Abstract
Objective
To evaluate the association between protein intake (amount and type) and antral follicle count (AFC).
Design
Prospective cohort.
Setting
Academic fertility center.
Population
265 women undergoing fertility treatments at an academic fertility center and participating in an ongoing study on environment and reproductive health.
Methods
We measured AFC in ultrasonographic evaluation among women undergoing infertility treatments. Women completed a previously validated semi-quantitative food frequency questionnaire. We used Poisson regression to evaluate the relation between protein intake and AFC while adjusting for age, body mass index, race, smoking status, and total energy intake.
Main Outcome Measures
Antral follicle count.
Results
Among 265 women (mean age: 35.0±3.9 years, 85% Caucasian), total protein intake (% energy) was unrelated to AFC. When protein from different food sources was considered separately, we found a negative association between dairy protein intake and AFC. The mean AFC was 14.4% (3.9%–23.7%) lower for women in the highest quintile of dairy protein intake than for women in the bottom quintile after adjusting for potential confounders (p-trend=0.04). This association was stronger among women who had never smoked (p-trend=0.002) but was not observed among previous smokers (p-trend=0.36). There were no associations between protein intake from either non-dairy animal or vegetable sources and AFC.
Conclusion
Higher dairy protein intake (≥ 5.24% of energy) was associated with lower antral follicle counts among women presenting for infertility treatment. These findings should be further investigated in prospective studies designed to also clarify the biology underlying the observed associations.
Keywords: antral follicle count, dairy intake, protein intake, ovarian reserve, ovary, female infertility
INTRODUCTION
Infertility affects 15.5% of couples seeking conception1 and bears significant financial and psychosocial repercussions for both the individuals involved and society in general.2–4 Despite a well-established notion that both nutrition and modifiable lifestyle factors impact female5–9 and male reproductive potential,10–12 research in this field is not extensive leaving couples planning pregnancy with few evidence-based resources to guide preconception diet advice.
Even though diminished ovarian reserve is one of the major causes of female infertility, the process leading to reproductive senescence is currently poorly understood. In light of emerging population trends towards delayed pregnancy,13 the identification of reversible factors (including diet) that affect the individual rates of reproductive decline might be of significant clinical value. Diets restricting the use of certain types of protein are gaining popularity, mainly due to increasing health and environmental awareness and compassion for animals. However, their effects on reproductive health and ovarian aging remain unknown. Animal studies suggest a possible adverse effect of a low-protein diet on conception rates, ovarian follicular numbers and ovarian reserve in adulthood,14–18 effects potentially mediated by accelerated accumulation of oxidative stress, altered ovarian telomere length and mitochondrial DNA copy number. Human data are lacking.
AFC and anti-mullerian hormone (AMH) are markers known to predict ovarian primordial follicle numbers better than basal follicle stimulating hormone (FSH) levels.19 AFC, in particular, appears to be even more sensitive than AMH in predicting both ovarian primordial follicle numbers19 and response to medication in in-vitro fertilization (IVF) cycles20 and has been found to be independently associated with age at natural menopause.21
Study Objective
The objective of the present analysis was to examine the relation between protein intake (both amount and source of dietary protein) and AFC (as a measure of ovarian reserve) in a group of women attending an infertility clinic.
METHODS
Study Population
Participants were women enrolled in the EARTH (Environment and Reproductive Health) study, an ongoing prospective cohort started in 2004 aimed at evaluating the effects of various environmental factors on reproductive health.22–24 Couples presenting to the Massachusetts General Hospital (MGH) for infertility treatments were invited to participate. Women between 18 and 45 years using their own gametes for intrauterine insemination or IVF were eligible to enroll. At enrollment, all participants underwent an anthropometric evaluation and completed a nurse-administered general health questionnaire where data on demographics, lifestyle, medical and reproductive history was collected. In 2007, a previously validated25 semi-quantitative food frequency questionnaire (FFQ) was introduced to assess participant’s dietary habits. Of 326 women who prospectively completed diet questionnaires and underwent ultrasound assessment, we excluded women with a prior oophorectomy (n=4), incomplete or missing AFC data (n=25), as well as women whose ultrasound for AFC determination was performed more than one year after FFQ completion (n=32), leaving 265 women for the present analysis. Included participants did not differ significantly from those excluded in age, body mass index (BMI), smoking status or race/ethnicity but did so in the prevalence of female factor infertility (p<0.01).
Diet assessment
All study participants completed a previously validated FFQ, thus providing information on how often, on average, they consumed specified amounts of each food, beverage and supplement included in the questionnaire during the year preceding their enrollment in the study. For each food, the questionnaire offered nine possible responses, ranging from never or less than once a month to six or more times per day. Nutrient content of each item was obtained from the nutrient database of the US Department of Agriculture26 (USDA) and supplemented with data from food manufacturers. Nutrient intakes were estimated by summing the contribution of all relevant food items and were expressed as daily intakes. Total protein intake, as well as protein intake from different food sources (dairy foods, animal foods, vegetables) was estimated and expressed as the percentage of energy consumed. In a validation study, the correlation between FFQ-assessed protein intake and protein intake assessed with prospectively collected diet records representing one year of diet was 0.44.27 Among the major food sources of protein, recall was better for dairy foods (skim milk r=0.88) and worse for vegetables (beans r= 0.34).28
Ultrasonographic Determination of Antral Follicle Counts
All women participating in the study underwent a standard infertility work-up which included the ultrasonographic determination of the AFC for ovarian reserve evaluation, either on the 3rd day of an unstimulated menstrual cycle or on the 3rd day of a progesterone withdrawal bleed (at which time a serum FSH level was measured as well). All transvaginal ultrasounds were performed by one of the MGH reproductive endocrinology and infertility physicians. No fertility medications were used in the cycle preceding the ultrasonographic determination of the AFC.
Statistical Analysis
We divided women into quintiles of protein intake (total, vegetable, and animal (dairy and non-dairy)). We first summarized participant characteristics by quintiles of total protein intake and tested for differences across quintiles using Kruskal-Wallis test for continuous variables and Chi-square test for categorical variables. Poisson regression models were used to examine the relation between protein intake and log(AFC), while adjusting for potential confounders. We compared AFC of women in increasing quintiles of protein intake in relation to those of women in the lowest quintile (reference). Population marginal means were utilized to present marginal population averages adjusted for the covariates in the model, and results were exponentiated to express them in the original count scale. Tests for linear trend were performed using the median values of protein intake in each quintile as a continuous variable. Protein intake was adjusted for total energy intake using the nutrient density method. Specifically, terms for fat intake (% of calories) and total energy intake were added to the models to allow the protein intake parameters to represent the isocaloric substitution of carbohydrates with the same amount of energy from protein. In addition, we estimated the effect of substituting a type of protein for another by including energy contribution from all protein types as continuous variable in the same model. The effect of substituting one type of protein for another was estimated using linear combinations of the regression coefficients; the 95% confidence interval of a substitution was estimated based on the variance of each regression coefficient and their covariance. Multivariable-adjusted models included additional terms for age, BMI, race, and smoking status. We evaluated whether the association between protein intake and AFC was modified by BMI (≥ 25 kg/m2 and < 25 kg/m2), age (≥ 35 and < 35 years) and smoking (current/former and never smokers) by introducing cross-product terms to the final multivariate models. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Two-sided p values ≤0.05 were considered statistically significant.
RESULTS
The study population was primarily Caucasian (85%) with a mean (±SD) age of 35.0 (±3.9) years. The majority of women (65%) were of normal weight defined as a BMI <25 kg/m2 (median (25th, 75th): 23.3 (21.2, 26.2) kg/m2), while 25%, and 10% of them were either overweight or obese, respectively. Most of the women had never smoked (72%). Mean (±SD) alcohol and caffeine intake were 7.7 (±8.9) g/day and 120 (±115) mg/day, respectively. Median intakes of protein, fat and carbohydrates (% energy) were 17, 33, and 50% energy, respectively (US mean protein intake: 14.6% energy).29 The most common infertility diagnosis was idiopathic (46%), followed by male factor (28%), ovulatory dysfunction (9%) and diminished ovarian reserve (7%). 38% of participants reported at least one prior pregnancy. Overall, 37% of participants had an AFC ≥ 15 whereas 6.4% had an AFC ≤ 5 and were thus expected to be either high or poor responders, respectively.
There were no appreciable differences in age, BMI, smoking status, reproductive history or intakes of caffeine, alcohol, total energy or total fat across quintiles of total protein intake (Table 1). As expected, carbohydrate intake decreased with increasing intake of protein (p-trend: <.0001).
Table 1.
Demographic characteristics of 265 EARTH study women according to quintile of PI.
| Total Protein Intake | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P-value1 | |
| N | 53 | 53 | 53 | 53 | 53 | |
| Median, % energy | 13.6 | 15.3 | 16.4 | 17.9 | 20.9 | |
| Range, % energy | 10.35,14.44 | 14.48,15.96 | 15.98,17.02 | 17.03,18.73 | 18.76,26.14 | |
| Mean ± SD or N (%) | ||||||
| Demographics | ||||||
| Age, years | 34.8±4.1 | 35.2±3.7 | 35.6±4.4 | 34.3±3.7 | 35.0±3.7 | 0.53 |
| BMI, kg/m2 | 23.9±3.9 | 24.0±3.8 | 23.2±3.4 | 25.3±6.2 | 25.3±5.0 | 0.26 |
| Ever smokers, N (%) | 14 (26) | 17 (32) | 8 (15) | 20 (38) | 16 (30) | 0.11 |
| M-V Exercise, hrs/week2 | 3.5±3.7 | 3.4±3.8 | 4.0±4.7 | 3.8±4.1 | 4.9±6.1 | 0.96 |
| White | 45 (85) | 46 (87) | 44 (83) | 45 (85) | 46 (87) | 0.98 |
| Diet | ||||||
| Alcohol, g/d | 6.5±7.3 | 9.1±9.3 | 7.6±10.4 | 9.4±10.6 | 6.0±5.8 | 0.21 |
| Caffeine, g/d | 116±100 | 119±88 | 112±122 | 133±115 | 120±144 | 0.48 |
| Total fat, % energy | 32.4±6.6 | 32.6±5.9 | 32.3±7.1 | 32.6±5.7 | 33.5±5.6 | 0.55 |
| Total carbohydrate, % energy | 54.5±7.4 | 51.0±6.4 | 50.4±7.2 | 48.0±6.9 | 44.6±6.3 | <.0001 |
| Total energy intake, kcal/d | 1840±586 | 1904±627 | 1777±632 | 1778±577 | 1627±564 | 0.15 |
| Reproductive history, N (%) | ||||||
| Prior pregnancy | 26 (49) | 19 (36) | 19 (36) | 18 (34) | 22 (42) | 0.51 |
| Day 3 FSH, IU/ml | 7.2±2.0 | 7.5±3.2 | 7.8±2.8 | 7.4±1.8 | 7.3±2.5 | 0.82 |
| Infertility diagnosis, N (%) | 0.16 | |||||
| Male factor | 11 (21) | 14 (26) | 14 (26) | 22 (42) | 13 (25) | |
| Diminished ovarian reserve | 3 (6) | 4 (8) | 7 (13) | 2 (4) | 2 (4) | |
| Ovulatory | 8 (15) | 7 (13) | 3 (6) | 1 (2) | 4 (8) | |
| Endometriosis, uterine, tubal | 3 (6) | 5 (9) | 4 (8) | 6 (11) | 9 (17) | |
| Unexplained | 28 (53) | 23 (43) | 25 (47) | 22 (42) | 25(47) | |
Kruskal–Wallis & x2-test: for continuous and categorical variables, respectively;
Moderate-Vigorous Exercise.
Total protein intake was unrelated to AFC in age-adjusted and multivariable-adjusted analyses (Table 2). While AFC was significantly lower among women in the second and third quintile of total protein intake when compared to women in the bottom quintile, there was not a clear pattern across quintiles of intake. Similarly, when this relation was examined separately for protein coming either from vegetable or animal sources, we noted no association between vegetable protein intake and AFC (Table 2) and while AFC was significantly lower among women in the 2nd and 5th quintile compared to the 1st, no significant trend was noted across quintiles of animal protein intake (Table 2).
Table 2.
Association between antral follicle counts (AFC) and protein intake in 265 women.
| Adjusted Mean Antral Follical Count (95% Confidence Intevals) by Protein Intake
| ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P- trend1 | |
| Subject, N | 53 | 53 | 53 | 53 | 53 | |
|
Total protein intake (% energy) | ||||||
| Range: | 10.35,14.44 | 14.48,15.96 | 15.98,17.02 | 17.03,18.73 | 18.76,26.14 | |
| MDLl2 | 14.8 (13.8, 15.8) | 13.0 (12.0, 14.0)* | 13.2 (12.2, 14.2)* | 13.8 (12.8, 14.8) | 14.1 (13.2, 15.2) | 0.90 |
| MDL23 | 14.7(13.7, 15.8) | 13.0 (12.0, 14.0)* | 13.1 (12.1, 14.1)* | 13.8 (12.9, 14.9) | 14.2 (13.2, 15.3) | 0.97 |
|
Vegetable protein intake (% energy) | ||||||
| Range: | 2.14,5.00 | 5.05,5.83 | 5.84,6.60 | 6.62,7.51 | 7.52,15.16 | |
| MDL14 | 13.8 (12.7, 14.9) | 13.4 (12.5, 14.5) | 14.0 (13.0, 15.1) | 13.8 (12.8, 14.8) | 13.8 (12.8, 15.0) | 0.84 |
| MDL25 | 14.1 (13.0, 15.4) | 13.7 (12.6, 14.9) | 14.3 (13.2, 15.5) | 14.1 (13.1, 15.3) | 14.1 (13.0, 15.4) | 0.86 |
|
Animal protein intake (% energy) | ||||||
| Range: | 0.16,7.84 | 7.86,9.48 | 9.49,10.89 | 10.89,12.98 | 12.99,22.01 | |
| MDL16 | 14.9 (13.7, 16.1) | 12.9 (12.0, 13.9)* | 13.5 (12.5, 14.5) | 14.5 (13.5, 15.6) | 13.1 (12.1, 14.1)* | 0.27 |
| MDL27 | 14.9 (13.7, 16.2) | 12.8 (11.9, 13.8)* | 13.4 (12.4, 14.4) | 14.5 (13.5, 15.6) | 13.1 (12.1, 14.2)* | 0.31 |
|
Dairy protein intake (% energy) | ||||||
| Range: | 0.00,2.31 | 2.33,3.20 | 3.21,4.12 | 4.12,5.24 | 5.24,9.27 | |
| MDL18 | 14.5 (13.5, 15.7) | 13.4 (12.5, 14.5) | 13.7 (12.7, 14.7) | 14.8 (13.7, 15.9) | 12.4 (11.5, 13.5)* | 0.04 |
| MDL29 | 14.8 (13.7, 16.0) | 13.7 (12.6, 14.8) | 13.9 (12.8, 15.1) | 14.9 (13.8, 16.2) | 12.6 (11.6, 13.8)* | 0.04 |
|
Non-dairy animal protein intake (% energy) | ||||||
| Range: | 0.03,4.12 | 4.15,5.57 | 5.58,7.13 | 7.15,8.98 | 9.01,21.04 | |
| MDL110 | 13.7 (12.7, 14.9) | 13.4 (12.4, 14.4) | 13.8 (12.8, 14.8) | 13.7 (12.7, 14.7) | 14.2 (13.2, 15.3) | 0.48 |
| MDL211 | 14.0 (12.8, 15.3) | 13.5 (12.5, 14.7) | 14.0 (13.0, 15.2) | 14.0 (12.9, 15.2) | 14.6 (13.4, 15.8) | 0.42 |
Estimated using median intake in each quartile as a continuous variable;
Model (MDL) 1: adjusted for total energy intake (TEI), age, and total fat intake (TFI);
Model 2: adjusted for TEI, TFI, age, BMI, smoking status (ever vs. never), race (white vs non-white);
Model 1: adjusted for TEI, TFI, age, and animal protein intake (PI);
Model 2: adjusted for TEI, TFI, age, BMI, smoking status (ever vs. never), race (white vs non-white), and animal PI;
Model 1: adjusted for TEI, TFI, and vegetable PI;
Model 2: adjusted for TEI, TFI, age, BMI, smoking status (ever vs. never), race (white vs non-white), and vegetable PI;
Model 1: adjusted for TEI, TFI, age, non-dairy PI, and vegetable PI;
Model 2: adjusted for TEI, TFI, age, BMI, smoking status (ever vs. never), race (white vs. non-white), non-dairy PI and vegetable PI;
Model 1: adjusted for TEI, TFI, age, dairy PI and vegetable PI;
Model 2: adjusted for TEI, TFI, age, BMI, smoking status (ever vs. never), race (white vs. non-white), dairy PI and vegetable PI;
P-value for trend <0.05 compared to women in the lowest quartile of PI.
We then further divided animal protein intake into protein coming from dairy and protein coming from other animal sources. Women in the highest quintile of dairy protein intake (≥5.24% of energy, or ≥2.3 cups of milk/day) had 14.4% (3.9%–23.7%, p=0.009) lower AFC than women in the lowest quintile after adjusting for potential confounders (Table 2). In addition, we estimated the effect of substituting 2% energy of dairy protein (around 1 cup of milk) with vegetable protein, and calculated that the AFC would be 5% higher (0.3%, 10%, p=0.04).
Last, we evaluated whether the relation between protein intake and AFC was modified by smoking, BMI and age. Smoking modified the association between dairy protein intake and AFC (p-interaction=0.003, fig. 1A–1B). Consumption of dairy protein was inversely associated with AFC among the 190 (72%) women who had never smoked (p-trend=0.002, fig. 1A), but not among the 75 (28%) women who had a history of ever smoking (p-trend=0.36, fig. 1B). There was no evidence of significant heterogeneity in the relation of dairy protein intake and AFC by BMI (p-interaction=0.12) or age (p-interaction=0.58).
Figure 1.
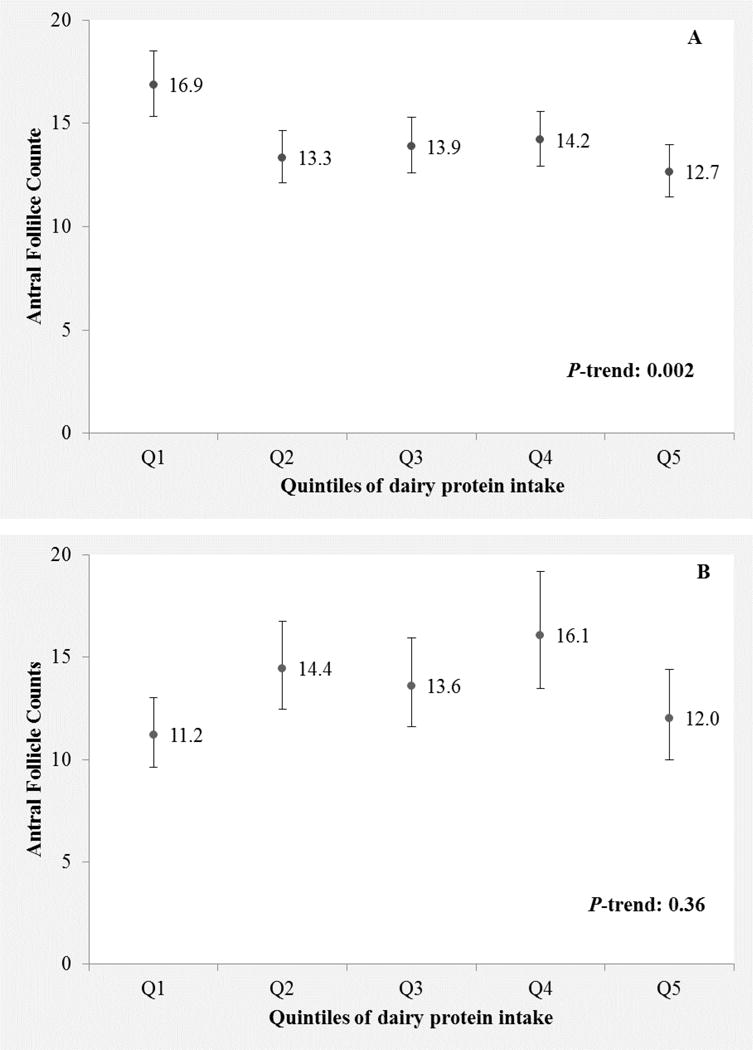
Antral follicle count (AFC) according to quintiles of dairy protein intake among never (Fig. 1A) and ever smokers (Fig. 1B). Values are adjusted antral follicle counts with 95% confidence intervals. Results are adjusted for total energy intake, age, BMI, smoking status (ever vs. never), race (white vs non-white), vegetable protein intake and non-dairy protein intake, with each of these covariates at their mean levels. Tests for trend were conducted across quintiles using the median intake in each quintile as a continuous variable.
DISCUSSION
In light of the increasing popularity of diets that limit certain protein sources and the growing trend towards delayed pregnancy, the identification of dietary factors that might affect reproductive aging can help guide preconception dietary counseling.
Main Findings
We evaluated the association between protein intake and AFC in a prospective cohort of reproductive age women attending an infertility clinic and found that overall greater consumption of dairy protein (≥5.24% energy, or ≥2.3 cups of milk/day) was associated with lower AFC. This association was stronger among women that never smoked. Neither vegetable nor animal protein intake from non-dairy sources were related to AFC in any of the analyses.
Studies investigating the effects of various dietary components on reproductive targets are emerging, however data on a potential effect of diet on ovarian reserve are scarce. To the best of our knowledge our study is the first to explore the possible association of dietary protein consumption with human ovarian reserve.
Strengths and Limitations
Our study has the following strengths: i) all data were derived from one large, fertility center treating a diverse population, ii) all AFC determinations were performed by infertility specialists only, following the same protocol thus minimizing “between operators” variability, and iii) the use of a previously validated FFQ with adequate validity and reproducibility for epidemiological study use.25,30 One of the biggest challenges when evaluating the effects of a particular dietary component on either a reproductive target or on disease risk in general, is the correlation among dietary nutrients (i.e.: low-fat dairy consumers might have an overall “healthier” diet, while high-fat food consumption might be associated with a sedentary and overall “less healthy” lifestyle). In order to address this, our study and each individual sub-analysis within it controlled not only for total energy intake and alcohol/tobacco consumption but for all other dietary factors that might have correlated with the dietary component under consideration.
A potential limitation of our study is the lack of AMH levels to correlate with the AFC findings, mainly because the assay was not commercially available during most of the study period and the test was neither required nor covered by insurance. However, the AFC is considered a robust and reliable ovarian reserve predictor, slightly more sensitive than AMH in predicting ovarian primordial follicle number19 and IVF response.20 Finally, the results should be interpreted with caution because the findings may not be generalizable to a spontaneously conceiving population, and the consumption of dairy may be reflective of other unknown dietary or lifestyle factors that might be affecting ovarian reserve.
Interpretation
While it is not possible in the present study to identify the underlying mechanism linking higher dairy protein intake to lower AFC, dairy products are a diverse food group in terms of factors that could potentially influence the ovarian reserve and there are several hypotheses that could explain the findings and include the following: 1) the presence in dairy products of measurable amounts of steroid hormones and growth factors that might have physiological and other effects in humans,31–33 and 2) the contamination of milk products by pesticides and endocrine disrupting chemicals34–35 that may negatively impact folliculogenesis and oocyte competence.
Regarding the former, studies suggest that commercial milk (derived from both pregnant and non-pregnant animals) contains large amounts of estrogens, progesterone and other placental hormones that are eventually released into the human food chain,36 with dairy intake accounting for 60–80% of the estrogens consumed.37 Dairy estrogens overcome processing, appear in raw whole cow’s38–39 and commercial milk products,40 are found in substantially higher concentrations with increasing amounts of milk fat, with no apparent difference between organic and conventional dairy products,39 and once inside the human body get converted to estrone and estradiol.41 Following absorption, bovine steroids may alter reproductive outcomes. Human studies documented associations between dairy consumption and both plasma steroid hormone concentrations42 and secretion of gonadotropins.36 It is therefore possible, that absorbed bovine steroids may target either the hypothalamic-pituitary-gonadal axis or directly the oocyte and the local, intra-ovarian/intra-follicular supporting environment, through mechanisms involving altered gene expression and modified neuroendocrine signaling.
Serum levels of growth factors are also altered by dietary protein intake. Increased intake of i) dairy products, ii) animal vs. vegetable and iii) milk vs. meat protein, was associated with higher serum levels of insulin-like growth factor-I (IGF-I).43–50 It is possible that the higher circulating IGF-I levels (resulting either from the diet itself or from stimulation of endogenous production44) adversely impact the ovary and its reserve (IGF-I regulates granulosa cell steroidogenesis and apoptosis during follicular development thus playing an essential role in reproduction).51 IGF-I is also known to influence fertility at multiple other levels within the reproductive system, including effects on the hypothalamic–pituitary-gonadal axis52–55 and the gonads.56–60
It is also possible that the observed association between dairy protein and AFC is mediated by the presence of environmental contaminants in the dairy. Dairy consumption has been associated with higher serum concentrations of certain organochlorine pesticides in both adult and pediatric populations61–65 and with higher bisphenol-A (BPA) concentrations among lactating mothers,66 while phthalates67 and bisphenol analogues have been detected in dairy products.35 In our previous study on this same cohort, higher urinary BPA concentrations were associated with lower AFC.68
Lastly, it is also possible that the differences in association with AFC of different sources of protein may simply reflect different degrees of measurement error. A validation study of the questionnaire used in this study found that the validity of recall for dairy foods was substantially higher than that for major sources of vegetable protein.28 This alone could lead to a situation where we would identify an association with dairy protein only when in reality protein intake in general was inversely related to AFC.
Whether the observed association between dairy consumption and lower AFC results from an effect of steroid hormones, growth factors, environmental contaminants or other factors (i.e.: timing and duration of exposure) remains to be determined and is well beyond the study’s scope. Finally, a 14% reduction in AFC might not be clinically significant at the individual level but can be of public health importance at the population level (across infertile women of reproductive age).
The fact that smoking modified the observed association between dairy protein intake and AFC was not entirely unexpected since smoking has been linked to both indicators of increased ovarian age and adverse reproductive outcomes.69–72 The fact that the observed effect reached significance only among women who were never smokers potentially suggests that smoking’s negative effect on ovarian reserve is a lot stronger and “masks” that of certain dietary habits.
CONCLUSION
We evaluated the relation between protein intake and ovarian reserve in a population of women presenting for infertility treatment and found an association between increased dairy intake (≥5% energy) and lower AFC. Given the lack of data on this topic, and the fact that multiple environmental and genetic factors, as well as complex neuroendocrine interactions, may alter the fate of the non-regenerating oocyte pool, it is imperative that these findings are reproduced in prospective studies designed to also clarify the biology underlying the observed associations. The latter might be crucial given that consumption of another species’ milk by humans is an evolutionary novel dietary behavior that has the potential to alter reproductive parameters and may have long-term adverse health effects.
Acknowledgments
The authors would like to thank Jennifer Ford, RN and Myra Keller, RN for their assistance with patient recruitment and specimen collection.
FUNDING
Supported by grants: ES009718, ES000002 from the National Institute of Environmental Health Sciences (NIEHS), P30DK46200 and T32DK00770 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and Ruth L. Kirschstein National Research Service Award T32 DK007703-16.
Footnotes
Presented in part at the 69th Annual Meeting of the American Society for Reproductive Medicine, which was held in Boston, MA, on October 12–17, 2013
DISCLOSURE OF INTERESTS
Author: MCA is employed by Nestle Research Center. Authors: IS, YHC, MB, PLW, RH and JEC report no conflicts of interest. The ICMJE disclosure forms are available as online supporting information.
CONTRIBUTION TO AUTHORSHIP
IS: substantially contributed to the conception, and design of the study, the acquisition, analysis and interpretation of the data, and wrote the manuscript.
YHC: substantially contributed to the design of the study, analysis and interpretation of the data, and the drafting of the manuscript.
MB: substantially contributed to the acquisition of the data.
MCA: substantially contributed to the analysis and interpretation of the data.
PW: substantially contributed to the analysis and interpretation of the data.
RH: substantially contributed to the conception, and design of the study, the acquisition, analysis and interpretation of the data.
JEC: substantially contributed to the conception, and design of the study, the acquisition, analysis and interpretation of the data.
All authors critically revised the manuscript for important intellectual content, read and approved the final manuscript.
ETHICAL APPROVAL
The study was approved by the Institutional Review Boards of the Massachusetts General Hospital and the Harvard T. H. Chan School of Public Health (IRB #: 1999P008167, original approval: 12/13/1996, most recent renewal: 03/01/2016), and informed consent was obtained from all participants prior to study enrollment.
References
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013 Apr;99(5):1324–1331. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domar AD. Creating a collaborative model of mental health counseling for the future. Fertil Steril. 2015;104(2):277–80. doi: 10.1016/j.fertnstert.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Chen TH, Chang SP, Tsai CF, Juang KD. Prevalence of depressive and anxiety disorders in an assisted reproductive technique clinic. Hum Reprod. 2004;19:2313–2318. doi: 10.1093/humrep/deh414. [DOI] [PubMed] [Google Scholar]
- 4.Verhaak CM, Lintsen AM, Evers AW, Braat DD. Who is at risk of emotional problems and how to know? Screening of women going for IVF treatment. Hum Reprod. 2010;25:1234–1240. doi: 10.1093/humrep/deq054. [DOI] [PubMed] [Google Scholar]
- 5.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur J Clin Nutr. 2009;63(1):78–86. doi: 10.1038/sj.ejcn.1602904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavarro J, Rich-Edwards JW, Rosner BA, Willett WC. Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr. 2007;85:231–7. doi: 10.1093/ajcn/85.1.231. [DOI] [PubMed] [Google Scholar]
- 7.Missmer S, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein M, Spiegelman D, et al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. 2010;25(6):1528–1535. doi: 10.1093/humrep/deq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavarro J, Rich-Edwards JW, Rosner BA, Willett WC. Protein Intake and Ovulatory Infertility. Am J Obstet Gynecol. 2008;198:210–212. doi: 10.1016/j.ajog.2007.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavarro J, Rich-Edwards JW, Rosner BA, Willett WC. A prospective study of dairy foods intake and anovulatory infertility. Hum Reprod. 2007;22(5):1340–1347. doi: 10.1093/humrep/dem019. [DOI] [PubMed] [Google Scholar]
- 10.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27(5):1466–74. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afeiche M, Williams PL, Mendiola J, Gaskins AJ, Jorgensen N, Swan SH, et al. Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod. 2013;28(8):2265–2275. doi: 10.1093/humrep/det133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afeiche MC, Bridges ND, Williams PL, Gaskins A, Tanrikut C, Petrozza JC, et al. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril. 2014;101(5):1280–7. doi: 10.1016/j.fertnstert.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathews TJ, Hamilton BE. Delayed Childbearing: More Women Are Having Their First Child Later in Life. NCHS Data Brief. 2009 Aug;(21):1–8. [PubMed] [Google Scholar]
- 14.Aiken CE, Tarry-Adkins JL, Ozanne SE. Suboptimal nutrition in utero causes DNA damage and accelerated aging of the female reproductive tract. FASEB J. 2013;27(10):3959–3965. doi: 10.1096/fj.13-234484. [DOI] [PubMed] [Google Scholar]
- 15.Aiken CE, Tarry-Adkins JL, Ozanne SE. Transgenerational Developmental Programming of Ovarian Reserve. Sci Rep. 2015;5:16175. doi: 10.1038/srep16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol. 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzmán C, García-Becerra R, Aguilar-Medina MA, Merchant-Larios H, Zambrano E. Maternal protein restriction during pregnancy and/or lactation negatively affects follicular ovarian development and steroidogenesis in the prepubertal rat offspring. Arch Med Res. 2014;45(4):294–300. doi: 10.1016/j.arcmed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Sui S, He B, Jia Y, Li R, Cai D, Li X, et al. Maternal protein restriction during gestation and lactation programs offspring ovarian steroidogenesis and folliculogenesis in the prepubertal gilts. J Steroid Biochem Mol Biol. 2014;143:267–76. doi: 10.1016/j.jsbmb.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Hansen KR, Hodnett GM, Knowlton N, Criag LT. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Mutlu MF, Erdem M, Erdem A, Yildiz S, Mutlu I, Arisoy O, et al. Antral follicle count determines poor ovarian response better than anti-müllerian hormone but age is the only predictor for live birth in in vitro fertilization cycles. J Assist Reprod Genet. 2013;30(5):657–65. doi: 10.1007/s10815-013-9975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wellons MF, Bates GW, Schreiner PJ, Siscovick DS, Sternfeld B, Lewis CE. Antral follicle count predicts natural menopause in a population-based sample: the Coronary Artery Risk Development in Young Adults Women’s Study. Menopause. 2013;20(8):825–830. doi: 10.1097/GME.0b013e31827f06c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mok-Lin A, Ehlrich S, Williams PL, Petrozza JC, Wright DL, Calafat AM, et al. Urinary Bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary Bisphenol A concentrations and implantation failure among women undergoing In Vitro Fertilization. Environ Health Perspect. 2012;120:978–983. doi: 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. Urinary Bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012;27(12):3583–92. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semi-quantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 26.United States Department of Agriculture, Agricultural Research Service. [Internet] USDA National Nutrient Database for Standard Reference, Release 26. Nutrient Data Laboratory; 2013. Home Page, available from http://www.ars.usda.gov/Services/docs.htm?docid=8964. [Google Scholar]
- 27.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz G, Rosner B, et al. Food-based validation of a Dietary Questionnaire: The Effects of week to Week Variation in Food Consumption. Inter J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 28.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 29.Fulgoni VL. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87(Suppl):1554S–7S. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]
- 30.Willett WC, Lenart E. Chapter 6: Reproducibility and Validity of Food Frequency Questionnaires. In: Willett WC, editor. Nutritional Epidemiology. 2nd. New York: Oxford University Press; 1998. [Google Scholar]
- 31.Ganmaa D, Tezuka H, Enkhmaa D, Hoshi K, Sato A. Commercial cow’s milk has uterotrophic activity on the uteri of young ovariectomized rats and immature rats. Int J Cancer. 2006;118:2363–5. doi: 10.1002/ijc.21659. [DOI] [PubMed] [Google Scholar]
- 32.Ganmaa D, Cui X, Feskanich D, Hankinson SE, Willett WC. Milk, dairy intake and risk of endometrial cancer: a 26-year follow-up. Int J Cancer. 2012;130(11):2664–71. doi: 10.1002/ijc.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melnik BC, John SM, Carrera-Bastos P, Cordain L. The impact of cow’s milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutr Metab. 2012;9(1):74. doi: 10.1186/1743-7075-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaum J, Schuda L, Wu C, Sars R, Ferrario J, Andrews K. A national survey of persistent, bioaccumulative, and toxic (PBT) pollutants in the United States milk supply. J Expo Anal Environ Epidemiol. 2003;13:177. doi: 10.1038/sj.jea.7500269. [DOI] [PubMed] [Google Scholar]
- 35.Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bipshenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61(19):4655–62. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- 36.Maruyama K, Oshima T, Ohyama K. Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows. Pediatr Int. 2010;52:33–38. doi: 10.1111/j.1442-200X.2009.02890.x. [DOI] [PubMed] [Google Scholar]
- 37.Hartmann S, Lacorn M, Steinhart H. Natural occurrence of steroid hormones in food. Food Chemistry. 1998;62(1):7–20. [Google Scholar]
- 38.Pape-Zambito DA, Magliaro AL, Kensinger RS. 17Beta-estradiol and estrone concentrations in plasma and milk during bovine pregnancy. J Dairy Sci. 2008;91(1):127–35. doi: 10.3168/jds.2007-0481. [DOI] [PubMed] [Google Scholar]
- 39.Pape-Zambito DA, Roberts RF, Kensinger RS. Estrone and 17beta-estradiol concentrations in pasteurized-homogenized milk and commercial dairy products. J Dairy Sci. 2010;93(6):2533–40. doi: 10.3168/jds.2009-2947. [DOI] [PubMed] [Google Scholar]
- 40.Daxenberger A1, Ibarreta D, Meyer HH. Possible health impact of animal oestrogens in food. Hum Reprod Update. 2001;7(3):340–55. doi: 10.1093/humupd/7.3.340. [DOI] [PubMed] [Google Scholar]
- 41.Andersson AM, Skakkebaek NE. Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol. 1999;140:477–85. doi: 10.1530/eje.0.1400477. [DOI] [PubMed] [Google Scholar]
- 42.Brinkman MT, Baglietto L, Krishnan K, English DR, Severi G, Morris HA, et al. Consumption of animal products, their nutrient components and postmenopausal circulating steroid hormone concentrations. Eur J Clin Nutr. 2010;64(2):176–83. doi: 10.1038/ejcn.2009.129. [DOI] [PubMed] [Google Scholar]
- 43.Crowe FL, Key TJ, Allen NE, Appleby PN, Roddam A, Overvad K, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1333–40. doi: 10.1158/1055-9965.EPI-08-0781. [DOI] [PubMed] [Google Scholar]
- 44.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor-I and insulin-like growth factor binding protein-3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–861. [PubMed] [Google Scholar]
- 45.Morimoto LM, Polly A, Newcomb PA, White E, Bigler J, Potter JD. Variation in plasma insulin-like growth factor-1 and insulin-like growth factor binding protein-3: personal and lifestyle factors. Cancer Causes Control. 2005;16:917–927. doi: 10.1007/s10552-005-2702-3. [DOI] [PubMed] [Google Scholar]
- 46.Norat T, Dossus L, Rinaldi S, Overvad K, Grønbaek H, Tjønneland A, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr. 2007;61:91–98. doi: 10.1038/sj.ejcn.1602494. [DOI] [PubMed] [Google Scholar]
- 47.Young NJ, Metcalfe C, Gunnell D, Rowlands MA, Lane JA, Gilbert R, et al. A cross-sectional analysis of the association between diet and insulin-like growth factor (IGF)-I, IGF-II, IGF-binding protein (IGFBP)-2, and IGFBP-3 in men in the United Kingdom. Cancer Causes Control. 2012;23:907–17. doi: 10.1007/s10552-012-9961-6. [DOI] [PubMed] [Google Scholar]
- 48.Hoppe C, Udam TR, Lauritzen L, Molgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am J Clin Nutr. 2004;80:447–452. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- 49.Hoppe C, Molgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr. 2004;58:1211–1216. doi: 10.1038/sj.ejcn.1601948. [DOI] [PubMed] [Google Scholar]
- 50.Beasley JM, Gunter MJ, Lacroix AZ, Prentice RL, Neuhouser ML, Tinker LF, et al. Associations of serum IGF-1 and IGFBP-3 levels with biomarker calibrated protein, dairy product and milk intake in the Women’s Health Initiative. Br J Nutr. 2013;7:1–7. doi: 10.1017/S000711451300319X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mani AM, Fenwick MA, Cheng Z, Sharma MK, Singh D, Wathes C. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction. 2010;139(1):139–51. doi: 10.1530/REP-09-0050. [DOI] [PubMed] [Google Scholar]
- 52.Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med. 2005;230:292–306. doi: 10.1177/153537020523000503. [DOI] [PubMed] [Google Scholar]
- 53.Hara N, Takizawa I, Isahaya E, Nishiyama T, Hoshii T, Ishizaki F, et al. Insulin-like growth factor-1 is associated with regulation of the luteinizing hormone production in men receiving androgen deprivation therapy with gonadotropin-releasing hormone analogues for localized prostate cancer. Urol Oncol. 2012;30:596–601. doi: 10.1016/j.urolonc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Hikake T, Hayashi S, Iguchi T, Sato T. The role of IGF1 on the differentiation of prolactin secreting cells in the mouse anterior pituitary. J Endocrinol. 2009;203:231–240. doi: 10.1677/JOE-09-0232. [DOI] [PubMed] [Google Scholar]
- 55.Srivastava VK, Hiney JK, Dees WL. Hypothalamic actions and interactions of alcohol and IGF-1 on the expression of glial receptor protein tyrosine phosphatase-β during female pubertal development. Alcohol Clin Exp Res. 2011;35:1812–1821. doi: 10.1111/j.1530-0277.2011.01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, et al. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27(3):511–23. doi: 10.1210/me.2012-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliver JE, Aitman TJ, Powell JF, Wilson CA, Clayton RN. Insulin-like growth factor I gene expression in the rat ovary is confined to the granulosa cells of developing follicles. Endocrinology. 1989;124(6):2671–9. doi: 10.1210/endo-124-6-2671. [DOI] [PubMed] [Google Scholar]
- 58.Zhou J, Chin E, Bondy C. Cellular pattern of insulin-like growth factor-I (IGF-I) and IGF-I receptor gene expression in the developing and mature ovarian follicle. Endocrinology. 1991;129(6):3281–8. doi: 10.1210/endo-129-6-3281. [DOI] [PubMed] [Google Scholar]
- 59.Hernandez ER, Roberts CT, Jr, LeRoith D, Adashi EY. Rat ovarian insulin-like growth factor I (IGF-I) gene expression is granulosa cell-selective: 5′-untranslated mRNA variant representation and hormonal regulation. Endocrinology. 1989;125:572–4. doi: 10.1210/endo-125-1-572. [DOI] [PubMed] [Google Scholar]
- 60.Villalpando I, Lira E, Medina G, Garcia-Garcia E, Echeverria O. Insulin-like growth factor 1 is expressed in mouse developing testis and regulates somatic cell proliferation. Exp Biol Med. 2008;233:419–426. doi: 10.3181/0708-RM-212. [DOI] [PubMed] [Google Scholar]
- 61.Arrebola JP, Martin-Olmedo P, Fernandez MF, Sanchez-Cantalejo E, Jimenez-Rios JA, Torne P, et al. Predictors of concentrations of hexachlorobenzene in human adipose tissue: a multivariate analysis by gender in southern Spain. Environ Int. 2009;35:27–32. doi: 10.1016/j.envint.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Arrebola JP, Mutch E, Rivero M, Choque A, Silvestre S, Olea N, et al. Contribution of sociodemographic characteristics, occupation, diet and lifestyle to DDT and DDE concentrations in serum and adipose tissue from a Bolivian cohort. Environ Int. 2012;38:54–61. doi: 10.1016/j.envint.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Gasull M, Bosch de Basea M, Puigdomenech E, Pumarega J, Porta M. Empirical analyses of the influence of diet on human concentrations of persistent organic pollutants: a systematic review of all studies conducted in Spain. Environ Int. 2011;37(7):1226–35. doi: 10.1016/j.envint.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Lam T, Williams PL, Burns JS, Sergeyev O, Korrick SA, Lee MM, et al. Predictors of serum chlorinated pesticide concentrations among prepubertal Russian boys. Environ Health Perspect. 2013;121(11–12):1372–7. doi: 10.1289/ehp.1306480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SA, Dai Q, Zheng W, Gao YT, Blair A, Tessari JD, et al. Association of serum concentration of organochlorine pesticides with dietary intake and other lifestyle factors among urban Chinese women. Environ Int. 2007;33:157–163. doi: 10.1016/j.envint.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Yi B, Kim C, Park M, Han Y, Park YJ, Yang M. Association between endocrine disrupting phenols in colostrums and maternal and infant health. Int J Endocrinol. 2013;2013:282381. doi: 10.1155/2013/282381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sathyanarayana S, Alcedo G, Saelens BE, Zhou C, Dills RL, Yu J, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol. 2013;23(4):378–84. doi: 10.1038/jes.2013.9. [DOI] [PubMed] [Google Scholar]
- 68.Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, et al. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol. 2013;42:224–31. doi: 10.1016/j.reprotox.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kinney A, Kline J, Kelly A, Reuss ML, Levin B. Smoking, alcohol and caffeine in relation to ovarian age during the reproductive years. Hum Reprod. 2007;22:1175–1185. doi: 10.1093/humrep/del496. [DOI] [PubMed] [Google Scholar]
- 70.Mishra GD, Dobson AJ, Schofield MJ. Cigarette smoking, menstrual symptoms and miscarriage among young women. Aust N Z J Public Health. 2000;24:413–20. doi: 10.1111/j.1467-842x.2000.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 71.Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas. 2000;35:3–9. doi: 10.1016/s0378-5122(00)00092-x. [DOI] [PubMed] [Google Scholar]
- 72.Whitcomb BW, Bodach SD, Mumford SL, Perkins NJ, Trevisan M, Wactawski-Wended J, et al. Ovarian function and cigarette smoking in the Bio Cycle study. Paediatr Perinat Epidemiol. 2010;24:433–440. doi: 10.1111/j.1365-3016.2010.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


