Abstract
Essential tremor and Parkinson’s disease–associated tremor are extremely prevalent within the field of movement disorders. The ventral intermediate (VIM) nucleus of the thalamus has been commonly used as both a neuromodulatory and neuroablative target for the treatment of these forms of tremor. With both deep brain stimulation and Gamma Knife radiosurgery, there is an abundance of literature regarding the surgical planning, targeting, and outcomes of these methodologies. To date, there have been no reports of frameless, linear accelerator (LINAC)–based thalomotomies for tremor.
The authors report the case of a patient with tremor-dominant Parkinson’s disease, with poor tremor improvement with medication, who was offered LINAC-based thalamotomy. High-resolution 0.9-mm isotropic MR images were obtained, and simulation was performed via CT with 1.5-mm contiguous slices. The VIM thalamic nucleus was determined using diffusion tensor imaging (DTI)–based segmentation on FSL using probabilistic tractography. The supplemental motor and premotor areas were the cortical target masks. The authors centered their isocenter within the region of the DTI-determined target and treated the patient with 140 Gy in a single fraction. The DTI-determined target had coordinates of 14.2 mm lateral and 8.36 mm anterior to the posterior commissure (PC), and 3 mm superior to the anterior commissure (AC)–PC line, which differed by 3.30 mm from the original target determined by anatomical considerations (15.5 mm lateral and 7 mm anterior to the PC, and 0 mm superior to the AC-PC line). There was faint radiographic evidence of lesioning at the 3-month follow-up within the target zone, which continued to consolidate on subsequent scans. The patient experienced continued right upper-extremity resting tremor improvement starting at 10 months until it was completely resolved at 22 months of follow-up.
Frameless LINAC-based thalamotomy guided by DTI-based thalamic segmentation is a feasible method for achieving radiosurgical lesions of the VIM thalamus to treat tremor.
Essential tremor is the most prevalent movement disorder, affecting nearly 5% of the adult population older than 65 years of age.13 The condition is generally progressive, resulting in increasing difficulties in performing activities of daily living (ADLs) to a point of functional dependence on others. Medical therapies for essential tremor have not advanced considerably in the past few decades, and nearly one-third of patients discontinue their medications due to intolerance or lack of efficacy.9 Neuromodulatory treatments such as deep brain stimulation (DBS)2–4,14,17,24 and motor cortex stimulation,15 as well as ablative therapies such as MRI-guided ultrasound7,12 and stereotactic radiosurgery (SRS),5 have been shown to have varying degrees of effectiveness in the treatment of essential tremor.19
A recent meta-analysis of SRS for essential tremor reported symptomatic improvement in approximately 80% of patients.5 All SRS treatments for tremor to date have been achieved using Gamma Knife radiosurgery (GKRS). Indeed, the only report of using linear accelerator (LINAC)–based focused radiation to the thalamus was reported by our institution in the treatment of thalamic pain syndromes.10 However, with the increasing ease of access and the appeal of a frameless system obviating the need for skull fixation with only submillimeter reductions in stereotactic accuracy,21 radiosurgical treatments using LINAC-based systems have gained considerable popularity in recent years.
It has long been accepted that accurate stereotaxy is crucial in the efficacy of neuromodulation.1,22 However, the apparent homogeneity of the internal thalamic nuclei on MR images typically used for clinical purposes (1.5–3.0 T) makes it difficult to ensure appropriate targeting without indirect confirmation with neurophysiological testing during DBS surgeries. As this is not possible when treating a patient with SRS, one becomes reliant on atlas coordinates that may not correlate with the individual functional anatomy of the patient. The inability to test the treatment target in SRS thalamotomies may be a potential factor that has limited its widespread adoption.
Diffusion tensor imaging (DTI) has been shown to help delineate the internal anatomy of the thalamus by tracing white matter tracts back from cortical areas and cerebellum involved in tremor to their thalamic nuclei, namely the ventral intermediate nucleus (VIM) of the thalamus.6,11,20 Our group has previously demonstrated that using probabilistic tractography, connectivity from the premotor and supplementary motor areas can be used to identify the regions of the thalamus that allow for the greatest amount of tremor reduction when stimulated by DBS.20 Although this was done in a retrospective manner, when done prospectively, DTI segmentation has the potential to guide and refine thalamic targeting for improved efficacy in neuromodulation and thalamotomies. Here, we integrate multiple novel approaches with 1 patient who underwent DTI segmentation–guided LINAC-based radiosurgery to the VIM thalamus for tremor-dominant Parkinson’s disease.
Case Report
History and Examination
The patient was an 81-year-old man with tremor-dominant Parkinson’s disease whose tremor had limited response to Sinemet and primidone. He was evaluated by a movement disorders neurologist (Y.B.) using the Unified Parkinson’s Disease Rating Scale. His resting tremors were rated as 3 (severe) in the right arm and 2 (mild, present most of the time) in the left arm; they were 1 (slight and infrequent) and 2 in the right and left lower extremities, respectively. Kinetic and postural tremors were rated as 0 (absent) and 1 (slight, present with action) in the right and left arms, respectively. The resting tremors in the bilateral upper extremities were continuous.
The patient was poorly compliant with medication. He performed poorly on neuropsychiatric/cognitive testing and was thus determined to be a high-risk candidate for DBS surgery. Given his poor response to medications, SRS thalamotomy was offered. The patient felt that the tremors were significantly affecting his ability to perform his ADLs, especially eating. As his medications only helped reduce his tremors 40%–60% when they were the most effective, he was interested in pursuing this option.
Radiosurgical Planning
Patient Simulation
Pretreatment MR images with and without contrast were obtained, including 0.9-mm isotropic MPRAGE T1-weighted images with and without contrast, and 1.5-mm susceptibility weighted imaging. Simulation was performed using a Somatom Sensation 16 (Siemens) open CT scanner with 1.5-mm contiguous slices. A custom thermoplastic mask was created and used to immobilize the patient’s head and neck. The mask was attached to a localization frame during CT simulation to create a stereotactic reference space within the images. The CT and MR images were fused in iPlan RT Image (Brainlab) software.
VIM Localization
The VIM thalamus was identified using DTI-based segmentation of the thalamus as was previously validated by our group.20 Briefly, using the publicly available imaging analysis software FSL (http://www.fmrib.ox.ac.uk/fsl/), we defined the left premotor and supplementary motor cortices as the cortical target mask, as this region was previously determined to have the most functional connectivity with areas of the thalamus that ameliorated tremor to the greatest degree in patients undergoing thalamic DBS.20 We then used FDT (FMRIB’s Diffusion Toolbox) within FSL to perform probabilistic diffusion tractography to determine the white matter paths with the greatest likelihood of connectivity between the predefined cortical regions and the thalamus, presumably the VIM nucleus thereof. The diffusion data were then registered to the thin-cut T1-weighted images using linear registration (FMRIB’s Linear Registration Tool [FLIRT]). The FSL function PROBTRACKX (5000 directions sampled, 0.2 curvature threshold, and a loop-check termination) was then used to generate a thalamic map with the greatest probability of connectivity to the aforementioned cortical regions, which would represent our radiosurgical target. This sensory thalamus was thresholded and binarized at 1500 with fslmaths to develop a pseudocolor map, where the lightest areas had the greatest probability of connectivity with the supplementary area and premotor cortex. We then centered our isocenter within this DTI-determined target (Fig. 1). The stereotactic coordinate of this DTI-determined target was centered at 14.2 mm lateral and 8.36 mm anterior to the PC, and 3 mm superior to the AC-PC line.
FIG. 1.
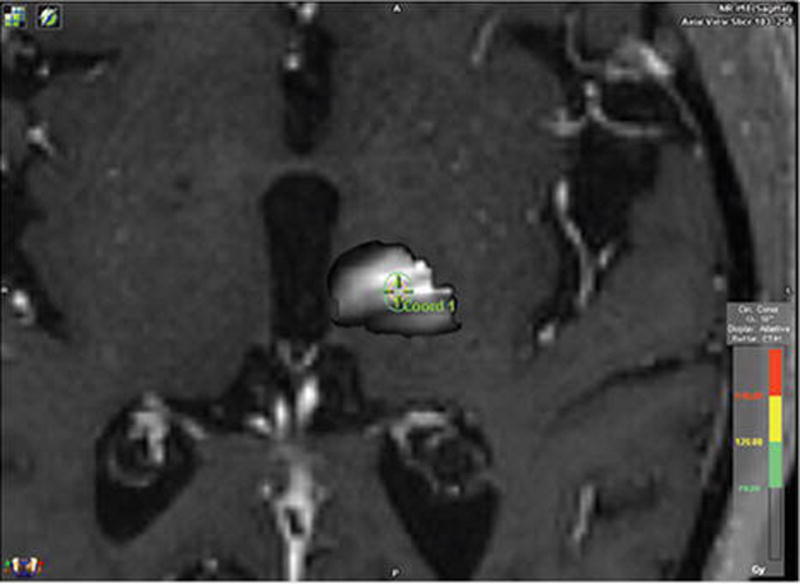
DTI-determined VIM thalamus. FSL was used to determine the areas of the thalamus with the greatest likelihood of connectivity with the premotor and supplementary motor areas. The areas of greatest likelihood for connectivity to these regions and thus the greatest likelihood of being the VIM are the whitest regions in our thalamic map. We attempted to center our isocenter as centrally as possible based on this connectivity map. Figure is available in color online only.
Planning
A 4-mm-diameter SRS conical collimator (SRS cone) and 6-MV photons on a Varian Novalis Tx linear accelerator were used. The plan was created with 11 equally spaced arcs of 160° arc length that resulted in a spherically shaped 50% isodose volume. The dose calculation grid was set to 1 mm, and a prescribed dose of 140 Gy (to the isocenter point) was delivered in a single fraction.
Patient Localization for Treatment Delivery
The ExacTrac X-Ray (Brainlab) was used to align the patient in the treatment room. Stereotactic radiography was performed prior to the delivery of each treatment arc, and a positioning tolerance of 0.5 mm was used. Gross positioning accuracy was also verified via cone-beam CT imaging (Varian) prior to delivery of the first treatment arc. A total of 20 ExacTrac verification images were acquired during treatment. Eight repositioning shifts were needed to correct out-of-tolerance positioning following arc deliveries. The mean (SD) translational deviations from treatment isocenter detected during treatment in the lateral, longitudinal, and vertical directions, respectively, were 0.08 (0.46) mm, 0.12 (0.30) mm, and 0.03 (0.28) mm. Beams were not delivered if the detected deviation was greater than 0.5 mm, and 8 couch shifts were needed to correct deviation during treatment. The total time within the radiation suite was 1 hour and 20 minutes, which included 20 minutes of setup time. The patient tolerated the procedure well without any significant discomfort.
Clinical Outcomes
The patient was seen and examined by the movement disorders neurologist at multiple time points after his DTI-guided frameless LINAC thalamotomy. The patient’s resting tremor was noted to have improved mildly at 1 month postoperatively, with moderate improvement at 5 months after SRS. He continued to improve at 10 months, 16 months, and 22 months postoperatively, with complete resolution of his resting tremor at 22 months; his family noted that he had much improved functional ability with his right upper extremity. There was no significant improvement in his postural or kinetic tremor. Of note, he developed a high-amplitude tremor on the right that was not previously appreciable, which limited his functional improvement, although his prior resting tremor was suppressed. His clinical course and quantified Fahn-Tolosa-Marin tremor scores are summarized in Table 1.8 Of note, the patient also experienced improvement in his appendicular rigidity on subsequent follow-up visits (Table 1).
TABLE 1.
Preoperative and postoperative Fahn-Tolosa-Marin Tremor Rating Scale scores for rest tremor, postural tremor, kinetic tremor, and rigidity
| Variable | Preop | 1 Mo | 5 Mos | 10 Mos | 16 Mos | 22 Mos |
|---|---|---|---|---|---|---|
| Resting tremor | ||||||
| RUE | 3 | 2 | 2 | 1 | 1 | 0 |
| LUE | 2 | 2 | 2 | 2 | 2 | 0 |
| RLE | 1 | 0 | 1 | 1 | 1 | 0 |
| LLE | 2 | 0 | 0 | 0 | 0 | 0 |
| Postural tremor | ||||||
| Rt | 1 | 1 | 1 | 1 | 1 | 1 |
| Lt | 1 | 1 | 0 | 0 | 0 | 0 |
| Kinetic tremor | ||||||
| Rt | 1 | 1 | 1 | 1 | 1 | 1 |
| Lt | 1 | 1 | 1 | 1 | 1 | 1 |
| Rigidity | ||||||
| RUE | 2 | 2 | 2 | 0 | 0 | 2 |
| LUE | 3 | 1 | 1 | 0 | 0 | 1 |
| RLE | 2 | 2 | 2 | 1 | 1 | 0 |
| LLE | 2 | 2 | 1 | 1 | 1 | 0 |
LLE = left lower extremity; LUE = left upper extremity; RLE = right lower extremity; RUE = right upper extremity.
Radiographic Outcomes
Traditional Versus DTI-Determined Target
Using standard methods for localizing the VIM nucleus of the thalamus, namely, targeting 11 mm lateral to the wall of the third ventricle, 25% of the AC-PC distance anterior to the PC, and at the level of the AC-PC line, our initial planned target was 15.5 mm lateral and 7 mm anterior to the PC, and 0 mm superior to the AC-PC line. The DTI-determined VIM target was 14.2 mm lateral and 8.36 mm anterior to the PC, and 3 mm superior to the AC-PC line. These 2 potential isocenters were approximately 3.3 mm apart from each other (Fig. 2).
FIG. 2.
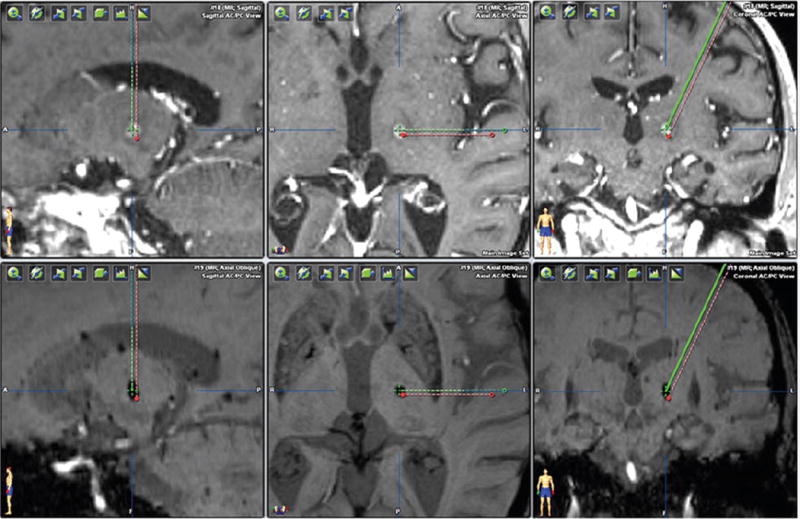
Comparison of structural MRI-determined versus DTI-determined VIM thalamic targets. The green crosshair indicates the DTI-determined VIM thalamus and shows good concordance with the ultimate radiosurgical lesion. The red dot represents the VIM thalamus target determined by standard measurements based on the AC-PC distance and third ventricle width. Figure is available in color online only.
Evolution of Lesion
Magnetic resonance imaging was performed serially at 3, 7, and 12 months postoperatively. At 3 months posttreatment, MRI demonstrated a 3-mm focus of T2 hyperintensity with subtle contrast enhancement within the region of the DTI-determined thalamic target (Fig. 3). Coregistration of the preoperative planning MRI and postprocedural MRI confirms that the area of signal change is within the focus of our treatment area to the 120-Gy line. Imaging at 7 months demonstrated more robust T2 hyperintensity and contrast enhancement with FLAIR signal changes within the planned area of SRS. Twelve-month postoperative images demonstrated T2 hypointensity and susceptibility-weighted imaging attenuation with continued contrast enhancement and stable lesion size. Of note, there was a mild increase in T2 hyperintensity within the posterior limb of the internal capsule at this time point.
FIG. 3.
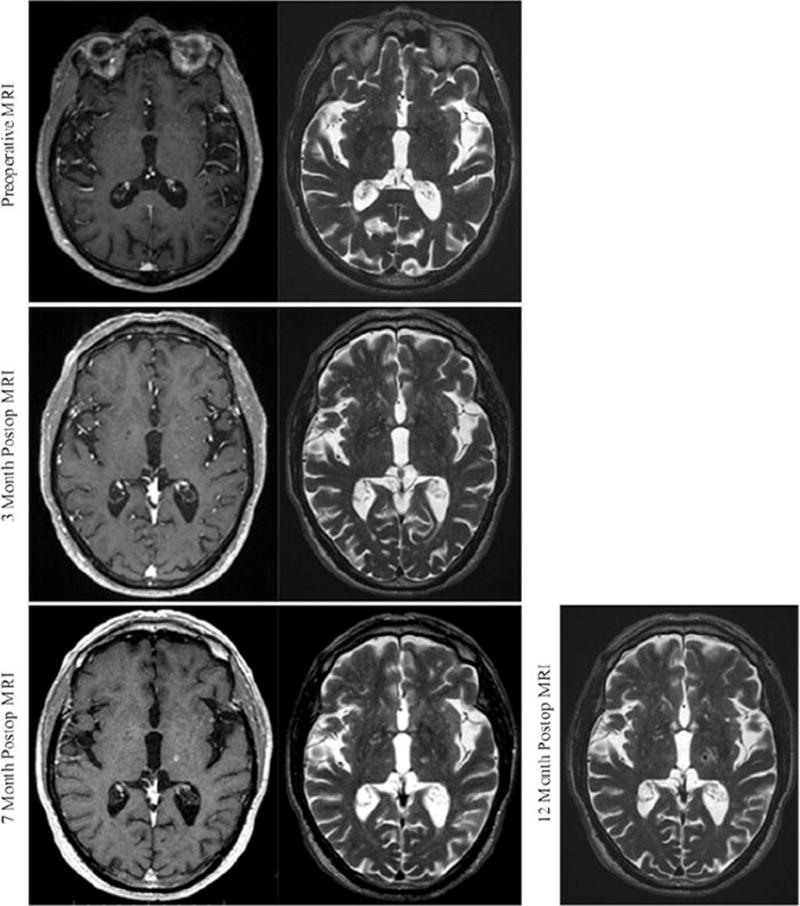
Radiographic evolution of the radiosurgical lesion. The patient was monitored radiographically, with MR images obtained preoperatively and at 3, 7, and 12 months postoperatively. T1-weighted with contrast and T2-weighted sequences were obtained at all time points except at 1 year postoperatively, when contrast scans were not obtained. At as early as 3 months, there is subtle contrast enhancement and T2 hyperintensity within the area of our intended target. These signal characteristics continue to solidify through 7 months following SRS. Twelve months postoperatively, there is T2 hypointensity within the radiosurgical target.
Discussion
Stereotactic radiosurgery using the Gamma Knife has been shown to be effective in reducing the degree of essential tremor in clinically significant ways in approximately 80% of patients.5 The experience, including hundreds of patients published in numerous clinical series, affirms that it is a safe and efficacious means of treating essential tremor in patients who are poor surgical candidates on account of medical or neurocognitive reasons.5 However, despite the vast literature regarding GKRS thalamotomies for essential tremor, there are no reports of frameless LINAC-based thalamotomies for tremor to date. This may stem from the fact that interventional treatments for essential tremor typically require a multidisciplinary team of specialized functional neurosurgeons, movement disorder neurologists, and radiation oncologists. As one is more likely to encounter such a team at larger academic medical institutions or tertiary referral centers where Gamma Knife devices are traditionally housed, it is natural that there is a preponderance of literature regarding this modality.18
Notwithstanding these historical trends, there is an increasing amount of evidence supporting the use of LINAC-based radiosurgery as a technology comparable to GKRS.18,25 Traditionally, LINAC was deemed less accurate with a prohibitively slow delivery time; however, with recent modifications, the accuracy and speed of this technology have been demonstrated to be comparable to that of the Gamma Knife.10 Moreover, the use of the head and neck thermoplastic mask allows for stereotactic registration and dose delivery without the use of a frame, obviating the need for a neurosurgeon to be present on the day of treatment. Although the accuracy of frame-based systems seems to be slightly superior to mask-based ones, the difference is on the order of fractions of millimeters and does not appear to be clinically significant. Indeed, Ramakrishna and colleagues21 as well as our group (unpublished data) have demonstrated a mean error magnitude of 0.7 ± 0.3 mm on hidden target tests with frameless LINAC systems.
Although it remains to be proven whether one technology has considerably better treatment outcomes for the wide variety of indications for which SRS is employed, we have shown that it is possible to make small, accurate, and precise subcentimeter lesions within the thalamus for the treatment of thalamic pain syndromes.10 In this report, we once again demonstrate that it is feasible to create focused lesions isolated to the region of interest within the thalamus using LINAC-based SRS. Accuracy in regard to stereotaxy as well as volume of area treated is of paramount importance, as numerous analyses of the literature demonstrate that larger lesions to the thalamus in the treatment of essential tremor were associated with significantly higher rates of complications, including hemiparesis, dysphagia, and dysarthria.5,26 Although we did appreciate new T2 hyperintensity within the posterior limb of the internal capsule consistent with either perilesional edema or radiation effect at the 12-month follow-up, this was not found to be clinically significant.
We have previously demonstrated that DTI-based segmentation of the thalamus can help delineate the functional anatomy therein, with the notable example of identifying the VIM thalamus and predicting DBS lead location and tremor response to stimulation.20 Here, we described the prospective use of this methodology to identify a radiosurgical target for the treatment of tremor. Sammartino et al. recently demonstrated a similar use of DTI to prospectively target the VIM for MR-guided focused ultrasound.23 Given the seemingly homogeneous appearance of the thalamus on 3-T MRI and the varying degrees of interpatient heterogeneity in thalamic nuclei16,20 (and unpublished data), intraoperative electrophysiological testing is critical to ensure adequate placement of leads during DBS surgery for essential tremor. However, lacking the opportunity for physiological testing during radiosurgery, other measures must be taken to help ensure that patient-appropriate targets are localized and treated. We believe that DTI-based segmentation can serve as such a tool.
Although this case report illustrates the feasibility of LINAC thalamotomies and DTI-based segmentation guided therapies, there are limitations to our report. The patient improved on measures of bilateral rigidity in addition to contralateral and ipsilateral resting tremor, suggesting that there were other factors, including greater response to and compliance with medications, that contributed to the improvement of his tremor. Indeed, he had increased his Sinemet dose from 25/100 mg 3 times a day on preoperative assessment to 25/250 mg 4 times a day at last followup, although he continued to take primidone 100 mg twice daily. However, the greatest amount of functional improvement was seen in the patient’s right upper-extremity resting tremor, supporting the role of the thalamic DBS in his tremor alleviation. Moreover, at final follow-up, his resting tremor had completely subsided despite only modest improvement in rigidity, unlike their gradual resolution together at earlier postoperative time points (Table 1). The value of radiosurgical thalamotomy may be limited by its time to efficacy. Still, this report serves as a proof of concept that supports the potential role of using tractography-guided segmentation in other ablative procedures, such as radiofrequency ablation or high-intensity focused ultrasound.
The presented case is one of the first to use DTI-based segmentation to preoperatively target the VIM of the thalamus, and subsequently use a frameless SRS delivery system to treat tremor. The use of the LINAC in conjunction with a thermoplastic mask allows for a frameless system and obviates the need for a neurosurgeon to be present on the day of treatment. In this case, resting tremor gradually resolved postoperatively with complete resolution at 22 months of follow-up. The efficacy of this procedure provides further validation for a means of preoperatively identifying and treating a thalamic target using patient-specific connectivity patterns, which would otherwise be impossible with the imaging modalities routinely used in the clinical setting, or without invasive intraoperative physiological testing. Future studies including a larger cohort of patients may assess improvements in treatment efficacy using such a means of preoperative targeting.
Acknowledgments
This work was supported by the National Institute of Biomedical Imaging and Bioengineering Award No. K23EB014326 (N.P.) and philanthropic support from Casa Colina Centers for Rehabilitation.
References
- 1.Abosch A, Yacoub E, Ugurbil K, Harel N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery. 2010;67:1745–1756. doi: 10.1227/NEU.0b013e3181f74105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baizabal-Carvallo JF, Kagnoff MN, Jimenez-Shahed J, Fekete R, Jankovic J. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatry. 2014;85:567–572. doi: 10.1136/jnnp-2013-304943. [DOI] [PubMed] [Google Scholar]
- 3.Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 4.Blomstedt P, Hariz GM, Hariz MI, Koskinen LO. Thalamic deep brain stimulation in the treatment of essential tremor: a long-term follow-up. Br J Neurosurg. 2007;21:504–509. doi: 10.1080/02688690701552278. [DOI] [PubMed] [Google Scholar]
- 5.Campbell AM, Glover J, Chiang VL, Gerrard J, Yu JB. Gamma Knife stereotactic radiosurgical thalamotomy for intractable tremor: a systematic review of the literature. Radiother Oncol. 2015;114:296–301. doi: 10.1016/j.radonc.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Coenen VA, Allert N, Mädler B. A role of diffusion tensor imaging fiber tracking in deep brain stimulation surgery: DBS of the dentato-rubro-thalamic tract (drt) for the treatment of therapy-refractory tremor. Acta Neurochir (Wien) 2011;153:1579–1585. doi: 10.1007/s00701-011-1036-z. [DOI] [PubMed] [Google Scholar]
- 7.Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369:640–648. doi: 10.1056/NEJMoa1300962. [DOI] [PubMed] [Google Scholar]
- 8.Fahn S, Tolosa E, Concepcion M. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s Disease and Movement Disorders. 2. Baltimore: Williams and Wilkins; 1993. pp. 271–280. [Google Scholar]
- 9.Fasano A, Deuschl G. Therapeutic advances in tremor. Mov Disord. 2015;30:1557–1565. doi: 10.1002/mds.26383. [DOI] [PubMed] [Google Scholar]
- 10.Frighetto L, De Salles A, Wallace R, Ford J, Selch M, Cabatan-Awang C, et al. Linear accelerator thalamotomy. Surg Neurol. 2004;62:106–114. doi: 10.1016/j.surneu.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Hyam JA, Owen SL, Kringelbach ML, Jenkinson N, Stein JF, Green AL, et al. Contrasting connectivity of the ventralis intermedius and ventralis oralis posterior nuclei of the motor thalamus demonstrated by probabilistic tractography. Neurosurgery. 2012;70:162–169. doi: 10.1227/NEU.0b013e3182262c9a. [DOI] [PubMed] [Google Scholar]
- 12.Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12:462–468. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 13.Louis ED, Ferreira JJ. How common is the most common adult movement disorder?. Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 14.Mandat T, Koziara H, Rola R, Bonicki W, Nauman P. Thalamic deep brain stimulation in the treatment of essential tremor. Neurol Neurochir Pol. 2011;45:37–41. doi: 10.1016/s0028-3843(14)60058-x. [DOI] [PubMed] [Google Scholar]
- 15.Moro E, Schwalb JM, Piboolnurak P, Poon YY, Hamani C, Hung SW, et al. Unilateral subdural motor cortex stimulation improves essential tremor but not Parkinson’s disease. Brain. 2011;134:2096–2105. doi: 10.1093/brain/awr072. [DOI] [PubMed] [Google Scholar]
- 16.Nowinski WL, Belov D, Thirunavuukarasuu A, Benabid AL. A probabilistic functional atlas of the VIM nucleus constructed from pre-, intra- and postoperative electrophysiological and neuroimaging data acquired during the surgical treatment of Parkinson’s disease patients. Stereotact Funct Neurosurg. 2005;83:190–196. doi: 10.1159/000091082. [DOI] [PubMed] [Google Scholar]
- 17.Ondo W, Jankovic J, Schwartz K, Almaguer M, Simpson RK. Unilateral thalamic deep brain stimulation for refractory essential tremor and Parkinson’s disease tremor. Neurology. 1998;51:1063–1069. doi: 10.1212/wnl.51.4.1063. [DOI] [PubMed] [Google Scholar]
- 18.Park HS, Wang EH, Rutter CE, Corso CD, Chiang VL, Yu JB. Changing practice patterns of Gamma Knife versus linear accelerator–based stereotactic radiosurgery for brain metastases in the US. J Neurosurg. 2016;124:1018–1024. doi: 10.3171/2015.4.JNS1573. [DOI] [PubMed] [Google Scholar]
- 19.Picillo M, Fasano A. Recent advances in essential tremor: surgical treatment. Parkinsonism Relat Disord. 2016;22(Suppl 1):S171–S175. doi: 10.1016/j.parkreldis.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Pouratian N, Zheng Z, Bari AA, Behnke E, Elias WJ, Desalles AA. Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. J Neurosurg. 2011;115:995–1004. doi: 10.3171/2011.7.JNS11250. [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishna N, Rosca F, Friesen S, Tezcanli E, Zygmanszki P, Hacker F. A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol. 2010;95:109–115. doi: 10.1016/j.radonc.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Richardson RM, Ostrem JL, Starr PA. Surgical repositioning of misplaced subthalamic electrodes in Parkinson’s disease: location of effective and ineffective leads. Stereotact Funct Neurosurg. 2009;87:297–303. doi: 10.1159/000230692. [DOI] [PubMed] [Google Scholar]
- 23.Sammartino F, Krishna V, King NK, Lozano AM, Schwartz ML, Huang Y, Hodaie M. Tractography-based ventral intermediate nucleus targeting: novel methodology and intraoperative validation. Mov Disord. 2016;31:1217–1225. doi: 10.1002/mds.26633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlaier J, Anthofer J, Steib K, Fellner C, Rothenfusser E, Brawanski A, et al. Deep brain stimulation for essential tremor: targeting the dentato-rubro-thalamic tract? Neuromodulation. 2015;18:105–112. doi: 10.1111/ner.12238. [DOI] [PubMed] [Google Scholar]
- 25.Solberg TD, Goetsch SJ, Selch MT, Melega W, Lacan G, DeSalles AA. Functional stereotactic radiosurgery involving a dedicated linear accelerator and gamma unit: a comparison study. J Neurosurg. 2004;101(Suppl):3373–380. [PubMed] [Google Scholar]
- 26.Young RF, Li F, Vermeulen S, Meier R. Gamma Knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg. 2010;112:1311–1317. doi: 10.3171/2009.10.JNS09332. [DOI] [PubMed] [Google Scholar]


