Abstract
Background
It has been postulated that short wait time before liver transplant (LT) for hepatocellular carcinoma (HCC) results in the inclusion of tumors with aggressive biology, but prolonged wait time could result in a shift to more aggressive tumor behavior. We therefore test the hypothesis that a wait time “sweet spot” exists with a lower risk for HCC recurrence compared to the other 2 extremes.
Methods
This multi-center study included 911 patients from 3 LT centers with short, medium and long wait times (median of 4, 7, and 13 months, respectively) who received MELD exception listing for HCC from 2002–2012.
Results
Wait time, defined as time from initial HCC diagnosis to LT, was <6 months in 32.4%, 6–18 months in 53.7%, and >18 months in 13.9%. Waitlist dropout was observed in 18.4% at a median of 11.3 months. Probability of HCC recurrence at 1 and 5 years were 6.4% and 15.5% with wait time <6 or >18 months (n=343) versus 4.5% and 9.8% with wait time of 6–18 months (n=397), respectively (p=0.049). When only pre-LT factors were considered, wait time <6 or >18 months (HR 1.6, p=0.043) and AFP >400 at HCC diagnosis (HR 3.0, p<0.001) predicted HCC recurrence in multivariable analysis.
Conclusion
This large multi-center study provides evidence of an association between very short (<6 months) or very long (>18 months) wait times and an increased risk for HCC recurrence post-LT. The so-called “sweet spot” of 6–18 months should be the target to minimize HCC recurrence.
INTRODUCTION
Since 2002, when the Milan criteria1 were adopted by United Network for Organ Sharing (UNOS) in granting priority listing status for liver transplant (LT) for patients with hepatocellular carcinoma (HCC) under the Model of End-stage Liver Disease (MELD) organ allocation system, the demand for liver allografts for the treatment of HCC has been steadily rising. HCC now accounts for >20% of all LT in the United States, compared to <5% before 20022. Consensus guidelines stipulate that LT for HCC should be reserved for those with a predicted 5-year survival comparable to non-HCC patients3,4. Despite adherence to the Milan criteria, post-LT survival for HCC remains slightly worse than for non-HCC indications5, and HCC recurrence still occurs in 10–20% of patients after LT6–8. In addition to tumor burden based on tumor numbers and diameter9, other well-established tumor characteristics associated with poor post-LT outcomes include presence of micro-vascular invasion6,9,10 and elevated alpha-fetoprotein (AFP)11–13. However, tumor stage (within or beyond Milan) is underestimated by preoperative imaging in 20–30%6,10, and the presence of micro-vascular invasion cannot be ascertained before LT14. Consequently, there is a need to identify other potentially modifiable pre-LT factors that impact HCC recurrence after LT15.
It has been postulated that short wait time or rapid LT results in the inclusion of tumors with aggressive biologic behavior and high risk for post-LT recurrence16,17. Several studies utilizing the UNOS database for HCC patients undergoing LT have demonstrated an association between shorter wait time and worse survival18,19 as well as a higher rate of HCC recurrence20. Prolonged wait time is associated with a dropout rate of 20% at 1 year from LT listing21, and may shift tumor biology to become more aggressive over time, leading to increased HCC recurrence after LT. The effects of wait time on post-LT outcomes are especially important in the context of projected increased wait times in the United States for HCC patients due to 2 recent UNOS policy changes: a mandatory 6 month wait time before granting MELD exception and an HCC MELD exception cap at 34 points22,23.
Given the concerns related to very short and long wait times, we hypothesize that a wait time “sweet spot” exists which begins after a minimum period of observation to exclude those with very aggressive tumor biology from LT. To test this hypothesis within the framework of existing regional inequalities, we evaluated the impact of wait time on post-LT HCC recurrence in patients with HCC receiving MELD exception at 3 LT centers with short, medium, and long wait times.
MATERIALS AND METHODS
Study Design
This is a multi-center cohort study of patients aged ≥18 years with HCC within Milan criteria based on imaging listed for LT with MELD exception from 2002–2012. The 3 centers chosen represented centers with long (UCSF-center 1), medium (Mayo Clinic Rochester–center 2), and short (Mayo Clinic Jacksonville–center 3) wait times, defined in this study as time from HCC diagnosis to LT. HCC diagnosis was determined to be a more appropriate start date since date of listing with MELD exception depends on when a patient with known HCC is referred to a LT center and other factors, and the patient may have already had local-regional treatments (LRT). Among the 1151 patients listed with MELD exception at the 3 centers, 240 patients were excluded - 214 with HCC beyond Milan criteria at diagnosis requiring down-staging, 8 lost to follow-up within 1 month of listing, and 18 with cholangiocarcinoma found on explant.
Decisions regarding management of patients with HCC awaiting LT were made at each center’s multi-disciplinary Tumor Board and all patients underwent contrast-enhanced computed tomography or magnetic resonance at a minimum of every 3 months. The general practice at all 3 centers was to use trans-arterial chemoembolization as first-line LRT with intent for continued treatment to achieve complete tumor necrosis prior to LT. Explant pathology was reviewed to determine histologic grade based on the modified Edmondson criteria24, tumor stage based on viable tumors, and presence of vascular invasion.
Outcomes
The primary outcome studied was post-LT HCC recurrence, which was obtained from each individual center’s LT database. Additional outcomes studied included post-LT survival and dropout from the transplant waiting list for any of the following reasons: death without LT, HCC tumor progression beyond Milan criteria, being too sick or medically unsuitable to undergo LT, noncompliance, patient decision not to undergo LT, or being lost to follow-up.
Statistical Analysis
Patient characteristics were summarized using medians and inter-quartile ranges (IQR) for continuous variables and proportions for categorical variables. The cumulative incidence of dropout was estimated using competing risks (CR)25. Post-LT survival and HCC recurrence were evaluated using the Kaplan-Meier method, both overall and stratified by multiple time cutoffs from HCC diagnosis to LT. Univariate and multivariable hazard ratios (HR) for predictors of post-LT HCC recurrence were determined by Cox proportional hazards regression. This was performed both for all variables as well as separately for only variables known prior to transplant (eg, exclusion of explant data and AFP at transplant). Multiple cutoffs for wait time were evaluated using Akaike information criterion (AIC) with lower AIC values indicating better model fit. Predictors of HCC recurrence with a univariate p value <0.1 were evaluated in the multivariate analysis with the final model selected by backward elimination (p for removal >0.05). Center was added to the final multivariate model to account for center variability. LOESS curve fitting (local polynomial regression) was used to evaluate the optimal wait time. Statistical analyses were performed using SAS v9.4 and Stata/IC 11.1.
RESULTS
Patient Characteristics
Baseline demographic and clinical characteristics of the 911 patients comprising the study population are summarized in Table 1. The median age was 58 (IQR 53–63) and 74.2% were men. Caucasian (59.2%), Asian (19.7%), and Hispanic (12.6%) race/ethnicity made up most of the study population with a higher percentage of noncaucasians in center 1 than the other 2 centers (p<0.001). Liver disease due to nonviral hepatitis was more common at center 2 (39.9%) and 3 (36.5%) than center 1 (13.9%) (p<0.001). At the time of HCC diagnosis, median MELD score was 11 (IQR 8–14) and median AFP was 13.0 ng/mL (IQR 5.3–69.0). More than half (52.1%) received a single LRT, 18.4% had ≥3 treatments, and 6.5% received no LRT. Only 9.5% underwent >1 LRT at center 3 (shortest wait time) compared to 47.6% at center 1 (longest wait time) and 65.0% at center 2 (p<0.001).
Table 1.
Baseline Characteristics of the Study Population at HCC Diagnosis (n=911)
| Overall (n=911) | Center 1 (n=495) | Center 2 (n=183) | Center 3 (n=233) | P value | |
|---|---|---|---|---|---|
| Age (IQR) | 58 (53–63) | 58 (53–62) | 57 (53–63) | 60 (54–67) | <0.001 |
| Male Gender (%) | 676 (74.2) | 377 (76.2) | 128 (70.0) | 171 (73.4) | 0.24 |
| Race/Ethnicity (%) | <0.001 | ||||
| Caucasian | 539 (59.2) | 208 (42.0) | 149 (81.4) | 182 (78.1) | |
| Asian | 179 (19.7) | 154 (31.1) | 17 (9.3) | 8 (3.4) | |
| Hispanic | 115 (12.6) | 88 (17.8) | 4 (2.2) | 23 (9.9) | |
| African American | 53 (5.8) | 30 (6.1) | 10 (5.5) | 13 (5.6) | |
| Other | 25 (2.7) | 15 (3.0) | 3 (1.6) | 7 (3.0) | |
| Etiology of Liver Disease (%) | <0.001 | ||||
| HCV | 540 (59.3) | 308 (62.2) | 93 (50.8) | 139 (59.7) | |
| HBV | 144 (15.8) | 118 (23.8) | 17 (9.3) | 9 (3.9) | |
| NAFLD | 88 (9.7) | 32 (6.5) | 19 (10.4) | 37 (15.9) | |
| Alcohol | 80 (8.8) | 24 (4.8) | 31 (16.9) | 25 (10.7) | |
| Other | 59 (6.5) | 13 (2.6) | 23 (12.6) | 23 (9.9) | |
| MELD Score (IQR) | 11 (8–14) | 11 (8–14) | 11 (8–13) | 12 (9–15) | 0.001 |
| AFP ng/ml (IQR) (n=902) | 13.0 (5.3–69.0) | 15.0 (6.0–85.5) | 12.0 (5.0–76.0) | 10.0 (5.2–45.4) | 0.06 |
| AFP <20 (%) | 514 (57.0) | 266 (54.1) | 105 (59.3) | 143 (61.4) | |
| AFP 20–100 | 203 (22.5) | 117 (23.8) | 30 (16.9) | 56 (24.0) | |
| AFP 101–400 | 105 (11.6) | 58 (11.8) | 26 (14.7) | 21 (9.0) | |
| AFP 401–1000 | 38 (4.2) | 18 (3.6) | 10 (5.6) | 10 (4.3) | |
| AFP>1000 | 42 (4.7) | 33 (6.7) | 6 (3.4) | 3 (1.3) | |
| # HCC Lesions (%) | 0.24 | ||||
| 1 | 648 (71.1) | 361 (72.9) | 130 (71.0) | 157 (67.4) | |
| 2 | 186 (20.4) | 101 (20.4) | 36 (19.7) | 49 (21.0) | |
| 3 | 77 (8.4) | 33 (6.7) | 17 (9.3) | 27 (11.6) | |
| # LRTs (%) | <0.001 | ||||
| 0 | 59 (6.5) | 27 (5.4) | 6 (3.3) | 26 (11.1) | |
| 1 | 475 (52.1) | 232 (46.9) | 58 (31.7) | 185 (79.4) | |
| 2 | 209 (22.9) | 124 (25.0) | 69 (37.7) | 16 (6.9) | |
| ≥3 | 168 (18.4) | 112 (22.6) | 50 (27.3) | 6 (2.6) |
Dropout while on the Waiting List
Ninety-six patients (10.5%) experienced dropout due to tumor progression and an additional 38 patients (4.2%) died while on the waiting list. Median time from HCC diagnosis to dropout due to tumor progression or death was 11.3 months (IQR 6.8–16.5). Median time to dropout was 11.6 months at center 1 versus 10.7 and 6.6 months at centers 2 and 3, respectively (p=0.004). Cumulative probabilities of dropout due to tumor progression or death by CR analysis overall were 3.2% within 6 months, 8.7% within 12 months, and 12.4% within 18 months of HCC diagnosis. When dropout was stratified by center, CR probability of dropout within 18 months was 19.5% at center 1, 4.5% at center 2, and 3.5% at center 3 (p value for center 2 or 3 vs 1 <0.001) (Table 2). Overall, 85.1% of those with waitlist dropout were from center 1. Thirty-four patients were censored at the time of waitlist removal for reasons including significant cardiopulmonary disease, noncompliance, or patient decision not to undergo LT.
Table 2.
Outcomes on the Liver Transplant Waitlist (n=911)
| Overall (n=911) | Center 1 (n=495) | Center 2 (n=183) | Center 3 (n=233) | p value | |
|---|---|---|---|---|---|
| Dropout (%) | 168 (18.4) | 143 (28.9) | 12 (6.6) | 13 (5.6) | <0.001 |
| Tumor Progression | 96 (10.5) | 83 (16.8) | 6 (3.3) | 7 (3.0) | |
| Liver Death | 38 (4.2) | 35 (7.1) | 2 (1.1) | 1 (0.4) | |
| Censored | 34 (3.7) | 25 (5.0) | 4 (2.2) | 5 (2.1) | |
| Median Time (mo) to Dropout (IQR) | 11.3 (6.8–16.5) | 11.6 (7.4–17.1) | 10.7 (6.4–16.4) | 6.6 (5.3–9.0) | 0.004 |
| Probability of Dropout (Tumor Progression or Liver Death) by Competing Risks (95% CI) | <0.001 | ||||
| Within 6 months | 3.2 (2.2–4.5) | 4.4 (2.9–6.5) | 1.6 (0.4–4.4) | 1.7 (0.6–4.1) | |
| Within 12 months | 8.7 (6.9–10.6) | 13.6 (10.8–16.8) | 1.6 (0.4–4.4) | 3.5 (1.6–6.5) | |
| Within 18 months | 12.4 (10.4–14.7) | 19.5 (16.1–23.1) | 4.5 (2.1–8.3) | 3.5 (1.6–6.5) | |
| Active on waitlist (%) | 3 (0.3) | 3 (0.6) | 0 | 0 | |
| Liver Transplant (%) | 740 (81.2) | 349 (70.5) | 171 (93.4) | 220 (94.4) | <0.001 |
| Median Time (mo) to Transplant (IQR) | 8.4 (4.6–14.1) | 12.9 (8.6–19.0) | 7.3 (4.2–11.1) | 4.5 (2.3–7.3) | <0.001 |
| Wait time to LT (%) | <0.001 | ||||
| <6 months | 240 (32.4) | 36 (10.3) | 69 (40.3) | 135 (61.4) | |
| 6–18 months | 397 (53.7) | 217 (62.2) | 99 (57.9) | 81 (36.8) | |
| >18 months | 103 (13.9) | 96 (27.5) | 3 (1.8) | 4 (1.8) |
Liver Transplantation and Wait Time
Of the 911 patients in the cohort, 740 (81.2%) underwent LT after a median of 8.4 months (IQR 4.6–14.1) from HCC diagnosis. Median wait time from HCC diagnosis to LT was 12.9 months at center 1, 7.3 months at center 2, and 4.5 months at center 3 (p<0.001). The median HCC MELD-exception was 31 at center 1, 28 at center 2, and 25 at center 3. Only 70.5% of patients underwent LT at center 1 compared to 93.4% at center 2 and 94.4% at center 3 (p<0.001). Only 6 of the 740 (0.8%) patients received live donor LT, all at center 1. The median time from HCC diagnosis to LT was <3 months in 13.2%, 3–6 months in 19.2%, 6–12 months in 35.3%, 12–18 months in 18.4%, and >18 months in 13.9%. When stratified by center, 61.4% of patients in center 3 had a wait time of <6 months versus 10.3% in center 1, whereas 27.5% of patients in center 1 had a wait time of >18 months compared to 1.8% at both centers 2 and 3 (p<0.001) (Table 2).
For the 740 LT recipients, the median donor age was 45 years (IQR 31–56). Cold ischemia time was 7.0 hours (5.9–8.6) and warm ischemia time was 35 minutes (29–42).
Explant Tumor Characteristics
Explant tumor characteristics are summarized in Table 3. Median AFP at LT was 8.1 ng/ml (IQR 4.0–28.7) and 12.3% had an AFP >100. In the explant, complete necrosis with no residual viable tumor because of LRT was seen in 24.2%. Viable tumors were within Milan criteria in 56.0% and under-staged to beyond Milan criteria in 19.8%. No viable tumor in explant was more common at center 1 (39%) than center 2 (21.6%) and 3 (2.7%) (p<0.001). The overall incidence of micro-vascular invasion was 10.8% but was significantly higher in center 3 (16.4%) (p=0.005). Among patients with viable tumors in the explant, most had either well differentiated (40.3%) or moderately differentiated HCC (45.5%). Of the 14.1% with poorly differentiated tumor grade, 50.6% were from center 2 (p<0.001).
Table 3.
Explant Characteristics and AFP at the Time of Liver Transplant (n=740)
| Overall (n=740) | Center 1 (n=349) | Center 2 (n=171) | Center 3 (n=220) | p value | |
|---|---|---|---|---|---|
| AFP at LT ng/ml (IQR)* | 8.1 (4.0–28.7) | 8.3 (3.8–35.0) | 7.7 (4.1–23.0) | 8.4 (4.3–28.3) | 0.49 |
| AFP <20 (%) | 488 (68.9) | 219 (68.2) | 122 (73.0) | 147 (66.8) | |
| AFP 20–100 | 132 (18.6) | 60 (18.7) | 27 (16.2) | 45 (20.4) | |
| AFP 101–400 | 57 (8.0) | 27 (8.4) | 14 (8.4) | 16 (7.3) | |
| AFP 401–1000 | 16 (2.2) | 9 (2.8) | 2 (1.2) | 5 (2.3) | |
| AFP>1000 | 15 (2.1) | 6 (1.9) | 2 (1.2) | 7 (3.2) | |
| Pathologic Stage (%)** | <0.001 | ||||
| No residual tumor | 178 (24.2) | 136 (39.0) | 36 (21.6) | 6 (2.7) | |
| Within Milan | 412 (56.0) | 156 (44.7) | 101 (60.5) | 155 (70.5) | |
| Beyond Milan | 146 (19.8) | 57 (16.3) | 30 (18.0) | 59 (26.8) | |
| Microvascular Invasion (%) | 80 (10.8) | 27 (7.7) | 17 (9.9) | 36 (16.4) | 0.005 |
| Histologic Grade (%) (of those with residual viable tumor)*** | <0.001 | ||||
| Well Differentiated | 225 (40.3) | 82 (38.7) | 20 (14.9) | 123 (58.0) | |
| Moderately Differentiated | 254 (45.5) | 107 (50.5) | 74 (55.2) | 73 (34.4) | |
| Poorly Differentiated | 79 (14.1) | 23 (10.8) | 40 (29.8) | 16 (7.5) |
n=708 (missing in 32 patients)
n=736 (missing in 4 patients)
n=558
Intention-to-treat and Posttransplant Survival
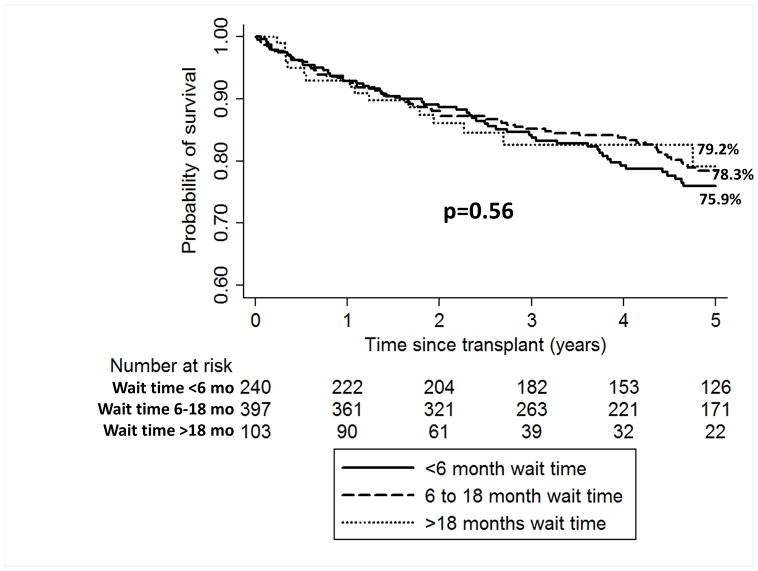
Intention-to-treat survival for the entire cohort was 92.7% (95% CI 90.8–94.3) at 1 year and 69.2% (65.9–72.2) at 5 years from HCC diagnosis. Median post-LT follow-up time was 4.4 years (IQR 2.3–6.7) and overall post-LT survival was 92.8% (95% CI 90.6–94.4) at 1 year and 77.4% (73.8–80.5) at 5 years. There were no significant differences seen when post-LT survival was stratified by wait time from HCC diagnosis to LT (Figure 1). Specifically, 1 and 5-year post-LT survival was 92.9% and 75.9% for wait time <6 months, 92.6% and 78.3% for wait time 6–18 months, and 93.0% and 79.2% for wait time >18 months (p=0.56).
Figure 1.
Kaplan-Meier post-LT survival estimates for LT recipients with wait time from HCC diagnosis to LT of <6 months, 6–18 months, and >18 months
Post-LT HCC Recurrence
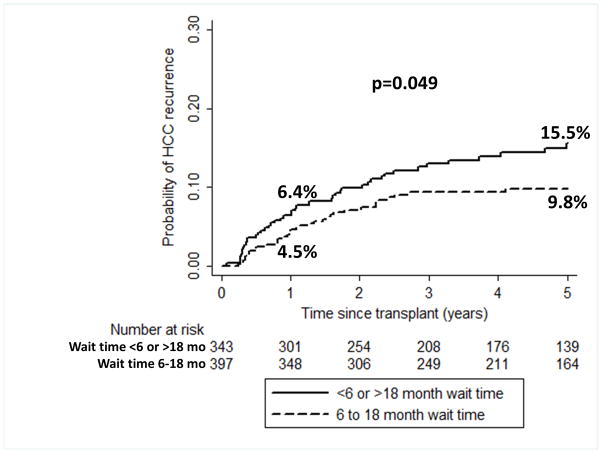
HCC recurrence occurred in 11.2% at a median of 13.0 months (IQR 6.0–26.7) from LT. Time to recurrence at center 1 was 17.9 months (8.5–26.9) as compared with 11.7 months (4.9–35.6) at center 2 and 10.4 months (4.4–24.2) at center 3 (p=0.44). The most common sites of initial recurrence were lung (44%), bone (30%), liver (26%), and peritoneum (26%). Overall post-LT recurrence within 1 and 5 years was 5.4% and 12.5%, respectively and 72.3% of all recurrences occurred within 2 years of LT. There were no significant differences in rates of recurrence seen when stratified by center. Specifically, recurrence rates within 5 years of LT were 13.2% at center 1, 9.5% at center 2, and 14.3% at center 3 (p=0.45). When stratified by wait time to LT, however, there was a trend towards increased probability of recurrence within 5 years with either wait time <6 months (14.5%) or >18 months (19.0%) as compared to a wait time of 6–18 months (9.8%) (p=0.09). When the short (<6 month) and long (>18 month) wait time, groups were combined (n=343), the 1 and 5 year cumulative probabilities of HCC recurrence were 6.4% and 15.5%, which was significantly higher than with wait time 6–18 months of 4.5% and 9.8%, respectively, (p=0.049) (Figure 2). LT recipients with wait time of <6 or >18 months comprised 46.3% of the overall cohort but accounted for 56.6% of the HCC recurrences.
Figure 2.
Cumulative probability of HCC recurrence for LT recipients with wait time from HCC diagnosis to LT of <6 or >18 months compared to those with wait time “sweet spot” of 6–18 months
Predictors of Post-LT HCC Recurrence
The results of univariate analysis of wait time as a predictor of HCC recurrence are summarized in Table 4. There was an insignificant trend towards increased recurrence seen with wait time <6 months (HR 1.44, 95% CI 0.90–2.31, p=0.13) and >18 months (HR 1.84, 95% CI 0.99–3.41, p=0.055). When these 2 wait time groups were combined, wait time of <6 or >18 months nearly reached statistical significance as a predictor of HCC recurrence (HR 1.54, 95% CI 0.995–2.37, p=0.052). This combined wait time of <6 or >18 months had the lowest AIC among the cutoffs tested (Table 4). There was no association seen with shorter wait times of <3, <4, or <5 months and increased recurrence risk.
Table 4.
Univariate Analysis of Wait Time from HCC Diagnosis to LT as Predictor of Post-LT HCC Recurrence by Cox Proportional Hazards Regression
| Wait Time from HCC Diagnosis to LT (months) | Univariate HR (95% CI) | p value | AIC |
|---|---|---|---|
|
| |||
| Wait >3 vs <3 | 0.89 (0.47–1.69) | 0.73 | 1047 |
|
| |||
| Wait >4 vs <4 | 1.13 (0.68–1.87) | 0.63 | 1044 |
|
| |||
| Wait >5 vs <5 | 1.23 (0.78–1.94) | 0.38 | 1046 |
|
| |||
| Wait >6 vs <6 | 1.26 (0.81–1.95) | 0.31 | 1046 |
|
| |||
| Wait <6 (vs 6–12) | 1.45 (0.86–2.45) | 0.17 | 1047 |
| Wait >12 (vs 6–12) | 1.34 (0.77–2.34) | 0.30 | 1047 |
| Wait <6 or >12 (vs 6–12) | 1.40 (0.87–2.25) | 0.17 | 1045 |
|
| |||
| Wait <6 (vs 6–18) | 1.44 (0.90–2.31) | 0.13 | 1045 |
| Wait >18 (vs 6–18) | 1.84 (0.99–3.41) | 0.055 | 1045 |
| Wait <6 or >18 (vs 6–18) | 1.54 (0.995–2.37) | 0.052 | 1043 |
The results of additional univariate and multivariable analysis of predictors of post-LT HCC recurrence are summarized in Table 5. Predictors of HCC recurrence in univariate analysis included microvascular invasion, explant tumor >Milan, and moderately and poorly differentiated tumor grade. Additionally, AFP at HCC diagnosis and at LT as a continuous variable and at all tested cutoffs including >100, >400, and >1000 was a significant predictor of HCC recurrence. Age, gender, MELD score, race/ethnicity, etiology of liver disease, number of lesions at HCC diagnosis, number of LRTs received, donor age, cold and warm ischemia time were not predictive of recurrence on univariate analysis nor was there any center effect seen.
Table 5.
Univariate and Multivariable Analysis of Predictors of Post-LT HCC Recurrence by Cox Proportional Hazards Regression
| Predictor | Univariate HR (95% CI) | p value | Multivariable HR (95% CI) | p value |
|---|---|---|---|---|
| Wait Time from HCC Diagnosis to LT <6 or >18 mos (vs 6–18 mos) | 1.54 (0.995–2.37) | 0.052 | 1.24 (0.79–1.95) | 0.35 |
| Patient + Tumor Characteristics at HCC Dx | ||||
| Age (per year) | 1.004 (0.98–1.03) | 0.80 | ||
| Female gender | 1.30 (0.84–2.01) | 0.23 | ||
| HBV (vs HCV) | 1.07 (0.56–2.03) | 0.85 | 1.36 (0.70–2.65) | 0.36 |
| Nonviral etiology (vs HCV) | 1.67 (1.04–2.68) | 0.03 | 1.48 (0.90–2.42) | 0.12 |
| MELD (per point) | 0.99 (0.94–1.04) | 0.72 | ||
| AFP>100 vs ≤100 | 1.46 (0.88–2.42) | 0.14 | ||
| AFP>400 vs ≤400 | 2.81 (1.58–4.99) | 0.0004 | ||
| AFP>1000 vs ≤1000 | 2.30 (1.002–5.28) | 0.049 | ||
| 2 lesions (vs 1) | 0.74 (0.40–1.35) | 0.32 | 0.57 (0.30–1.06) | 0.07 |
| 3 lesions (vs 1) | 1.82 (0.96–3.47) | 0.07 | 1.45 (0.74–2.86) | 0.28 |
| Explant Characteristics and AFP at LT | ||||
| AFP>100 vs ≤100 | 4.13 (2.59–6.58) | <0.001 | 2.79 (1.72–4.55) | <0.001 |
| AFP>400 vs ≤400 | 5.63 (3.26–9.74) | <0.001 | ||
| AFP>1000 vs ≤1000 | 11.43 (5.88–22.23) | <0.001 | ||
| Microvascular Invasion | 7.31 (4.67–11.43) | <0.001 | 3.58 (2.06–6.21) | <0.001 |
| Within Milan Criteria* | 1.91 (0.92–3.95) | 0.08 | ||
| Beyond Milan Criteria* | 6.32 (3.04–13.12) | <0.001 | 2.49 (1.10–5.63) | 0.03 |
| Moderate Differentiation** | 3.31 (1.64–6.68) | <0.001 | ||
| Poor Differentiation** | 5.93 (2.74–12.86) | <0.001 | ||
| Donor Factors | ||||
| Donor Age (per year) | 1.01 (0.99–1.02) | 0.29 | ||
| Cold Ischemia Time (per hour) | 0.96 (0.89–1.05) | 0.39 | ||
| Warm Ischemia Time (per min) | 0.99 (0.91–1.01) | 0.46 |
vs no residual viable tumor;
vs complete tumor necrosis
Predictors of HCC recurrence in multivariable analysis included microvascular invasion (HR 3.58, 95% CI 2.06–6.21, p<0.001), explant tumor >Milan criteria (HR 2.49, 95% CI 1.10–5.63, p=0.03), and AFP >100 at transplant (HR 2.79, 95% CI 1.72–4.55, p<0.001). No wait time categories predicted HCC recurrence in this multivariable analysis. However, when only factors known prior to LT were included (Table 6), wait time <6 or >18 months (HR 1.60, 95% CI 1.01–2.51, p=0.043) as well as AFP >400 at HCC diagnosis (HR 3.04, 95% CI 1.68–5.50, p<0.001) were the only factors predicting HCC recurrence after adjusting for center, tumor number, and etiology of liver disease. Additionally, while wait time <6 or >18 months was not associated with the presence of microvascular invasion or explant stage beyond Milan criteria on logistic regression, it was associated with AFP >100 at LT (HR 1.63, 95% CI 1.04–2.56, p=0.03) as compared to those with wait time 6–18 months.
Table 6.
Multivariable Analysis of Predictors of Post-LT HCC Recurrence by Cox Proportional Hazards Regression with Only Factors Known Prior to LT*
| Predictor | Multivariable HR (95% CI) | p value |
|---|---|---|
| Wait Time to LT <6 or >18 mo | 1.60 (1.01–2.51) | 0.04 |
| AFP at HCC diagnosis >400 vs ≤400 | 3.04 (1.68–5.50) | <0.001 |
Model adjusted for center, etiology of liver disease, and number of lesions
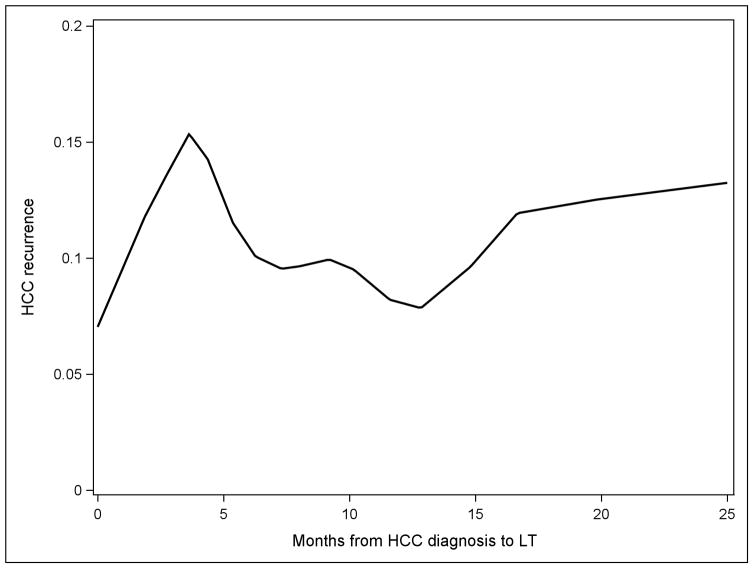
LOESS curve fitting (Figure 3) showed recurrence risks increasing for the first 4–5 months after HCC diagnosis, then decreasing from 6–12 months with the lowest risk around 13 months. The risk then increased slightly to about 18 months, suggesting that recurrence risk may be lowest in the 6–18-month period.
Figure 3.
LOESS curve fitting (local polynomial regression) showing the relationship between wait time from HCC diagnosis to LT and HCC recurrence risk
DISCUSSION
In 2010, the proportion of patients with HCC undergoing LT within 3 months of listing with MELD exception ranged from <20% in regions with long wait times to >90% in regions with short wait times26. There are perceived risks of transplanting patients with HCC too quickly without a minimal period of observation for tumor progression, as illustrated in the “fast-tracking” and “ablate and wait” concepts16,17, leading to the inclusion of aggressive tumors for LT with a high risk for post-LT recurrence. Several studies using the UNOS database have demonstrated that short wait time is associated with inferior post-LT outcomes. Halazun et al,18 showed that 5-year post-LT survival was significantly worse for patients in short wait time regions (66%) compared to those transplanted in long wait time regions (70%). Similarly, Schlansky et al,19 demonstrated that wait time from listing to LT of <3 months was associated with worse overall post-LT survival. It may be argued that there are factors beyond HCC recurrence accounting for the worse post-LT survival seen in those with short wait times, but a third study by Samoylova et al,20 found that a wait time of >4 months was associated with a 40% decrease in HCC recurrence. Nevertheless, the difference in HCC recurrence rates between patients waiting >4 months versus those waiting ≤4 months did not persist at 2 years after LT. These studies are limited by a lack of explant histopathologic data, a likely underestimation of HCC recurrence rate in the only study that assessed HCC recurrence20, and using the date of HCC listing rather than HCC diagnosis as the starting point in estimating wait time for LT.
In this large multi-center study, we aimed to assess the impact of wait time from HCC diagnosis to LT on HCC recurrence in centers with short, medium, and long wait times. Not surprisingly, we found micro-vascular invasion, elevated AFP and explant stage beyond Milan criteria to be significant predictors of HCC recurrence on multivariable analysis. However, micro-vascular invasion and explant tumor stage are unknown prior to LT. When we focused only on factors known prior to the time of LT, wait time of <6 or >18 months and AFP at HCC diagnosis were the only significant predictors of HCC recurrence on multivariable analysis. Patients with wait time <6 or >18 months, which comprised nearly half of the cohort, had a nearly 60% increased risk of HCC recurrence within 5 years of LT compared to those with wait time 6–18 months (16% vs 10%). The importance of performing LT within this “sweet spot” was further strengthened by the finding that short or long wait time was significantly associated with increased AFP at LT. Each center had a sizable percentage of patients within the “sweet spot” (62% of center 1, 58% of center 2, and 37% of center 3), and we observed no significant center effects in the analysis of HCC recurrence. Further, LOESS curve fitting suggested that performing LT in this “sweet spot” may be associated with the lowest recurrence risk, although this needs confirmation.
The observation of inferior posttransplant outcomes when the wait time is prolonged (> 18 months) has not been previously reported. The natural course of HCC following LRT provides some support of the hypothesis of a shift in tumor biologic behavior over time. LRT is recommended as a bridge to LT for HCC when the expected wait time is at least 6 months3 and TACE is the most commonly used treatment modality. Over 93% in the present study received at least 1 LRT before LT. In a study by Terzi et al,29 involving 148 patients with a single nodule treated only with TACE (92% within Milan criteria), relapse following initial complete response occurred in almost 2/3 of patients after a median of 9 months, and nearly 2/3 had distant intrahepatic relapse rather than just local recurrences at the prior treatment site. Distant intrahepatic tumor recurrence following initial LRT may represent 1 of many facets of a change in tumor aggressiveness.
Our findings may have important implications under the current climate of organ allocation for HCC. Given the growing body of evidence that patients with HCC are given an unfair advantage in organ allocation over non-HCC patients listed for LT26–28, UNOS implemented policy changes in October 2015 in the attempt to improve disparity in access to LT. They include a mandatory wait time of 6 months from listing before granting MELD exception and a cap of HCC MELD exception at 34 points22,23. Our results suggest that this mandatory delay in time to LT, if staying within a window of 6 to 18 months from the time of HCC diagnosis, should not compromise post-LT outcome and may even further improve LT benefits for HCC. On the other hand, in regions where the wait time is already prolonged for 12 to 18 months for HCC patients receiving MELD-exception21, time to LT may be further delayed under the cap 34 policy, potentially leading to worse outcomes after LT. Nevertheless, our results should not imply exclusion from LT those who achieved good disease control but waited longer than 18 months.
One of the main goals of these UNOS policy changes is to narrow the gap in the waitlist removal rates between HCC and non-HCC patients. For patients with HCC, our study clearly demonstrated an imbalance in the rates of waitlist dropout by regions with different wait times. We found low rates of waitlist dropout of 3–5% that were nearly identical between centers 2 and 3 with median wait times of 7.3 and 4.4, respectively. It has been shown that the rate of waitlist dropout for HCC patients is not linear, but tends to be low in the first 6 months on the waitlist, then increases exponentially over time30. In this study, center 1 with the longest wait time (median 13 months) accounted for 85% of all the waitlist dropouts and had a 20% probability of waitlist dropout at 18 months of HCC diagnosis. Certainly, efforts to reduce regional disparities such as with redistricting may help alleviate the unequal distribution of waitlist dropout currently found in regions with prolonged wait times compared to those with much shorter wait times. Another option to reduce regional disparities while balancing LT rates between HCC and non-HCC patients would be to assign a fixed MELD exception slightly below the median MELD at LT based on region and blood type. Finally, identifying new biomarkers for assessment of tumor aggressiveness 31 and predicting post-LT outcome is of critical importance in future research. DCP (des-gamma-carboxyprothrombin)32 and selected noncoding RNAs (microRNAs and long noncoding RNAs)33 hold promise and should be further investigated.
To our knowledge, this is the largest US multi-center study to date on LT for HCC. We used HCC recurrence as the primary end-point in assessing the impact of wait time rather than relying on overall survival as evident in most UNOS-based studies. In contrast to prior studies using UNOS data, we used the date of HCC diagnosis rather than date of LT listing as the starting point in defining wait time. Our definition takes into consideration the lag time between HCC diagnosis and referral to the LT center, and possible factors that might result in a delay in LT listing. We therefore believe that our definition of wait time results in a more accurate assessment of its impact on HCC recurrence and that changes should be considered for starting wait time from the date of HCC diagnosis rather than date of listing, like kidney transplant policy changes implemented in December 2014 by UNOS/OPTN in which wait time included time spent after starting dialysis.
Our study has limitations, most notably the retrospective study design and lack of information provided for response to LRT, which has been suggested to correlate with risk of HCC recurrence after LT34–36. Analysis of response to LRT is difficult to perform in this multi-center cohort due to multiple time points for evaluation in the center with prolonged waiting time and insufficient duration to observe the full effects of LRT in the center with short waiting time. Like the findings from Terzi et al,37 the number of LRT received was not predictive of HCC recurrence. While our study included a large cohort of 911 patients (740 received LT), there were only 83 cases of HCC recurrence which might have limited our ability to detect a stronger association between wait time and HCC recurrence. Finally, we have not yet validated the wait time “sweet spot”. A single-center study attempted to identify the “optimal” wait time for LT in HCC patients using an intention-to-treat survival analysis38, but we are concerned about the statistical bias as wait time itself is part of the observation. For example, a patient must survive 6 months from HCC diagnosis to be in the 6–18-month wait time group and patients dying within 3 months of diagnosis would automatically be included in the <3-month wait time group. We therefore chose not to perform an intention-to-treat survival analysis per different wait times as a result of this statistical bias.
In summary, this large multi-center study provides evidence of an association between post-LT HCC recurrence and very short (<6 months) or long (>18 months) wait times from HCC diagnosis to LT. Patients in these categories had an overall 60% increased risk of HCC recurrence compared to those with a wait time of 6–18 months and had higher AFP levels at the time of LT. The so-called wait time “sweet spot” of 6–18 months should be the target to minimize HCC recurrence after LT.
Acknowledgments
Grants and Financial Support: This work was supported by the Biostatistics Core of the UCSF Liver Center (P30 DK026473).
ABBREVIATIONS
- UNOS
United Network for Organ Sharing
- LT
liver transplantation
- HCC
Hepatocellular carcinoma
- MELD
Model for End Stage Liver Disease
- AFP
alpha-fetoprotein
- LRT
loco-regional therapy
- IQR
inter-quartile range
- CR
competing risks
- HR
hazard ratio
- AIC
Akaike information criterion
Footnotes
Disclosures: The authors declare no conflicts of interest
Authorship Page
Concept and design: Mehta, Heimbach, Lee, Harnois, Burns, Sanchez, Roberts, Yao
Acquisition, analysis, or interpretation of data: Mehta, Heimbach, Lee, Dodge, Harnois, Burns, Sanchez, Roberts, Yao
Drafting of the manuscript: Mehta, Dodge, Yao
Critical revision of the manuscript for important intellectual content: Mehta, Heimbach, Lee, Dodge, Harnois, Burns, Sanchez, Roberts, Yao
Statistical Analysis: Mehta, Heimbach, Lee, Dodge, Yao
Administrative, technical, or material support: Heimbach, Lee, Harnois, Burns, Sanchez
Supervision: Roberts, Yao
References
- 1.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Massie AB, Caffo B, Gentry SE, et al. MELD exceptions and rates of waiting list outcomes. Am J Transpl. 2011;11(11):2362–71. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13(1):11–22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16(3):262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 5.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134(5):1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Welch K, Hussain H, et al. Incidence and risk factors of hepatocellular carcinoma recurrence after liver transplantation in the MELD era. Dig Dis Sci. 2012;57(3):806–812. doi: 10.1007/s10620-011-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143(2):182–188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246(3):502–509. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986–994. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20(8):945–951. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplant for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35(9):987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 14.Gouw AS, Balabaud C, Kusano H, et al. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17(Suppl 2):S72–80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 15.Mehta N, Yao FY. Moving past “One size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl. 2013;19(10):1055–1058. doi: 10.1002/lt.23730. [DOI] [PubMed] [Google Scholar]
- 16.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S277–282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl. 2010;16(8):925–929. doi: 10.1002/lt.22103. [DOI] [PubMed] [Google Scholar]
- 18.Halazun KJ, Patzer RE, Rana AA, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology. 2014;60(6):1957–62. doi: 10.1002/hep.27272. [DOI] [PubMed] [Google Scholar]
- 19.Schlansky B, Chen Y, Scott DL, Austin D, Naugler WE. Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry. Liver Transpl. 2014;20(9):1045–1056. doi: 10.1002/lt.23917. [DOI] [PubMed] [Google Scholar]
- 20.Samoylova ML, Dodge JL, Yao FY, Roberts JP. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2014;20(8):937–944. doi: 10.1002/lt.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl. 2013;19(12):1343–1353. doi: 10.1002/lt.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heimbach JK, Hirose R, Stock PG, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61(5):1643–1650. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Organ Procurement and Transplantation Network. Policies. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Updated March 1 2017.
- 24.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 26.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transpl. 2010;10(7):1643–1648. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 27.Volk ML. Unfair priority for HCC: A problem whose ideal solution remains unsolved. Am J Transpl. 2010;10(7):1507–1508. doi: 10.1111/j.1600-6143.2010.03154.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012;18(4):434–443. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terzi E, Piscaglia F, Forlani L, et al. TACE performed in patients with a single nodule of hepatocellular carcinoma. BMC Cancer. 2014;14:601–614. doi: 10.1186/1471-2407-14-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9(7):684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 31.Chauhan R, Lahiri N. Tissue- and serum-associated biomarkers of hepatocellular carcinoma. Biomark Cancer. 2016;8(Suppl 1):37–55. doi: 10.4137/BIC.S34413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaiteerakij R, Zhang X, Addissie BD, et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2015;21(5):599–606. doi: 10.1002/lt.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Felden J, Heim D, Schulze K, et al. High expression of micro RNA-135A in hepatocellular carcinoma is associated with recurrence within 12 months after resection. BMC Cancer. 2017;17(1):60. doi: 10.1186/s12885-017-3053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19(10):1108–1118. doi: 10.1002/lt.23706. [DOI] [PubMed] [Google Scholar]
- 35.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12(8):1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 36.Kim DJ, Clark PJ, Heimbach J, et al. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transpl. 2014;14(6):1383–1390. doi: 10.1111/ajt.12684. [DOI] [PubMed] [Google Scholar]
- 37.Terzi E, Kim WR, Sanchez W, et al. Impact of multiple transarterial chemoembolization treatments on hepatocellular carcinoma for patients awaiting liver transplantation. Liver Transpl. 2015;21(2):248–257. doi: 10.1002/lt.24041. [DOI] [PubMed] [Google Scholar]
- 38.Salvalaggio PR, Felga G, Axelrod DA, Della Guardia B, Almeida MD, Rezende MB. List and liver transplant survival according to waiting time in patients with hepatocellular carcinoma. Am J Transpl. 2015;15(3):668–677. doi: 10.1111/ajt.13011. [DOI] [PubMed] [Google Scholar]