40–49 | 1.17 (0.98, 1.40) | 0.09 |
| 50–59 | 1.44 (1.20, 1.73) | <0.001 |
| 60–69 | 1.49 (1.20, 1.85) | <0.001 |
| 70 or older | 1.58 (1.21, 2.05) | <0.001 |
|
| ||
| Female donor | 0.97 (0.83, 1.14) | 0.74 |
|
| ||
| Donor race (ref. Caucasian) | 0.23* | |
| African American | 0.89 (0.75, 1.07) | 0.21 |
| Asian | 1.23 (0.86, 1.76) | 0.26 |
| Hispanic/Latino | 1.16 (0.95, 1.42) | 0.15 |
| Other race | 1.12 (0.54, 2.30) | 0.76 |
|
| ||
| Donor height (cm) | 1.00 (0.99, 1.00) | 0.46 |
|
| ||
| Donation after cardiac death | 1.14 (0.86, 1.51) | 0.37 |
|
| ||
| Donor cause of death (ref. all others) | 0.08* | |
| Anoxia | 0.86 (0.71, 1.04) | 0.11 |
| Cardiovascular accident | 0.85 (0.73, 0.99) | 0.04 |
| Split liver | 0.72 (0.39, 1.33) | 0.30 |
|
| ||
| Donor location (ref. local) | 0.12* | |
| Regional share | 1.19 (1.01, 1.41) | 0.04 |
| National share | 1.12 (0.86, 1.45) | 0.39 |
|
| ||
| Cold ischemia time (hours) | 1.00 (0.98, 1.02) | 0.87 |
p-value from overall test of significance for all levels of the factor.
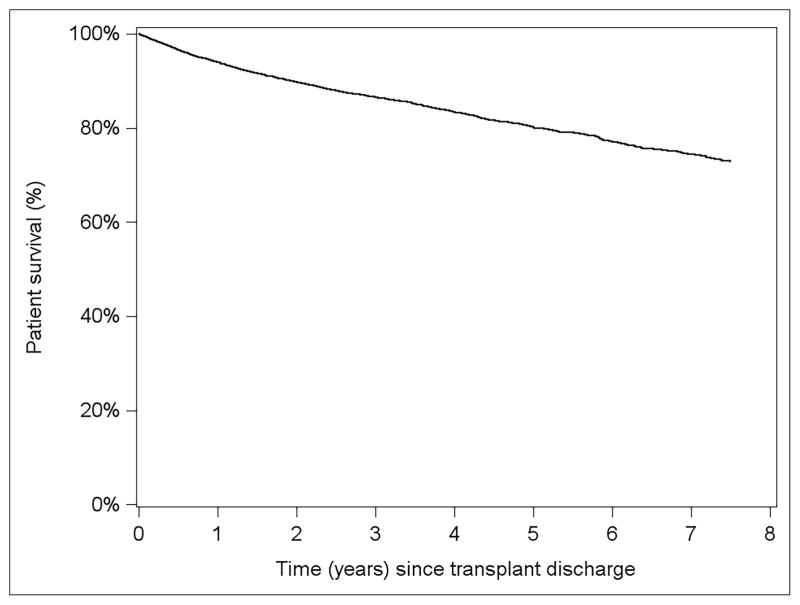
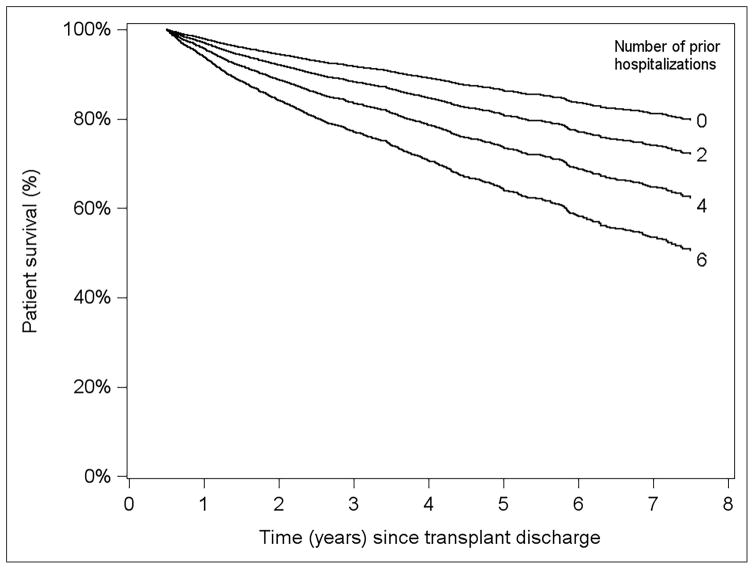
Figure 3 displays overall survival curves for a hypothetical reference-covariate patient; i.e., a LT recipient whose characteristics are described by the reference level of each categorical predictor listed in Table 3, and 0 for each continuous predictor; since all continuous predictors are scored on the natural log scale, the reference level equals 1. With respect to the horizontal (time) axis, time 0 represents 6 months post-LT, with the hospitalization counts pertaining to the first 6 months of follow-up. It can be seen that, all else equal, conditional survival depends strongly on a patient’s hospitalization experience during the first 6 post-LT months. For instance, a patient not hospitalized in the first 6 months is estimated to have 5-year survival of approximately 90%. In contrast, a recipient with 6 prior hospitalizations has 5-year survival probability of ≈60% (Figure 4).
Figure 3.
Adjusted patient survival from incident model starting at time of discharge from index LT hospitalization.
Model was adjusted for recipient factors(non-ESRD, 59 years old, white, male with BMI 26.5, non-cholestatic liver disease, not on life support at LT, not in hospital at LT, non-diabetic, slight ascites, not on dialysis with serum creatinine 1.0 mg/dl, bilirubin 2.9 mg/dl, albumin 2.9g/dl, INR 1.5, non-status 1, no portal vein thrombosis, no TIPSS) and donor factors (Donor age 44 years, Male donor, white donor, 172 cm tall, non-DCD, cause of death=trauma, whole liver, local transplant and 8 hours of cold ischemia time)
Figure 4.
Adjusted patient survival for various numbers of hospitalizations within first six months of LT from model conditional on survival at six months post-LT
Model was adjusted for recipient factors(non-ESRD, 59 years old, white, male with BMI 26.5, non-cholestatic liver disease, not on life support at LT, not in hospital at LT, non-diabetic, slight ascites, not on dialysis with serum creatinine 1.0 mg/dl, bilirubin 2.9 mg/dl, albumin 2.9g/dl, INR 1.5, non-status 1, no portal vein thrombosis, no TIPSS) and donor factors (Donor age 44 years, Male donor, white donor, 172 cm tall, non-DCD, cause of death=trauma, whole liver, local transplant and 8 hours of cold ischemia time)
Discussion
This is the one of the first studies to examine the burden of all-cause hospitalization and its impact on patient outcomes among LT recipients at the national level. In the population of LT recipients with Medicare as primary or secondary insurance, hospitalization rates were highest in the first six months after LT and declined to a plateau after the first post-transplant year. Importantly, a higher rate of early hospitalization was the most significant independent predictor of mortality beginning six months after LT. Out of all the independent recipient factors for early hospitalization, diagnosis of hepatitis C, diabetes and high BMI are the most actionable and modifiable risk factors identified in our study.
Although directly acting antiviral agents (DAA) have revolutionized the treatment for hepatitis C with excellent response rates among patients with compensated and decompensated cirrhosis as well as in post-transplant setting, (13–17) hepatitis C still remains the leading indication for LT in the current period(18). Based upon a recent modeling study, it has been proposed that with the implementation of birth cohort testing for hepatitis C and the availability of highly effective therapies, hepatitis C could become a rare disease in the next twenty two years.(19) Biggins et al. found that the rates of new registrations for hepatitis C without HCC that were born from 1941–1955 are expected to decline, with projected stability of rates in those born 1956–1960. But those with hepatitis C with hepatocellular carcinoma the rates of new registrations are expected to be steady in patients born from 1941–1950, and projected to increase in patients born from 1951–1960.(20) Our results show that hepatitis C is an important risk factor for early hospitalizations. With the effectiveness of DAA, hepatitis C is now a potentially modifiable risk factor. If these patients are treated while on the waiting list or shortly after LT it is possible that the risk of early hospitalization associated with hepatitis C may reduce over time.
Our study did not examine whether the diabetes was controlled or uncontrolled in these patients because of the lack of availability of more granular data. However, good control of diabetes may affect the early hospitalization rates after LT. Similarly, there was a trend towards higher hospitalization in those with higher BMI. Our study also showed that higher MELD score and RRI score at transplant were associated with a higher rate of early hospitalization.(6, 21) RRI is a risk score that predicts the risk of ESRD and ESRD is an independent predictor hospitalization.(11) Since incident ESRD after LT is associated with high hospitalization rates(7), it could be plausible that ESRD status during the first six months instead of RRI may have accounted for the hospitalization.
Since 2009, many studies used the 30-day cut off for early hospitalization because readmission over longer period of time (i.e. 60 days or 120 days) are less likely to be related to index hospitalization for a medical condition or surgical procedure. However, solid organ transplantation is very different from any other surgical or medical condition because based upon the organ type; it may take them up to 6 months to get to their steady state. Therefore, unlike previous studies (4–6, 21), our study, examined the hospitalization within first six months after LT.
Our study did not find any association between race and early hospitalization rates. Consistent with previous studies (22, 23), our study found that African-American race was associated with a 38% increased risk of death after adjusting for recipient and donor factors. Historically, African-Americans have lower response rates to the peg-interferon based treatment. However, the conditional mortality model in our study was adjusted for hepatitis C. One study suggested that donor race mismatch in African Americans hepatitis C positive recipients affect survival; but this observation was not significant in African American hepatitis C negative recipients.(24) We did not explore the potentially complex relationship between donor-recipient mismatch and African-American race, with respect to post-LT survival; such analysis is outside the scope the objectives of our current report.
The number of hospitalizations in the first 6 post-LT months, and being in the hospital at the 6-month post-LT point were easily the strongest predictors of mortality after adjusting for recipient and donor factors. Post-transplant outcomes, including patient survival and graft survival, are tracked by the Scientific Registry of Transplant Recipients (SRTR) and Centers for Medicare & Medicaid Services (CMS) using program-specific reports that are based upon recipient and donor characteristics. These regulatory tools ensure compliance with current performance standards for transplant programs.(25, 26) However, hospitalization rates are not included in the assessment of transplant programs.
Wilson et al. combined the data from University Health Consortium and SRTR and showed a significant hospital-level variation in 30-day and 90-day readmission rates.(21) While we cannot modify most recipient and donor risk factors, knowledge of risk may result in process improvement that could identify LT recipients at risk for early hospitalization, stimulating more effective care-coordination and pre-emptive multidisciplinary management. A recent pilot study by Russo et al. examined a prospective protocol designed to reduce readmission rates after LT by expanding outpatient services and alternatives to readmission. Under the protocol, LT recipients staying less than two midnights were considered as ‘observation status’ and not ‘inpatient readmission’. In their study of 46 patients after implementation of the protocol, readmission was reduced from 31% (pre-protocol) to 20%.(27) This change in the definition resulted in increase in the proportion of readmission as observation status (31% vs. 66%) during the protocol implementation time. However, this study did not examine the effect of these changes on patient mortality.(27, 28)
Limitations of our study include the observational retrospective design that results in the potential for bias due to patient selection and unmeasured patient characteristics, use of Medicare as a primary or secondary payer that may not be generalizable to all LT recipients and missing data in the two administrative datasets that may affect the results. It is very difficult to study the burden of hospitalization using single center data because of small sample size or using the 5% nationwide inpatient sample because LT are not very well represented in the dataset. We compared the baseline characteristics of LT recipients with Medicare as primary or secondary insurance to non-Medicare recipients, and except for slightly older age among those with Medicare as primary and secondary insurance, all other factors were similar. Missingness in this dataset varied from 0%–8%. Finally, our study cohort is from 2003–2010 but that does not limit the relevancy of our results since hepatitis C is still the leading indication for LT (18) and the majority of the LT candidates and recipients have detectable viral load at the time of LT.
In conclusion, the burden of early hospitalization after liver transplantation is strongly associated with patient survival. Although not all post-LT hospitalization can be prevented, treating hepatitis C with DAA while on the waiting list or after LT, good diabetes control and weight management along with developing effective multidisciplinary transitional care after hospitalization through ambulatory clinics may attenuate early post-LT hospitalization and resource utilization and improve survival.
Acknowledgments
Grant Support: Dr. Sharma is supported by National Institutes of Health (NIH) KO8 DK-088946 and RO3 DK 102480. Dr. Schaubel supported, in part, by National Institutes of Health (NIH) grant R01 DK-70869.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. This paper was in part presented as free communication at American Transplant Congress, 2017 held at Chicago, Illinois.
Abbreviations
- ACA
Affordable Care Act
- BMI
Body Mass index
- CMS
Centers for Medicare and Medicaid Services
- DRI
Donor Risk Index
- DCD
Donation after Cardiac Death
- INR
International Normalized Ratio
- LT
Liver transplantation
- MELD
Model for End-stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- RRI
Renal Risk Index
- SRTR
Scientific Registry of Transplant Recipients
- TIPSS
Trans jugular Intrahepatic Portosystemic Shunt
Footnotes
Conflict of interests: None
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305:504–505. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 3.Bentley STH, SG, Hauboldt RA. Milliman Research Report. Milliman Inc; 2011. U.S. organ and tissue transplant cost estimates and discussion; pp. 1–14. [Google Scholar]
- 4.Shankar N, Marotta P, Wall W, Albasheer M, Hernandez-Alejandro R, Chandok N. Defining readmission risk factors for liver transplantation recipients. Gastroenterol Hepatol (N Y) 2011;7:585–590. [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira AA, Bhattacharya R, Carithers R, Reyes J, Perkins J. Clinical factors predicting readmission after orthotopic liver transplantation. Liver Transpl. 2012;18:1037–1045. doi: 10.1002/lt.23475. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Hosmer A, Parks T, Sonnenday CJ, Sharma P. Predictors of Early Hospitalization After Deceased Donor Liver Transplantation. Dig Dis Sci. 2015 doi: 10.1007/s10620-015-3753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodrich NP, Schaubel DE, Smith AR, Merion RM, Sharma P. National Assessment of Hospitalization Rates for Incident End Stage Renal Disease after Liver Transplantation. Transplantation. 2016;100:2115–2121. doi: 10.1097/TP.0000000000001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, Snyder JJ, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 2013;27:50–56. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for mean and rate function of recurrent events. Journal of Royal Statistical Society Series B. 2000;62:711–730. [Google Scholar]
- 10.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Goodrich NP, Schaubel DE, Guidinger MK, Merion RM. Patient-Specific Prediction of ESRD after Liver Transplantation. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2013040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma P, Perlemutter A. Renal Risk Index Calculator. 2013. Website to calculate renal risk index- a risk score that predicts end stage renal disease after liver transplant. [Google Scholar]
- 13.Lawitz E, Poordad F, Gutierrez JA, Kakuda TN, Picchio G, Beets G, Vandevoorde A, et al. Simeprevir, daclatasvir and sofosbuvir for hepatitis C virus-infected patients with decompensated liver disease. J Viral Hepat. 2017;24:287–294. doi: 10.1111/jvh.12645. [DOI] [PubMed] [Google Scholar]
- 14.Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, McPhee F, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493–1505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 16.Fontana RJ, Brown RS, Jr, Moreno-Zamora A, Prieto M, Joshi S, Londono MC, Herzer K, et al. Daclatasvir combined with sofosbuvir or simeprevir in liver transplant recipients with severe recurrent hepatitis C infection. Liver Transpl. 2016;22:446–458. doi: 10.1002/lt.24416. [DOI] [PubMed] [Google Scholar]
- 17.Ciesek S, Proske V, Otto B, Pischke S, Costa R, Luthgehetmann M, Polywka S, et al. Efficacy and safety of sofosbuvir/ledipasvir for the treatment of patients with hepatitis C virus re-infection after liver transplantation. Transpl Infect Dis. 2016;18:326–332. doi: 10.1111/tid.12524. [DOI] [PubMed] [Google Scholar]
- 18.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17(Suppl 1):174–251. doi: 10.1111/ajt.14126. [DOI] [PubMed] [Google Scholar]
- 19.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161:170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggins SW, Bambha KM, Terrault NA, Inadomi J, Shiboski S, Dodge JL, Gralla J, et al. Projected future increase in aging hepatitis C virus-infected liver transplant candidates: a potential effect of hepatocellular carcinoma. Liver Transpl. 2012;18:1471–1478. doi: 10.1002/lt.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson GC, Hoehn RS, Ertel AE, Wima K, Quillin RC, 3rd, Hohmann S, Paterno F, et al. Variation by center and economic burden of readmissions after liver transplantation. Liver Transpl. 2015;21:953–960. doi: 10.1002/lt.24112. [DOI] [PubMed] [Google Scholar]
- 22.Quillin RC, 3rd, Wilson GC, Wima K, Hanseman DJ, Sutton JM, Shaw JJ, Cuffy MC, et al. Independent effect of black recipient race on short-term outcomes after liver transplantation. Surgery. 2015;157:774–784. doi: 10.1016/j.surg.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Wong RJ, Ahmed A. Combination of racial/ethnic and etiology/disease-specific factors is associated with lower survival following liver transplantation in African Americans: an analysis from UNOS/OPTN database. Clin Transplant. 2014;28:755–761. doi: 10.1111/ctr.12374. [DOI] [PubMed] [Google Scholar]
- 24.Pang PS, Kamal A, Glenn JS. The effect of donor race on the survival of Black Americans undergoing liver transplantation for chronic hepatitis C. Liver Transpl. 2009;15:1126–1132. doi: 10.1002/lt.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Axelrod DA, Kalbfleisch JD, Sun RJ, Guidinger MK, Biswas P, Levine GN, Arrington CJ, et al. Innovations in the assessment of transplant center performance: implications for quality improvement. Am J Transplant. 2009;9:959–969. doi: 10.1111/j.1600-6143.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- 26.Kasiske BL, McBride MA, Cornell DL, Gaston RS, Henry ML, Irwin FD, Israni AK, et al. Report of a consensus conference on transplant program quality and surveillance. Am J Transplant. 2012;12:1988–1996. doi: 10.1111/j.1600-6143.2012.04130.x. [DOI] [PubMed] [Google Scholar]
- 27.Russo MW, Levi DM, Pierce R, Casingal V, Eskind L, deLemos A, Schmeltzer PA, et al. A prospective study of a protocol that reduces readmission after liver transplantation. Liver Transpl. 2016;22:765–772. doi: 10.1002/lt.24424. [DOI] [PubMed] [Google Scholar]
- 28.Tapper EB. Early readmissions after liver transplantation and the power of quality improvement. Liver Transpl. 2016;22:717–719. doi: 10.1002/lt.24430. [DOI] [PubMed] [Google Scholar]