Abstract
Objectives
To characterize cerebral microbleeds (CMBs) in lacunar stroke patients in the SPS3 trial and to assess their relationship with recurrent stroke and death, and response to assigned treatment.
Methods
SPS3 is a randomized, clinical trial conducted between 2003 and 2011. Patients with recent MRI-documented lacunar infarcts were randomly assigned in a factorial design to target levels of systolic blood pressure (SBP; 130–149 mmHg vs <130 mmHg; open-label) and to antiplatelet treatment (aspirin/clopidogrel vs aspirin/placebo; double-blinded). The current analysis involves 1278 trial participants who had a baseline axial T2*- GRE MRI sequence allowing for CMB detection.
Results
CMBs were present in 30% of 1278 patients (mean age 63 y). Male gender (OR 1.7, 95%CI 1.3–2.3), history of hypertension (1.6, 1.2–2.3), increased systolic blood pressure (1.2 per 20 mmHg, 1.1–1.4), non-diabetic (1.4, 1.1–1.9), multiple old lacunar infarcts(1.9, 1.5–2.5) and moderate(1.7, 1.2–2.3) or severe (4.2, 3.0–5.9) white matter hyperintensities on MRI were independently associated with CMBs. During a mean follow-up of 3.3 y, overall stroke recurrence was 2.5% per patient-y. Patients with CMBs had an adjusted two-fold increased risk of recurrent stroke (HR 2.1, 1.4–3.1). CMBs were not a risk factor for death. There were no statistically significant interactions between CMBs and treatment assignments.
Interpretation
Patients with lacunar stroke and CMBs likely harbor a more advanced form of cerebral small vessel disease in need of efficacious therapeutic strategies.
Introduction
Cerebral microbleeds (CMBs) are remnants of prior cerebral microhemorrhages at the level of arterioles and capillaries visualized on blood-sensitive magnetic resonance imaging (MRI) sequences.1 CMBs have evolved as radiological markers of cerebral small vessel disease (CSVD), representing most notably, hypertensive arteriopathy (arteriolosclerosis) and/or cerebral amyloid angiopathy (CAA).2 CMB prevalence is highest in stroke subtypes associated with CSVD and strongly tied to hypertension.3 Previous studies characterizing CMBs in lacunar stroke have observed prevalence rates ranging from 23–54%,3–10 with higher prevalence observed within East-Asian populations.
CMBs may have important clinical implications as predictors of recurrent stroke and mortality.11–14 Data also suggest that stroke patients with CMBs may respond differently to secondary stroke preventative therapies.15,16 However, the therapeutic effect of stroke preventive therapies in patients with CMBs have yet to be reported from a randomized controlled trial. Moreover, methodological limitations, imposed by observational design, small sample sizes, and/or absence of clear classification of stroke subtype, hamper our current understanding of CMBs in lacunar stroke and the independent contribution of CMBs to the aforementioned outcomes beyond simply marking underlying CSVD.
Accordingly, we sought to characterize CMBs in a well-defined population of symptomatic CSVD by examining lacunar stroke patients within the Secondary Prevention of Small Subcortical Strokes (SPS3) trial and to assess the relationship between CMBs and recurrent stroke and death, as well as response to assigned treatments. We hypothesized that lacunar stroke patients with CMBs would have higher rates of recurrent stroke and death during follow-up and that they would benefit more from aggressive blood pressure management.
Methods
Study design
The rationale, design, participant characteristics, and main results of the SPS3 trial have been reported elsewhere.17–20 The full trial protocol is available in the supplement. In brief, SPS3 was an international randomized clinical trial investigating optimal blood pressure target and antiplatelet regimen in 3020 patients with recent, symptomatic, magnetic resonance imaging (MRI) confirmed lacunar infarcts. Eligible participants were patients aged ≥30 years with a recent (≤180 days) MRI documented lacunar infarct, without major-risk cardioembolic source, ipsilateral extracranial carotid stenosis ≥50% or evidence of prior cortical stroke. Additional exclusion criteria included disabling stroke (modified Rankin score of four or higher), significant cognitive impairment (MMSE ≥2 standard deviations below the mean for age and education), and previous non-traumatic intracranial macrohemorrhage. Local ethics committees for human research at all recruitment centers approved the study protocol, and study participation required written informed consent.
SPS3 participants were eligible for the current analysis if they had an interpretable axial T2*-weighted gradient echo (GRE) sequence allowing for CMB detection as part of their baseline MRI.
Randomization and masking
Eligible participants were randomly assigned, in a two-by-two factorial design, to both an antiplatelet intervention (1:1; aspirin 325 mg plus clopidogrel 75 mg vs aspirin 325 mg plus placebo; double-blind) and a target level of systolic blood pressure control (1:1; lower [<130 mm Hg] vs higher [130–149 mm Hg]; open- label).19 Individual site staff personnel were unaware of randomization assignments, which were stored electronically at the SPS3 statistical center.
Procedure
Full study procedures have been published elsewhere.19,20 Briefly, participants were seen at least every three months for blood pressure measurement, adjustment of drug treatment, dispensing of antiplatelet drugs, and detection of adverse events. Adherence to assigned antiplatelet treatment was estimated as 94% by pill count.17 Permanent discontinuation of assigned antiplatelet drug occurred in 30% of patients.17 After one year the mean systolic blood pressure was 138 mm Hg (95% CI 137–139) in the higher target group and 127 mm Hg (126–128) in the lower target group, with a mean difference of 11 mm Hg (SD 16) at last study visit.18
Participant characteristics
Patients with lacunar stroke (deficit lasting >24 hours) or subcortical transient ischemic attack (deficit lasting <24) documented by infarct on MRI were randomized into the trial. Diabetes was defined as a history of diabetes mellitus at the time of the qualifying stroke, fasting serum glucose ≥120 mg/dL at the time of stroke, and/or initiation of antidiabetic medications during the first three months of follow-up. Blood pressure at entry (baseline) was the average of two screening systolic blood pressure determinations, an average of three at first visit and of three at second visit one week later. Severity of hypertension was adjusted by adding 5 mm Hg for each antihypertensive medication (up to a maximum of four) at the time of the determination. Normotensive was defined as less than 120 mm Hg, prehypertensive as 120 to less than 140 mm Hg, stage I hypertension as 140 to less than 160 mm Hg, and stage II hypertension as 160 mm Hg or more. Race and ethnicity were determined primarily by self-report, using the two Census 2000 questions regarding race and Hispanic/Latino ethnicity as previously described.20
Imaging Acquisition and Analysis
Participants underwent structural brain MRI before study entry as part of their clinical management without pre-specified protocol for data acquisition. Participant eligibility was determined locally with MRI scans subsequently submitted for central review. Determination of location of qualifying infarct, old subcortical infarcts, and white matter hyperintensities (WMH) were done centrally.21 Old lacunar infarcts were defined as hypointense areas on FLAIR and/or T1 measuring ≥3 mm, but no more than 15 mm in maximum dimension. Hypointense lesions at the level of the anterior commissure, convexity, or midbrain, were considered enlarged perivascular spaces and not classified infarcts, unless surrounded by a hyperintense halo on FLAIR. Intra-rater reliability of old lacunar infarcts was assessed by having a neuroradiologist (C. B.) re-evaluate a set of 75 MRIs at the end of the study, unaware of his earlier interpretations. Intra-rater reliability for presence/absence of lacunes on Flair/T1 was good (κ = 0·64, 88% agreement).
WMHs were evaluated visually on FLAIR images using the age- related white matter change (AWRMC) (range 0–16). ARWMC scale categories were defined a priori as: zero to four, mild disease; five to eight, moderate disease; and 9 or more, severe disease. Inter- rater agreement was good-excellent on a sample of 40 MRIs (4 raters; range: κ = 0·64, 77% agreement to κ = 0·89, 95% agreement).
CMBs were rated according to the Brain Observer Microbleed Rating Scale (BOMBS) with each CMB determined to be “certain” or “uncertain”, and as lobar (isolated to cortex/grey white junction and/or subcortical white matter) or deep (isolated to basal ganglia grey matter, internal/external capsule, thalamus, brainstem, and/or cerebellum).22 Total numbers of each and overall of deep and lobar CMBs (regardless of size and certainty) were then computed, and severity of disease was coded a priori as absent (0 CMBs), mild (1–2 CMBs), moderate (3–10 CMBs), or severe (> 10 CMBs), and location was coded as lobar only, deep only, or mixed.23 CMB readings were done by one rater (AS) blinded to baseline features and outcomes with excellent intra-rater reliability for CMB presence on two separate reads of the same images separated by >12 months (n = 55, κ =0.82, 91% agreement), and previously reported excellent inter-rater reliability for CMB presence in a separate cohort.24
Outcomes
The primary outcome of interest was all stroke recurrence (first ischemic stroke or intracranial hemorrhage), and secondary outcome of interest was all-cause mortality. Ischemic stroke was clinically defined as a focal neurologic deficit of sudden onset persisting for more than 24 hours, and without evidence of hemorrhage on neuroimaging. Intracranial hemorrhages included those in intracerebral, subdural, epidural, and subarachnoid locations as documented on neuroimaging. A central adjudication committee that was unaware of the treatment assignments adjudicated all reported outcomes.
Statistical analysis
Patient demographic and clinical characteristics were compared between groups in cross-sectional analyses using a chi-square test for categorical variables and a t-test or ANOVA for continuous. Forward and backward stepwise multivariable binomial logistic regression analyses (likelihood ratio test) were used to identify variables independently associated with CMB(s). Multivariable Cox-proportional hazards models were used to estimate the contribution of CMB(s) to risk of i) recurrent stroke and ii) death during follow-up after adjusting for assigned treatment groups and other covariates as noted. A pre-specified sensitivity analysis was also performed excluding patients with only ‘uncertain’ CMBs. All analyses followed the intention-to-treat paradigm. All tests were two-sided, and statistical significance was accepted at the 0.05 level. Analyses were performed with SPSS version 22.0.
Role of the funding source
The sponsor was not involved in the current analysis and manuscript. NINDS however did participate in the design, data collection, primary analyses and interpretation of the SPS3 trial.
Results
Overall, 42% of enrolled participants between 2003 and 2011 (1278 of 3020) had images available to assess CMBs (34% of US/Canada enrollees, 62% of Latin America enrollees, and 48% of Spain enrollees) and were included in these analyses. (Figure 1) Participants had a mean age of 63 (SD 11), were 65% male, and had histories of hypertension, diabetes, and prior stroke of 75%, 36%, and 10%, respectively. Included participants were more often Hispanic, male, with greater severity of hypertension, and underrepresentation of smoking and renal dysfunction, but did not otherwise differ significantly with respect to baseline characteristics and MRI findings from those excluded (Table 1).
Figure 1.
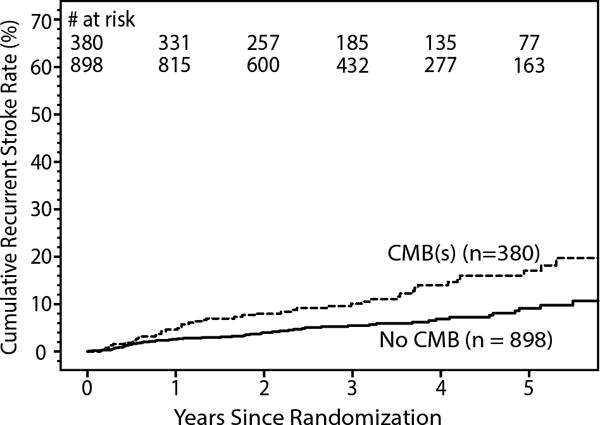
Kaplan-Meier curve for recurrent stroke by cerebral microbleed (CMB) status.
Table 1.
Comparison of baseline characteristics of participants included in the study sample versus those excluded.
| Included cohort (n = 1278) |
Excluded cohort (n = 1742) |
p-value | |
|---|---|---|---|
|
| |||
| Male (%) | 65 | 61 | 0.03 |
|
| |||
| Mean age, years, mean (SD) | 63 (11) | 63 (11) | 0.5 |
|
| |||
| Ethnicity-race (%) | < 0.001 | ||
| Non-Hispanic white | 44 | 56 | |
| Hispanic | 39 | 24 | |
| Black | 15 | 18 | |
| Other/multiple | 3 | 2 | |
|
| |||
| Current smoker (%) | 18 | 23 | 0.001 |
|
| |||
| Alcohol use, ≥ 7drinks/week (%) | 12 | 13 | 0.3 |
|
| |||
| Parental history of stroke (%) | 28 | 31 | 0.1 |
|
| |||
| Hypertension, % | 75 | 75 | 0.9 |
|
| |||
| Blood pressure at entry, mmHg, mean (SD) | 143 (20)/78 (11) | 143 (18)/78 (10) | 0.6/0.9 |
|
| |||
| Severity of hypertension (%) | 0.001 | ||
| Normal-prehypertensive | 17 | 22 | |
| Stage 1 | 42 | 40 | |
| Stage 2 | 41 | 37 | |
|
| |||
| Estimated GFR < 60 mL/min/1.73 m2, % | 13 | 17 | 0.01 |
|
| |||
| Prior lacunar stroke (%) | 10 | 11 | 0.3 |
|
| |||
| Prior subcortical TIA (%) | 5 | 5 | 1.0 |
|
| |||
| Diabetes mellitus (%) | 36 | 37 | 0.6 |
|
| |||
| Ischemic heart disease (%) | 9 | 11 | 0.1 |
|
| |||
| Hyperlipidemia (%) | 46 | 51 | 0.01 |
|
| |||
| Multiple old lacunar infarcts on MRI (%) | 42 | 38 | 0.08 |
|
| |||
| White matter hyperinstensities (ARWMC score) | |||
| mean (SD) | 5.6 (4.1) | 5.4 (4.2) | 0.3 |
| 0–4 | 49 | 51 | 0.7 |
| 5–8 | 29 | 28 | |
| 9+ | 22 | 22 | |
Abbreviations: ARWMC; age related white matter changes; GFR, glomerular filtration rate; MRI, magnetic resonance imaging; S3, small subcortical infarcts; SD, standard deviation
Thirty percent (n=380) of the 1278 patients had at least one CMB. Patient characteristics independently associated with CMB(s) were increasing age, male sex, Hispanic ethnicity, hypertension, higher baseline systolic blood pressure, prior lacunar stroke, and non-diabetic status compared to those with no CMB. (Table 2–3) Patients with CMB(s) also more often had multiple old lacunar infarcts on MRI and more WMHs. (Table 2–3).
Table 2.
Baseline characteristics and MRI findings by cerebral microbleed status
| No CMB (n = 898) |
CMB(s) (n = 380) |
p-value | |
|---|---|---|---|
|
| |||
| Male (%) | 63 | 71 | 0.009 |
|
| |||
| Mean age, years, mean (SD) | 62 (10) | 64 (11) | 0.01 |
|
| |||
| Ethnicity-race (%) | 0.04 | ||
| Non-Hispanic white | 46 | 39 | |
| Hispanic | 37 | 44 | |
| Black | 14 | 16 | |
| Other/multiple | 3 | 2 | |
|
| |||
| Current smoker (%) | 19 | 15 | 0.09 |
|
| |||
| Alcohol use, ≥ 7drinks/week (%) | 12 | 11 | 0.4 |
|
| |||
| Parental history of stroke (%) | 28 | 29 | 0.8 |
|
| |||
| Hypertension, % | 72 | 82 | < 0.001 |
|
| |||
| Severity of hypertension (%) | 0.009 | ||
| Normo-prehypertensive | 17 | 16 | |
| Stage 1 | 45 | 37 | |
| Stage 2 | 38 | 47 | |
|
| |||
| Estimated GFR < 60 mL/min/1.73 m2, % | 12 | 18 | 0.002 |
|
| |||
| Blood pressure at entry, mmHg, mean (SD) | 141 (18)/77 (10) | 147 (23)/80 (12) | < 0.001/< 0.001 |
|
| |||
| Prior lacunar stroke (%) | 8 | 14 | < 0.001 |
|
| |||
| Prior subcortical TIA (%) | 5 | 6 | 0.5 |
|
| |||
| Diabetes mellitus (%) | 39 | 30 | 0.005 |
|
| |||
| Ischemic heart disease (%) | 10 | 9 | 0.6 |
|
| |||
| Hyperlipidemia (%) | 48 | 41 | 0.02 |
|
| |||
| Multiple old lacunar infarcts on MRI (%) | 34 | 59 | < 0.001 |
|
| |||
| White matter hyperintensities (ARWMC score) | |||
| mean (SD) | 4.7 (3.4) | 7.7 (4.7) | < 0.001 |
| 0–4 | 57 | 30 | < 0.001 |
| 5–8 | 28 | 30 | |
| 9+ | 14 | 40 | |
Abbreviations: ARWMC; age related white matter changes; GFR, glomerular filtration rate; MRI, magnetic resonance imaging; SD, standard deviation; TIA, transient ischemic attack
Table 3.
Multivariable models of patient characteristics and MRI findings independently associated with cerebral microbleed(s).
| A) Participant characteristics | Odds ratio (95% CI) |
|---|---|
|
| |
| Male | 1.7 (1.3, 2.3) |
|
| |
| Age, per 10 year increase | 1.2 (1.0, 1.3) |
|
| |
| History of hypertension | 1·7 (1·2, 2·3) |
|
| |
| Systolic blood pressure, per 20 mmHg increase | 1.3 (1.2, 1.5) |
|
| |
| Prior lacunar stroke | 2·0 (1.3, 2.9) |
|
| |
| Diabetes | 0.6 (0.5, 0.8) |
|
| |
| B) Participant and MRI findings |
Odds ratio (95% CI) |
|
| |
| Male sex | 1.7 (1.3, 2.3) |
|
| |
| History of hypertension | 1.6 (1.2, 2.3) |
|
| |
| Systolic blood pressure, per 20 mmHg increase | 1.2 (1.1, 1.4) |
|
| |
| Diabetes | 0.7 (0.5, 0.9) |
|
| |
| Multiple old lacunar infarcts on MRI | 1.9 (1.5, 2.5) |
|
| |
| White matter hyperintensities (ARWMC score) | |
| 0–4 | reference group |
| 5–8 | 1.7 (1.2, 2.3) |
| 9+ | 4.2 (3.0, 5.9) |
Abbreviations: ARWMC, age related white matter changes; MRI, magnetic resonance imaging
Of those with CMBs, severity of disease (CMB count burden) was mild in 57% (n=217), moderate in 31% (n = 118), and severe in 12% (n = 45). Participant characteristics did not vary by severity except that patients with more severe disease were more often Hispanic and less often diabetic (Table 4). More severe disease was associated with more advanced CSVD (WMHs and multiple old lacunar infarcts) on MRI. (Table 4)
Table 4.
Baseline characteristics according to cerebral microbleed severity
| Severity | Mild (1–2) (n = 217) |
Moderate (3–10) (n = 118) |
Severe (> 10) (n = 45) |
p–value |
|---|---|---|---|---|
|
| ||||
| Male (%) | 71 | 64 | 82 | 0.08 |
|
| ||||
| Age, years, mean (SD) | 65 (10) | 63 (12) | 64 (12) | 0.2 |
|
| ||||
| Ethnicity-race (%) | 0.02 | |||
| Non-Hispanic white | 39 | 44 | 27 | |
| Hispanic | 40 | 42 | 67 | |
| Black | 18 | 14 | 7 | |
| Other/multiple | 3 | 1 | 0 | |
|
| ||||
| Current smoker (%) | 14 | 19 | 9 | 0.2 |
|
| ||||
| Alcohol use, ≥ 7drinks/week (%) | 9 | 14 | 9 | 0.3 |
|
| ||||
| Parental history of stroke (%) | 29 | 26 | 36 | 0.4 |
|
| ||||
| History of hypertension (%) | 82 | 82 | 87 | 0.7 |
|
| ||||
| Severity of hypertension (%) | ||||
| Normal-prehypertensive | 20 | 14 | 4 | |
| Stage 1 | 34 | 38 | 47 | 0.1 |
| Stage 2 | 46 | 48 | 49 | |
|
| ||||
| Estimated GFR < 60 mL/min/1.73 m2, % | 16 | 19 | 24 | 0.4 |
|
| ||||
| Blood pressure at entry, mmHg, mean (SD) | 146 (23)/79 (12) | 149 (23)/81 (12) | 148 (20)/83 (13) | 0.6/0.1 |
|
| ||||
| Prior lacunar stroke (%) | 12 | 16 | 20 | 0.3 |
|
| ||||
| Prior subcortical TIA (%) | 6 | 7 | 4 | 0.9 |
|
| ||||
| Diabetes mellitus (%) | 35 | 30 | 11 | 0.008 |
|
| ||||
| Ischemic heart disease (%) | 10 | 6 | 11 | 0.4 |
|
| ||||
| Hyperlipidemia (%) | 45 | 34 | 40 | 0.2 |
|
| ||||
| Multiple old lacunar infarcts on MRI (%) | 51 | 62 | 84 | < 0.001 |
|
| ||||
| White matter hyperintensities (ARWMC score) | ||||
| mean (SD) | 6.4 (4.3) | 8.6 (4.8) | 11.5 (3.6) | < 0.001 |
| 0–4 | 39 | 25 | 2 | < 0.001 |
| 5–8 | 34 | 26 | 18 | |
| 9+ | 27 | 48 | 80 | |
Abbreviations: ARWMC; age related white matter changes; GFR, glomerular filtration rate; MRI, magnetic resonance imaging; SD, standard deviation; TIA, transient ischemic attack.
Location of CMBs was lobar only in 21% (n = 81), deep only in 44% (n = 167), and mixed in 35% (n = 132). Participants with lobar only CMB(s) were more likely to have diabetes (at a rate proportionally similar to patients without CMBs), but otherwise did not differ with other locations. (Table 4) Participants with mixed CMBs had more advanced CSVD [more CMB(s), WMHs and multiple old lacunar infarcts] on MRI.
Associations with recurrent stroke and death
During a mean follow-up of 3.3 years, 100 participants (annualized rate 2.5% per patient-year) had a recurrent stroke (83 ischemic, 16 hemorrhagic, 1 uncertain etiology): 54 of the 898 with no CMB, 46 of the 380 with CMB(s). (Figure 1) Participants with CMB(s) were at increased risk of recurrent stroke (adjusted HR 2.1, 95% CI 1.4, 3.1) after adjusting for assigned treatment groups and clinical risk factors for recurrent stroke in the SPS3 trial (male sex, black race, diabetes, and prior symptomatic lacunar stroke or transient ischemic attack25). Risk of recurrent stroke (adjusted HR 2.3, CI 1.5, 3.6) was not appreciably changed when the patients with hemorrhagic strokes (11 in no CMB group and 5 in CMB group) were excluded, nor when the 57 patients with only ‘uncertain’ CMB(s) were excluded (adjusted HR 2.0, CI 1.3, 3.1). After adjusting for multiple old lacunar infarcts on MRI (>1) and WMHs (ARWMC groups; coded as 0–4, 5–8 and 9+) as well as assigned treatment group and clinical risk factors, participants with CMB(s) remained at increased risk of stroke (adjusted HR 1.9, 95% CI 1.2, 2.9). Adjusting for old lacunar infarcts on MRI and WMHs (ARWMC score) as continuous variables instead of categorical in this same model did not affect the increased risk of stroke with CMB(s) observed (adjusted HR 1.9, 95% CI 1.2, 2.9). Recurrent stroke risk appeared highest in patients with > 10 CMBs and lobar only CMBs (Table 5, Figure 2); however, there was inadequate power to detect significant differences between CMB burden or location subgroups after excluding patients without CMBs from the analysis. Given the few number of hemorrhagic strokes we were unable to carry out any meaningful analyses for this outcome in isolation. All cause-mortality risk was unaffected by CMB(s) (adjusted HR 1.2, CI 0.8, 2.0). (Table 5)
Table 5.
Baseline characteristics according to cerebral microbleed topography
| Topography | Strictly lobar (n = 81) |
Strictly Deep (n = 167) |
Mixed (n = 132) |
p-value |
|---|---|---|---|---|
|
| ||||
| Male (%) | 69 | 71 | 71 | 0.9 |
|
| ||||
| Age, years, mean (SD) | 66 (11) | 64 (11) | 64 (11) | 0.2 |
|
| ||||
| Ethnicity-race (%) | 0.2 | |||
| Non-Hispanic white | 40 | 46 | 37 | |
| Hispanic | 44 | 38 | 51 | |
| Black | 15 | 19 | 11 | |
| Other/multiple | 1 | 3 | 1 | |
|
| ||||
| Current smoker (%) | 20 | 13 | 14 | 0.3 |
|
| ||||
| Alcohol use, ≥ 7drinks/week (%) | 5 | 13 | 11 | 0.1 |
|
| ||||
| Parental history of stroke (%) | 32 | 29 | 26 | 0.7 |
|
| ||||
| History of hypertension (%) | 85 | 78 | 86 | 0.2 |
|
| ||||
| Severity of hypertension (%) | 0.3 | |||
| Normal-prehypertensive | 20 | 18 | 11 | |
| Stage 1 | 38 | 35 | 39 | |
| Stage 2 | 43 | 47 | 51 | |
|
| ||||
| Estimated GFR < 60 mL/min/1.73 m2, % | 20 | 17 | 18 | 0.9 |
|
| ||||
| Blood pressure at entry, mmHg, mean (SD) | 145 (25)/78 (12) | 147 (22)/81 (11) | 149 (22)/81 (12) | 0.5/0.05 |
|
| ||||
| Prior lacunar stroke (%) | 16 | 11 | 17 | 0.2 |
|
| ||||
| Prior subcortical TIA (%) | 8 | 5 | 6 | 0.8 |
|
| ||||
| Diabetes mellitus (%) | 44 | 29 | 23 | 0.005 |
|
| ||||
| Ischemic heart disease (%) | 11 | 8 | 8 | 0.7 |
|
| ||||
| Hyperlipidemia (%) | 47 | 43 | 34 | 0.1 |
|
| ||||
| Multiple old lacunar infarcts on MRI (%) | 56 | 52 | 69 | 0.01 |
|
| ||||
| White matter hyperintensities (ARWMC score) | ||||
| mean (SD) | 6.8 (4.5) | 6.9 (4.3) | 9.2 (4.9) | < 0.001 |
| 0–4 | 36 | 34 | 22 | < 0.001 |
| 5–8 | 35 | 32 | 24 | |
| 9+ | 29 | 34 | 54 | |
|
| ||||
| Severity of CMB count (%) | < 0.001 | |||
| mild (1–2) | 84 | 78 | 14 | |
| moderate (3–10) | 12 | 19 | 58 | |
| severe (> 10) | 4 | 3 | 28 | |
Abbreviations: ARWMC; age related white matter changes; GFR, glomerular filtration rate; MRI, magnetic resonance imaging; SD, standard deviation; TIA, transient ischemic attack.
Figure 2.
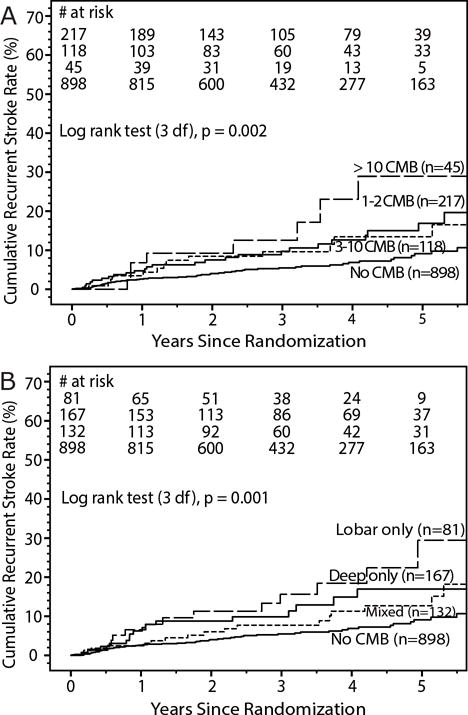
Kaplan-Meier curves for recurrent stroke by cerebral microbleed (CMB) severity (A) and topography (B) subgroups.
Effect of randomized interventions
Overall, in our subgroup of SPS3 participants with adequate MRI sequences for CMB detection, more aggressive blood pressure lowering resulted in a statistically significant reduction in stroke recurrence (HR 0.6, CI 0.4, 0.9) but not all cause mortality (HR 0.8, CI 0.5–1.3). (Figure 3) Dual antiplatelet therapy with aspirin and clopidogrel did not reduce stroke recurrence compared to aspirin alone (HR 1.0, CI 0.7, 1.5), whereas all cause-mortality was increased in patients assigned dual antiplatelet therapy (HR 2.3, CI 1.4, 3.7). (Figure 3) Aggressive blood pressure lowering significantly reduced risk of stroke recurrence in patients with CMB(s) (HR 0.5, CI 0.3, 0.9) but not patients without CMB(s) (HR 0.7, CI 0.4, 1.3); however, the difference in the magnitude of the effect was statistically insignificant (interaction, p=0.34). When assessing patients with moderate-severe CMB burden (≥3 CMBs), aggressive blood pressure lowering significantly reduced risk of stroke (HR 0.2, CI 0.1–0.8) with a trend suggesting that there may exist a different treatment effect on risk of recurrent stroke in those with moderate-severe disease compared to patients without CMBs (interaction, p = 0.087).
Figure 3.
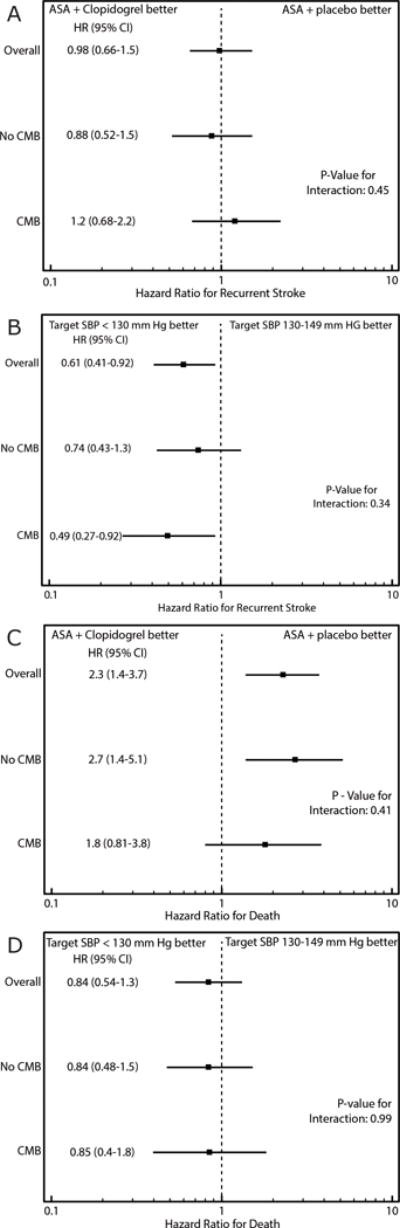
Stroke recurrence (A, B) and all-cause mortality (C, D), and response to treatment assignment by cerebral microbleed (CMB) status.
No effect on all-cause mortality was detected in patients with CMB(s) (HR 0.9, CI 0.4, 1.8) or patients without CMB(s) (HR 0.8, CI 0.5, 1.5) (interaction, p=0.99). (Figure 3)
Risk of stroke recurrence did not differ for those assigned dual antiplatelet therapy versus aspirin alone amongst patients with CMB(s) (HR 1.2, CI 0.7, 2.2) and those without CMB(s) (HR 0.9, CI 0.5, 1.5), (interaction, p=0.45). There was no strong suggestion that the effect of dual antiplatelet therapy in those with moderate-severe CMB burden (HR 1.9, CI 0.8, 4.6) differed from those with no CMBs (interaction, p=0.16). Dual antiplatelet therapy appeared not to increase all cause-mortality in patients with CMBs (HR 1.8, CI 0.8, 3.8) in contrast to those without CMBs (HR 2.7, CI 1.4, 5.1), however the effect modification was not statistically significant (interaction, p=0.41). (Figure 3)
Discussion
In this large and well-characterized cohort of patients with recent lacunar stroke we found CMBs to be highly prevalent, associated with overrepresentation of hypertensive disease and underrepresentation of diabetes mellitus, as well as an independent predictor of stroke recurrence. In this subgroup of SPS3 participants who underwent MRI sequences allowing for CMB detection, intensive blood pressure control was associated with a statistically significant reduction in recurrent stroke (HR 0.6, 95% CI 0.4, 0.9), but there was no statistically significant interaction with the presence of CMBs.
Similar to previous reports, we found a high prevalence of CMBs in lacunar stroke patients.3 The observed 30% prevalence rate of CMBs is similar to that found in other lacunar stroke cohorts from Caucasian populations.6,7 Our results demonstrate that even within a cohort restricted to well-characterized symptomatic CSVD, CMBs are strongly associated with advanced hypertensive disease and MRI markers of CSVD. The mechanism underlying the lower prevalence of diabetes mellitus in patients with lacunar stroke who have CMBs on MRI – a novel observation – is however uncertain. One plausible explanation is that in patients with diabetes, lacunar strokes result more frequently from branch orifice atheromatous disease26–28 rather than hypertensive arteriopathy - the latter being the predominant vascular pathology underlying strictly deep/mixed CMBs.2 Hence, the perceived inverse association may result from diabetics in this population having a different non-CMB inducing vascular pathology contributing to their lacunar strokes, which we hypothesize to be branch orifice atheromatous disease.
The presence of CMBs on baseline MRI was associated with a two-fold increased risk of stroke during follow-up. This association remained unchanged after excluding patients with incident intracerebral hemorrhage (ICH). Similarly, a recent systematic review/meta-analysis reported a CMB-related 1.8-fold increase in risk of recurrent ischemic stroke after ischemic stroke/transient ischemic attack.11 Observing a nearly identical relationship within a sample of lacunar stroke, that is independent of other MRI markers of advanced CSVD, suggests that the association between CMBs and recurrent ischemic stroke go above and beyond CMBs simply marking underlying CSVD and is hypothesis generating. One possibility is that extravasated hemosiderin, which has been reported to migrate through perivascular spaces inducing an intense inflammatory reaction, could contribute to the pathogenesis of vascular compromise, and future cerebral vascular injury.29 Alternatively, CMBs on MRI may mark higher CSVD severity that is not represented by the other MRI markers. The observed trend for higher rates of recurrent stroke in patients with strictly lobar CMBs also suggests a possible synergistic relationship between hypertensive arteriopathy and CAA, possibly by way of impaired cerebral vascular reactivity.30 As our current therapeutic strategies are insufficient in mitigating recurrent ischemic stroke risk in patients with lacunar stroke, these hypotheses need to be explored further in future experimental and clinical studies, as they could potentially lead to novel therapeutic targets to halt CSVD and related neuronal injury.31 Conversely, CMBs were not predictive of all caused-mortality within our cohort, suggesting that underlying CSVD might have confounded their previously reported associations with mortality in broader populations.12–14 Our low number of ICH during follow-up negated the possibility of any meaningful analysis confined to risk of incident ICH.
To our knowledge our reported findings are the first assessing the effect of secondary stroke preventative therapies in patients with CMBs within the context of a randomized controlled trial. In contrast to prior observational studies demonstrating greater degree of adverse events with the use of antithrombotic agents in patients with CMBs,16 we did not observe a statistically significant treatment interaction between CMB presence and dual vs. mono antiplatelet therapy. Accordingly, our results caution against deriving firm conclusions regarding conservative antithrombotic therapy regimens in patients with CMBs solely from observational data that can be prone to residual confounding, particularly since most ischemic stroke patients with CMBs have higher future absolute rates of ischemic events, rather than hemorrhagic ones.11 Although, no statistically significant treatment interactions were observed between the tested blood pressure targets and CMB presence, there did exist a trend for patients with CMBs to have greater therapeutic effect with intensive blood pressure control, particularly in patients with moderate-severe CMB burden. However, we restate that the apparent difference in response to BP lowering according to CMB status was not statistically significant, rendering our observations hypothesis generating and requiring further exploration in larger samples.
Our results were limited by the unavailability of MRI sequences allowing for CMB assessment in all SPS3 trial participants, which was largely center dependent according to localized MRI sequence protocols. This may have introduced selection bias into our sample. For instance, included participants within our sample were more often Hispanic, compared to the larger SPS3 population. Moreover, the distribution of SPS3 recruitment sites across the Americas and Spain, limits the generalizability of our findings to the worldwide population, such as East-Asian populations who have a high prevalence of CSVD, and may be more prone to ICH.32 The SPS3 trial occurred between 2003–2011 and technological advancements in CMB detection since then also may limit generalizability. Importantly, the non-standardization of GRE sequence acquisition parameters and the unavailability of data on these parameters or MRI field strengths, which were never captured, for inclusion into our multivariate analyses is a major limitation that may have resulted in heterogeneous CMB detection rates across the various recruitment centers and confounded our results. Our observation of greater severity of WMD on MRI in patients with mixed CMBs could be confounded by the fact that this subgroup by definition only includes patients with multiple CMBs (2 or more), whereas those categorized as strictly lobar or deep can include patients with a single CMB. We categorized patients with ‘uncertain’ CMBs as CMB positive due to similarities observed in their baseline characteristics with patients with ‘certain’ CMBs, which is imperfect. Reassuringly however, sensitivity analyses excluding patients with only ‘uncertain’ CMBs did not alter our findings. We rated WMH using a visual ARWMC rating scale, rather than volumetric measurements. Although visual scales are more clinically applicable, it is possible that the absence of volumetric measurements limited our ability to appropriately adjust for degree of WMH in our analyses. A final limitation is that our sample was underpowered to appropriately assess risk by CMB burden and topography, and treatment interactions in the CMB burden and topography subgroups, as well as for the outcome of ICH.
Results from this largest well-defined cohort of lacunar stroke suggest that CMBs are highly prevalent in patients with lacunar stroke and an independent predictor of stroke recurrence. Accordingly, the presence of CMBs in patients with lacunar stroke likely represents a more aggressive form of CSVD in need of efficacious therapeutic strategies. Until such therapies become available, our data suggest that it would be reasonable to reinforce guideline recommendations suggesting intensive BP reduction to SBP < 130 mmHg in lacunar stroke patients, particularly in the presence of CMBs on MRI.
Table 6.
Risk of recurrent stroke and mortality by cerebral microbleed (CMB) status
| Recurrent stroke
|
Mortality
|
|||||
|---|---|---|---|---|---|---|
| # of events | Crude HR* (95% CI) |
Adjusted HR** (95% CI) |
Adjusted HR*** (95% CI) |
# of deaths | Crude HR* (95% CI) |
|
|
| ||||||
| CMB Count | ||||||
| None (n = 898) | 54 | reference | reference | reference | 51 | reference |
| 1–2 (n = 217) | 25 | 1.9 (1.2, 3.1) | 2.0 (1.2, 3.2) | 1.8 (1.1, 3·0) | 15 | 1.2 (0.7, 2.2) |
| 3–10 (n = 118) | 13 | 1.7 (0.9, 3.1) | 1.8 (1.0, 3.2) | 1.7 (0·9, 3·2) | 9 | 1.2 (0.6, 2.4) |
| > 10 (n = 45) | 8 | 3.0 (1.4, 6.3) | 4.0 (1.8, 8.7) | 3.7 (1·6, 8·6) | 3 | 1.4 (0.4, 4.4) |
|
| ||||||
| CMB Topography | ||||||
| None (n = 898) | 54 | reference | reference | reference | 51 | reference |
| Lobar only (n = 81) | 13 | 2.9 (1.6, 5.3) | 2.7 (1.5, 5.0) | 2.2 (1.1, 4.3) | 7 | 1.5 (0.7, 3.3) |
| Deep only (n = 167) | 17 | 1.6 (0.9, 2.8) | 1.7 (1.0, 2.9) | 1.6 (0.9, 2.8) | 8 | 0.8 (0.4, 1.7) |
| Mixed (n = 132) | 16 | 1.9 (1.1, 3.4) | 2.2 (1.2, 3.9) | 2.0 (1.1, 3.7) | 12 | 1.6 (0.9, 3.0) |
Abbreviations: HR=hazard ratio. CI=confidence interval
Adjusted for assigned treatments
Adjusted for assigned treatments and clinical risk factors male sex, black race, diabetes, and prior symptomatic lacunar stroke or transient ischemic attack.25
Adjusted for assigned treatments, clinical risk factors male sex, black race, diabetes, and prior symptomatic lacunar stroke or transient ischemic attack,25 and MRI risk factors multiple S3 and white matter hyperintensities (ARWMC score).
Acknowledgments
Trial Registration Number: ClinicalTrials.gov identifier, NCT00059306
Funding: Supported by a Cooperative Agreement from the National Institute of Neurological Disorders and Stroke (U01 NS38529-04A1). Dr. Shoamanesh is supported by an endowed chair from the Marta and Owen Boris Foundation.
Footnotes
Author contributions:
1) Concept and design of study: AS, LAP, RGH and ORB. 2) Acquisition and analysis of data: AS, LAP, RGH, ORB, CB, LC, LAM, MS, JMF, DCA, and CSK. 3) Drafting of the manuscript: AS and LAP.
Potential Conflicts of Interest: Dr. Shoamanesh has nothing to disclose. Dr. Pearce has nothing to disclose. Dr. Bazan has nothing to disclose. Dr. Catanese has nothing to disclose. Dr. McClure has nothing to disclose. Dr. Sharma has nothing to disclose. Dr. Marti-Fabregas has nothing to disclose. Dr. Anderson has nothing to disclose. Dr. Kase has nothing to disclose. Dr. Hart has nothing to disclose. Dr. Benavente has nothing to disclose.
References
- 1.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet neurology. 2009 Feb;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovascular diseases. 2011;32(6):528–534. doi: 10.1159/000331466. [DOI] [PubMed] [Google Scholar]
- 3.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130(Pt 8):1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 4.Kato H, Izumiyama M, Izumiyama K, et al. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33(6):1536–1540. doi: 10.1161/01.str.0000018012.65108.86. [DOI] [PubMed] [Google Scholar]
- 5.Naka H, Nomura E, Wakabayashi S, et al. Frequency of asymptomatic microbleeds on T2*-weighted MR images of patients with recurrent stroke: association with combination of stroke subtypes and leukoaraiosis. AJNR Am J Neuroradiol. 2004;25(5):714–719. [PMC free article] [PubMed] [Google Scholar]
- 6.Schonewille WJ, Singer MB, Atlas SW, Tuhrim S. The prevalence of microhemorrhage on gradient-echo magnetic resonance imaging in acute lacunar infarction. Journal of stroke and cerebrovascular diseases. 2005;14(4):141–144. doi: 10.1016/j.jstrokecerebrovasdis.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Lewis SC, Keir SL, et al. Cerebral microbleeds are associated with lacunar stroke defined clinically and radiologically, independently of white matter lesions. Stroke. 2006;37(10):2633–2636. doi: 10.1161/01.STR.0000240513.00579.bf. [DOI] [PubMed] [Google Scholar]
- 8.Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, et al. Higher ambulatory blood pressure relates to new cerebral microbleeds: 2-year follow-up study in lacunar stroke patients. Stroke. 2013;44(4):978–983. doi: 10.1161/STROKEAHA.111.676619. [DOI] [PubMed] [Google Scholar]
- 9.Mok VC, Lau AY, Wong A, et al. Long-term prognosis of Chinese patients with a lacunar infarct associated with small vessel disease: a five-year longitudinal study. International journal of stroke. 2009;4(2):81–88. doi: 10.1111/j.1747-4949.2009.00262.x. [DOI] [PubMed] [Google Scholar]
- 10.Imaizumi T, Inamura S, Nomura T. Contribution of Deep Microbleeds to Stroke Recurrence: Differences between Patients with Past Deep Intracerebral Hemorrhages and Lacunar Infarctions. Journal of stroke and cerebrovascular diseases. 2015;24(8):1855–64. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Wilson D, Charidimou A, Ambler G, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: A meta-analysis. Neurology. 2016;87(14):1501–1510. doi: 10.1212/WNL.0000000000003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altmann-Schneider I, Trompet S, de Craen AJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42(3):638–644. doi: 10.1161/STROKEAHA.110.595611. [DOI] [PubMed] [Google Scholar]
- 13.Akoudad S, Ikram MA, Koudstaal PJ, et al. Cerebral microbleeds and the risk of mortality in the general population. European journal of epidemiology. 2013;28(10):815–821. doi: 10.1007/s10654-013-9854-3. [DOI] [PubMed] [Google Scholar]
- 14.Song TJ, Kim J, Song D, et al. Association of cerebral microbleeds with mortality in stroke patients having atrial fibrillation. Neurology. 2014;83(15):1308–1315. doi: 10.1212/WNL.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 15.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41(6):1222–1228. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 16.Soo YO, Yang SR, Lam WW, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255(11):1679–1686. doi: 10.1007/s00415-008-0967-7. [DOI] [PubMed] [Google Scholar]
- 17.The SPS3 Investigators. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The SPS3 Group. Benavente OR, Coffey CS, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benavente OR, White CL, Pearce L, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. International journal of stroke. 2011;6(2):164–175. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White CL, Szychowski JM, Roldan A, et al. Clinical features and racial/ethnic differences among the 3020 participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. Journal of stroke and cerebrovascular diseases. 2013;22(6):764–774. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benavente OR, Pearce LA, Bazan C, et al. Clinical-MRI correlations in a multiethnic cohort with recent lacunar stroke: the SPS3 trial. International journal of stroke. 2014;9(8):1057–1064. doi: 10.1111/ijs.12282. [DOI] [PubMed] [Google Scholar]
- 22.Cordonnier C, Potter GM, Jackson CA, et al. improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS) Stroke. 2009;40(1):94–99. doi: 10.1161/STROKEAHA.108.526996. [DOI] [PubMed] [Google Scholar]
- 23.Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. International journal of stroke. 2013;8(5):348–356. doi: 10.1111/j.1747-4949.2012.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoamanesh A, Martinez-Ramirez S, Oliveira-Filho J, et al. Interrelationship of Superficial Siderosis and Microbleeds in Cerebral Amyloid Angiopathy. Neurology. 2014;83(20):1838–1843. doi: 10.1212/WNL.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart RG, Pearce LA, Bakheet MF, et al. Predictors of stroke recurrence in patients with recent lacunar stroke and response to interventions according to risk status: secondary prevention of small subcortical strokes trial. Journal of stroke and cerebrovascular diseases. 2014;23(4):618–624. doi: 10.1016/j.jstrokecerebrovasdis.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peress NS, Kane WC, Aronson SM. Central nervous system findings in a tenth decade autopsy population. Progress in brain research. 1973;40(0):473–483. doi: 10.1016/S0079-6123(08)60707-4. [DOI] [PubMed] [Google Scholar]
- 27.Rincon F, Sacco RL, Kranwinkel G, et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovascular diseases. 2009;28(1):65–71. doi: 10.1159/000219299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan U, Porteous L, Hassan A, Markus HS. Risk factor profile of cerebral small vessel disease and its subtypes. Journal of neurology, neurosurgery, and psychiatry. 2007;78(7):702–706. doi: 10.1136/jnnp.2006.103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrag M, McAuley G, Pomakian J, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta neuropathologica. 2010;119(3):291–302. doi: 10.1007/s00401-009-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumas A, Dierksen GA, Gurol ME, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Annals of neurology. 2012;72(1):76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. International journal of stroke. 2015;10(4):469–478. doi: 10.1111/ijs.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44(4):995–1001. doi: 10.1161/STROKEAHA.111.000038. [DOI] [PubMed] [Google Scholar]


