Abstract
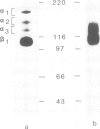
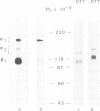
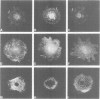
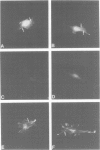
Integrins can mediate the attachment of cells to collagen type I. In the present study we have investigated the possible differences in collagen type I recognition sites for the alpha 1 beta 1 and alpha 2 beta 1 integrins. Different cyanogen bromide (CB) fragments of the alpha 1 (I) collagen chain were used in cell attachment experiments with three rat cell types, defined with regard to expression of collagen binding integrins. Primary rat hepatocytes expressed alpha 1 beta 1, primary rat cardiac fibroblasts alpha 1 beta 1 and alpha 2 beta 1, and Rat-1 cells only alpha 2 beta 1. All three cell types expressed alpha 3 beta 1 but this integrin did not bind to collagen--Sepharose or to immobilized collagen type I in a radioreceptor assay. Hepatocytes and cardiac fibroblasts attached to substrata coated with alpha 1(I)CB3 and alpha 1(I)CB8; Rat-1 cells attached to alpha 1(I)CB3 but only poorly to alpha 1(I)CB8-coated substrata. Cardiac fibroblasts and Rat-1 cells spread and formed beta 1-integrin-containing focal adhesions when grown on substrata coated with native collagen or alpha 1(I)CB3; focal adhesions were also detected in cardiac fibroblasts cultured on alpha 1(I)CB8. The rat alpha 1 specific monoclonal antibody 3A3 completely inhibited hepatocyte attachment to alpha 1(I)CB3 and alpha 1(I)CB8, as well as the attachment of cardiac fibroblasts to alpha 1(I)CB8, but only partially inhibited the attachment of cardiac fibroblasts to alpha 1(I)CB3. 3A3 IgG did not inhibit the attachment of Rat-1 cells to collagen type I or to alpha 1(I)CB3.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Yamada K. M. Analysis of the role of glycosylation of the human fibronectin receptor. J Biol Chem. 1989 Oct 25;264(30):18011–18018. [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Aumailley M., Mann K., von der Mark H., Timpl R. Cell attachment properties of collagen type VI and Arg-Gly-Asp dependent binding to its alpha 2(VI) and alpha 3(VI) chains. Exp Cell Res. 1989 Apr;181(2):463–474. doi: 10.1016/0014-4827(89)90103-1. [DOI] [PubMed] [Google Scholar]
- Aumailley M., Timpl R., Sonnenberg A. Antibody to integrin alpha 6 subunit specifically inhibits cell-binding to laminin fragment 8. Exp Cell Res. 1990 May;188(1):55–60. doi: 10.1016/0014-4827(90)90277-h. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Rapid fractionation of collagen chains and peptides by high-performance liquid chromatography. Anal Biochem. 1986 Apr;154(1):338–344. doi: 10.1016/0003-2697(86)90534-8. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg T. K., Rubin K., Lundgren E., Borg K., Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984 Jul;104(1):86–96. doi: 10.1016/0012-1606(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons C. M., Cawston T. E., Barnes M. J. The platelet reactivity of collagen type I: evidence for multiple platelet-reactive sites in the type I collagen molecule. Thromb Haemost. 1986 Aug 20;56(1):95–99. [PubMed] [Google Scholar]
- Forsberg E., Paulsson M., Timpl R., Johansson S. Characterization of a laminin receptor on rat hepatocytes. J Biol Chem. 1990 Apr 15;265(11):6376–6381. [PubMed] [Google Scholar]
- Gehlsen K. R., Dillner L., Engvall E., Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988 Sep 2;241(4870):1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- Gerton G. L., Wardrip N. J., Hedrick J. L. A gel eluter for recovery of proteins separated by polyacrylamide gel electrophoresis. Anal Biochem. 1982 Oct;126(1):116–121. doi: 10.1016/0003-2697(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J. C., Plow E. F. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988 Feb 25;59(1):1–6. [PubMed] [Google Scholar]
- Gullberg D., Terracio L., Borg T. K., Rubin K. Identification of integrin-like matrix receptors with affinity for interstitial collagens. J Biol Chem. 1989 Jul 25;264(21):12686–12694. [PubMed] [Google Scholar]
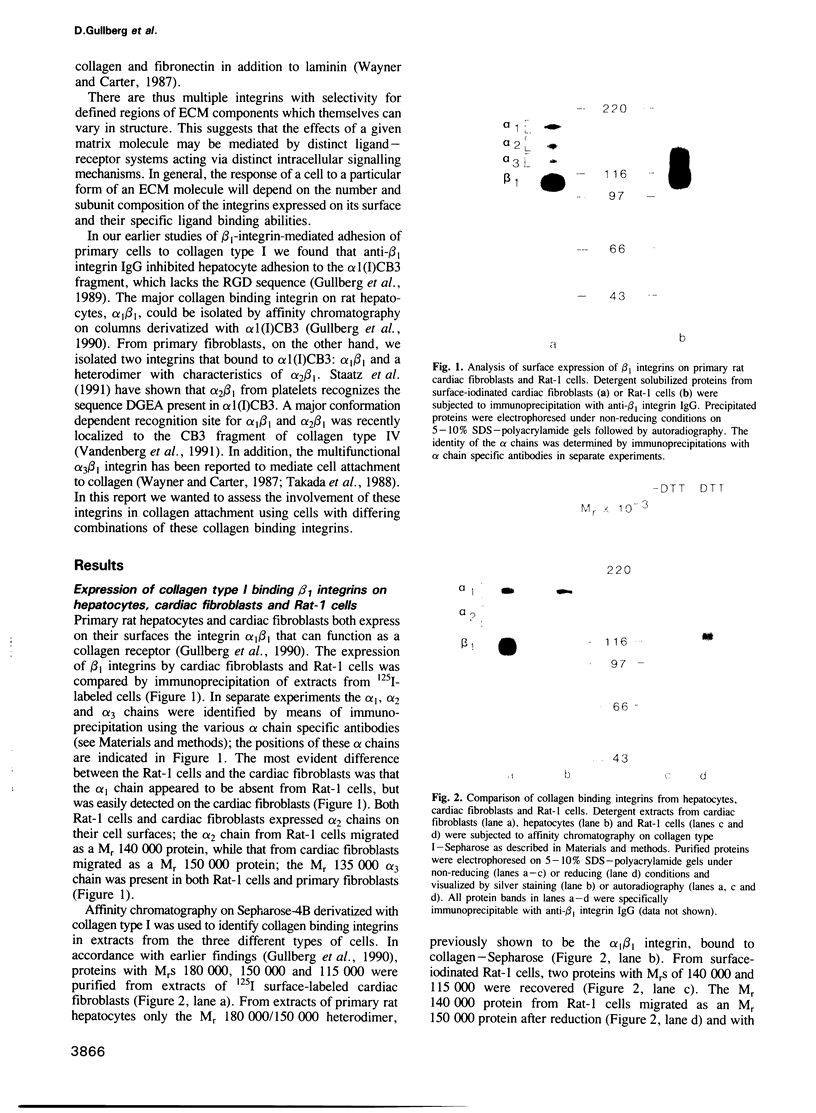
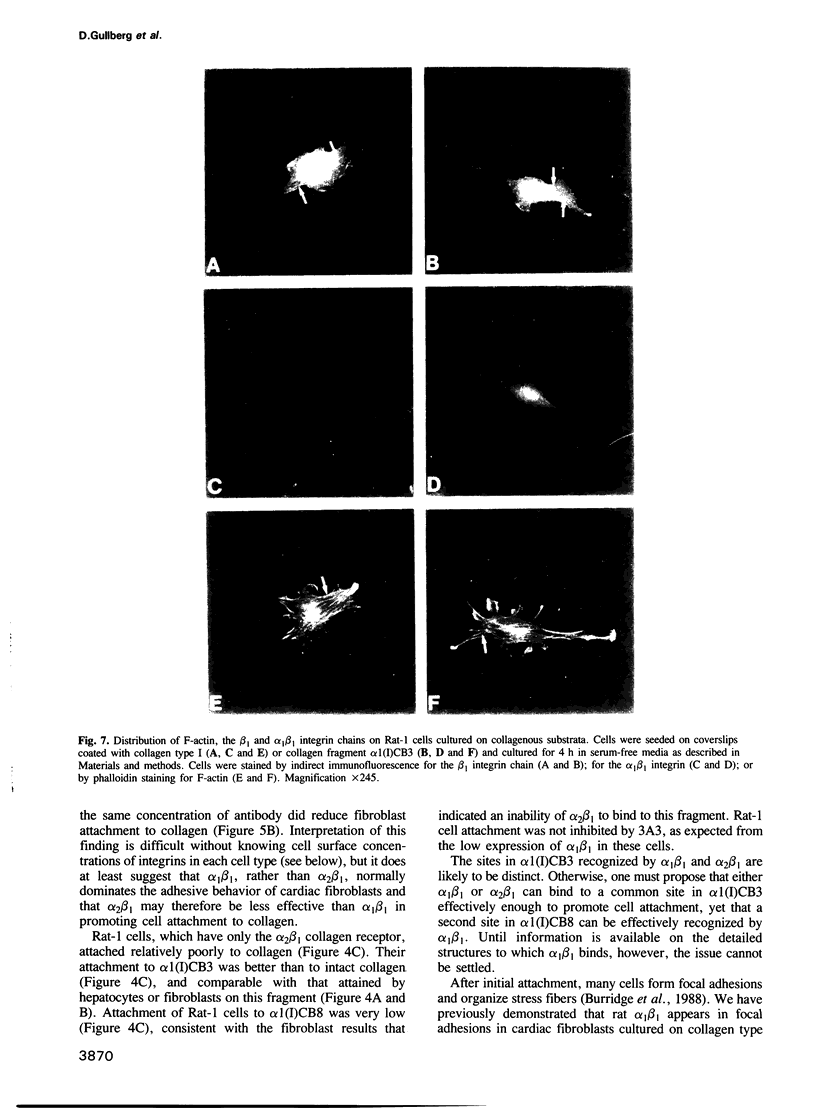
- Gullberg D., Turner D. C., Borg T. K., Terracio L., Rubin K. Different beta 1-integrin collagen receptors on rat hepatocytes and cardiac fibroblasts. Exp Cell Res. 1990 Oct;190(2):254–264. doi: 10.1016/0014-4827(90)90194-f. [DOI] [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
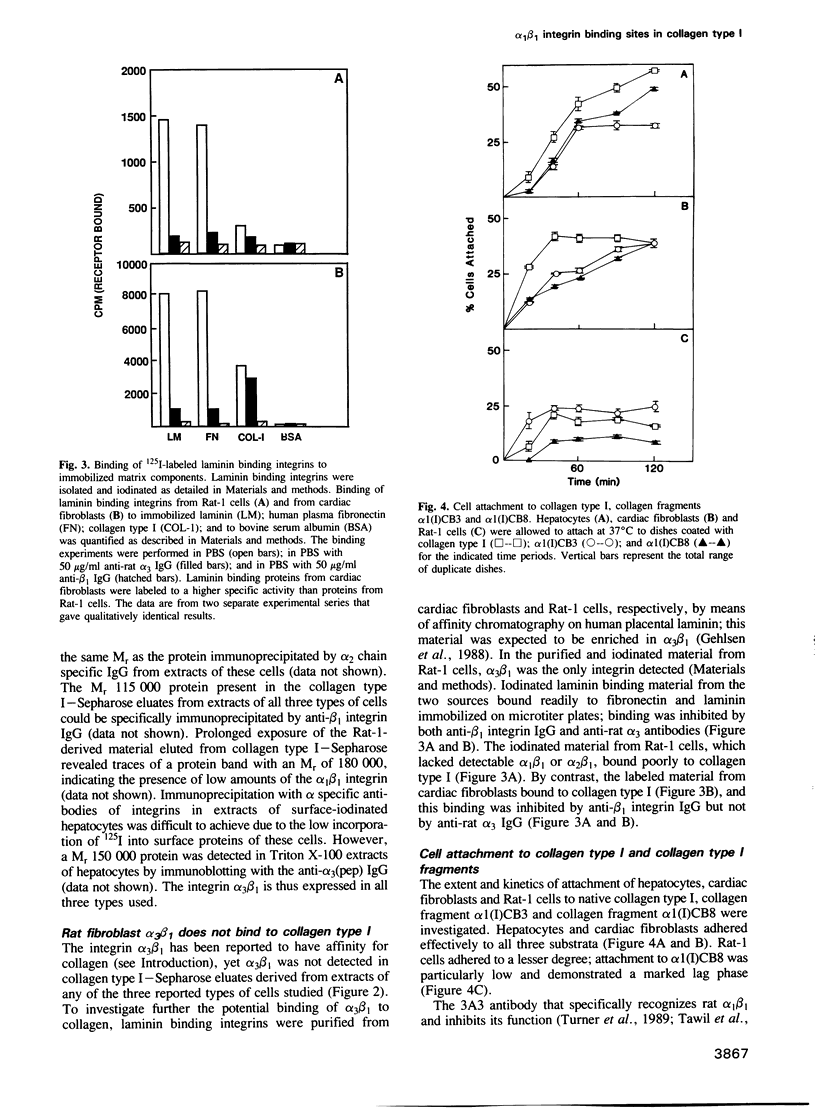
- Hynes R. O., Marcantonio E. E., Stepp M. A., Urry L. A., Yee G. H. Integrin heterodimer and receptor complexity in avian and mammalian cells. J Cell Biol. 1989 Jul;109(1):409–420. doi: 10.1083/jcb.109.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
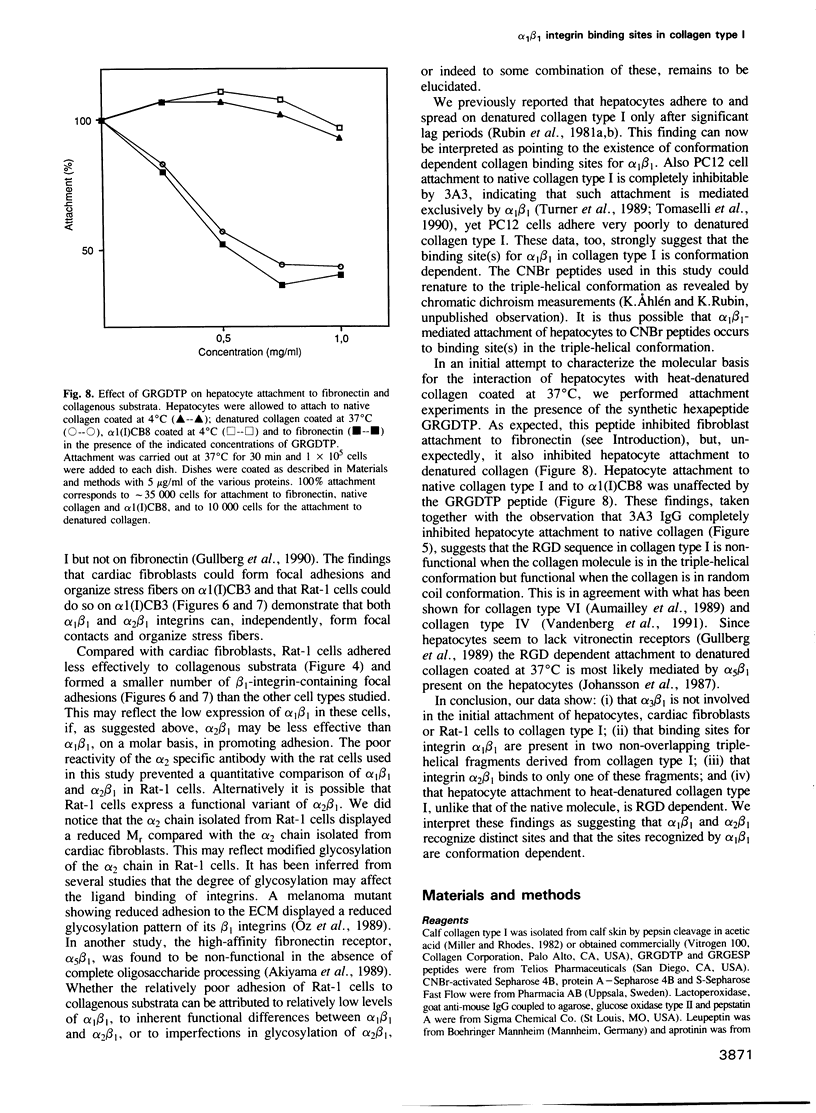
- Johansson S., Forsberg E., Lundgren B. Comparison of fibronectin receptors from rat hepatocytes and fibroblasts. J Biol Chem. 1987 Jun 5;262(16):7819–7824. [PubMed] [Google Scholar]
- Kramer R. H., Marks N. Identification of integrin collagen receptors on human melanoma cells. J Biol Chem. 1989 Mar 15;264(8):4684–4688. [PubMed] [Google Scholar]
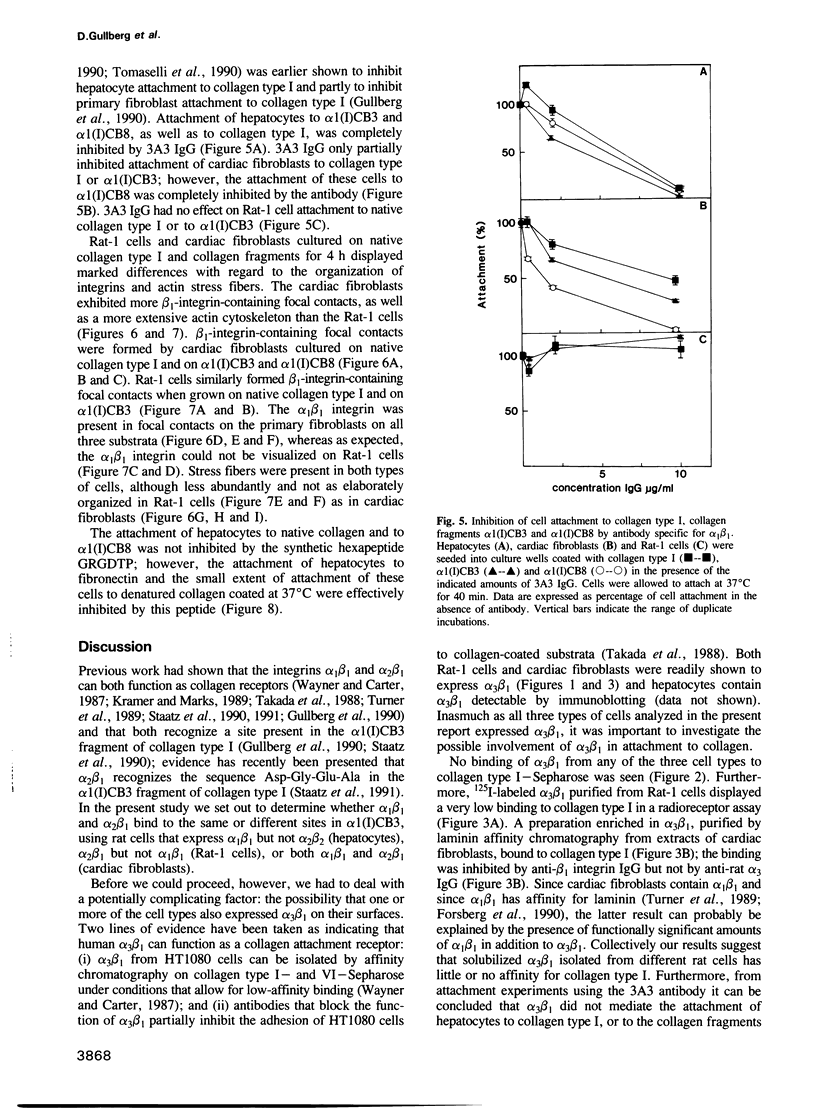
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J. W., Vestal D. J., Cheresh D. A., Bodary S. C. cDNA sequence of the human integrin beta 5 subunit. J Biol Chem. 1990 Oct 5;265(28):17126–17131. [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Obrink B. Hepatocyte--collagen adhesion. Methods Enzymol. 1982;82(Pt A):513–529. doi: 10.1016/0076-6879(82)82083-1. [DOI] [PubMed] [Google Scholar]
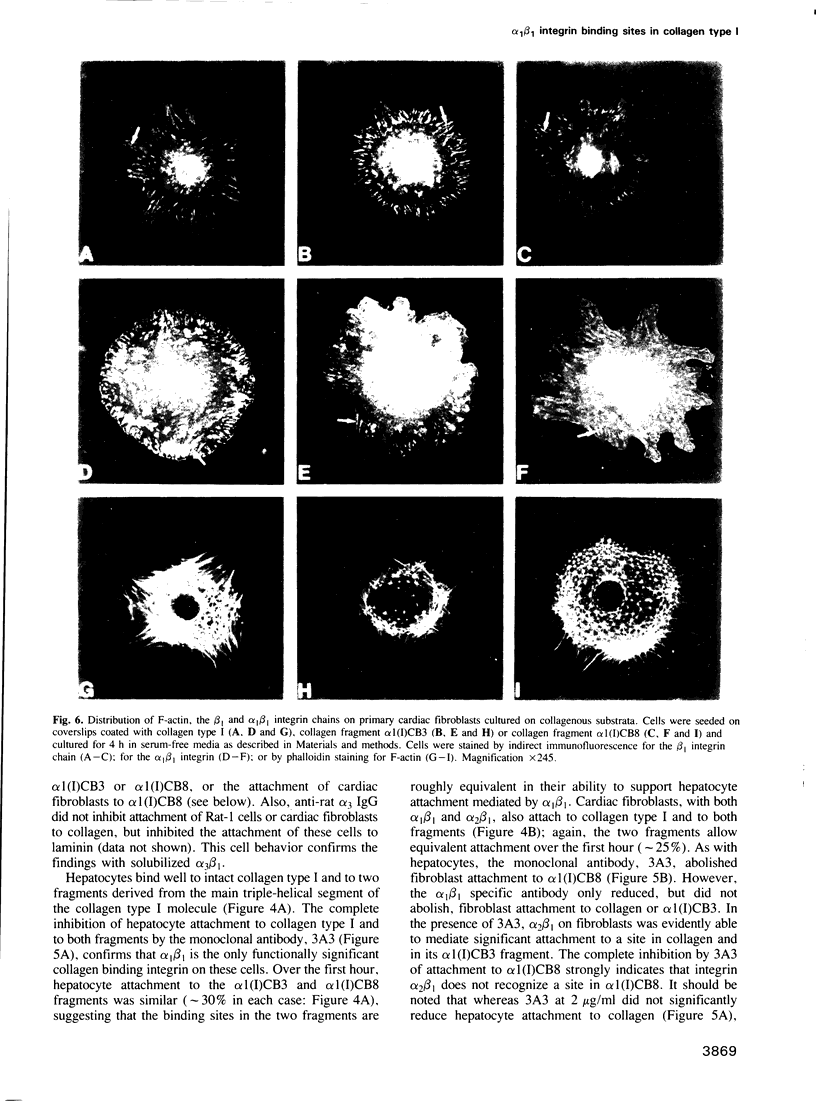
- Oldberg A., Franzén A., Heinegård D., Pierschbacher M., Ruoslahti E. Identification of a bone sialoprotein receptor in osteosarcoma cells. J Biol Chem. 1988 Dec 25;263(36):19433–19436. [PubMed] [Google Scholar]
- Oz O. K., Campbell A., Tao T. W. Reduced cell adhesion to fibronectin and laminin is associated with altered glycosylation of beta 1 integrins in a weakly metastatic glycosylation mutant. Int J Cancer. 1989 Aug 15;44(2):343–347. doi: 10.1002/ijc.2910440226. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Modderman P. W., Damsky C. H., Aumailley M., Timpl R. Integrin recognition of different cell-binding fragments of laminin (P1, E3, E8) and evidence that alpha 6 beta 1 but not alpha 6 beta 4 functions as a major receptor for fragment E8. J Cell Biol. 1990 Jun;110(6):2145–2155. doi: 10.1083/jcb.110.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staatz W. D., Fok K. F., Zutter M. M., Adams S. P., Rodriguez B. A., Santoro S. A. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991 Apr 25;266(12):7363–7367. [PubMed] [Google Scholar]
- Staatz W. D., Walsh J. J., Pexton T., Santoro S. A. The alpha 2 beta 1 integrin cell surface collagen receptor binds to the alpha 1 (I)-CB3 peptide of collagen. J Biol Chem. 1990 Mar 25;265(9):4778–4781. [PubMed] [Google Scholar]
- Takada Y., Huang C., Hemler M. E. Fibronectin receptor structures in the VLA family of heterodimers. Nature. 1987 Apr 9;326(6113):607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Tawil N. J., Houde M., Blacher R., Esch F., Reichardt L. F., Turner D. C., Carbonetto S. Alpha 1 beta 1 integrin heterodimer functions as a dual laminin/collagen receptor in neural cells. Biochemistry. 1990 Jul 10;29(27):6540–6544. doi: 10.1021/bi00479a028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli K. J., Hall D. E., Flier L. A., Gehlsen K. R., Turner D. C., Carbonetto S., Reichardt L. F. A neuronal cell line (PC12) expresses two beta 1-class integrins-alpha 1 beta 1 and alpha 3 beta 1-that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990 Nov;5(5):651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Flier L. A., Carbonetto S. Identification of a cell-surface protein involved in PC12 cell-substratum adhesion and neurite outgrowth on laminin and collagen. J Neurosci. 1989 Sep;9(9):3287–3296. doi: 10.1523/JNEUROSCI.09-09-03287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg P., Kern A., Ries A., Luckenbill-Edds L., Mann K., Kühn K. Characterization of a type IV collagen major cell binding site with affinity to the alpha 1 beta 1 and the alpha 2 beta 1 integrins. J Cell Biol. 1991 Jun;113(6):1475–1483. doi: 10.1083/jcb.113.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]