Scientific Abstract
Approximately 30% of individuals with autism spectrum disorder (ASD) have elevated whole blood serotonin (5-HT) levels. Genetic linkage and association studies of ASD and of whole blood 5-HT levels as a quantitative trait have revealed sexual dimorphism. Few studies have examined the presence of a sex difference on hyperserotonemia within ASD. To assess whether the rate of hyperserotonemia is different in males than in females with ASD, we measured whole blood 5-HT levels in 292 children and adolescents with ASD, the largest sample in which this biomarker has been assessed. Based upon previous work suggesting that hyperserotonemia is more common prior to puberty, we focused our analysis on the 182 pre-pubertal children with ASD. 42% of pre-pubertal participants were within the hyperserotonemia range. In this population, we found that males were significantly more likely to manifest hyperserotonemia than females (p = 0.03). As expected, no significant difference was found in the post-pubertal population. Additional work will be needed to replicate this intriguing finding and to understand whether it could potentially explain differences in patterns of ASD risk between males and females.
Keywords: Serotonin, 5-HT, Autism Spectrum Disorder, Hyperserotonemia
Autism spectrum disorder (ASD) describes a heterogeneous group of neurodevelopmental conditions characterized by impairments in social interaction and communication, as well as the presence of stereotyped behaviors and restricted interests (American Psychiatric Association, 2013). Elevated whole blood serotonin (5- hydroxytryptamine or 5-HT) was the first biomarker demonstrated in autism (Schain & Freedman, 1961). A recent systematic review and meta-analysis found that approximately 30% of individuals with ASD have elevated whole blood 5-HT levels, termed hyperserotonemia, but it also found significant heterogeneity across study populations, with rates higher than 50% in three of the nineteen published samples (Gabriele, Sacco, & Persico, 2014). Importantly, within neurodevelopmental disorders, hyperserotonemia appears to be specific for ASD and is not associated with intellectual disability in the absence of ASD (Mulder et al., 2004).
Serotonin is a monoamine neurotransmitter produced from the essential amino acid tryptophan. Although 5-HT is best known for its role in neurotransmission, the majority of 5-HT in the human body is actually found in the periphery (Muller et al. 2015). Peripheral 5-HT is produced by enterochromaffin cells in the gastrointestinal track, which release it into the enteric circulation (Gershon, 2013). More than 99% of the 5-HT in the blood is contained in platelets, which lack biosynthetic capacity but take up 5-HT via the serotonin transporter (SERT, 5-HTT) (Anderson, Feibel, & Cohen, 1987). A variety of different mechanisms could therefore underlie hyperserotonemia in ASD: increased 5-HT synthesis in enterochromaffin cells, potentially including altered handling of its precursor, tryptophan; increased 5-HT release from enterochromaffin cells; increased uptake of 5-HT into platelets without an increase in platelet free plasma 5-HT; diminished catabolism of 5-HT; or decreased release of 5-HT from platelets (Anderson et al. 1987; Cook et al. 1988; Cook & Leventhal, 1996; Marazziti et al. 2000; Croonenberghs et al. 2005; Cross et al. 2008; Janusonis, 2008; Boccuto et al. 2013).
Since 5-HT does not cross the blood-brain barrier, peripheral hyperserotonemia is unlikely to have a direct effect on brain function after the development of the blood brain barrier, but it may indicate a common factor that could influence the 5-HT system both in the brain and in the periphery (Cook & Leventhal, 1996; Muller, Anacker, & Veenstra-VanderWeele, 2015). Indirect effects of blood serotonin on the brain are also possible, since serotonin plays an important role in gut function and immune regulation, with potential downstream effects on the brain (Baganz & Blakely, 2013; Gershon, 2013; Arreola et al. 2015; Margolis et al. 2016).
While elevated whole blood 5-HT levels have been repeatedly observed in ASD, potential moderators of this biomarker have rarely been examined. Available data suggests that group differences in 5-HT levels are larger in samples of younger children and that hyperserotonemia is more commonly observed before puberty, but age effects were too rarely assessed to be included in the recent meta-analysis (McBride et al. 1998; Hammock et al. 2012; Gabriele et al. 2014). Importantly, hyperserotonemia has been documented in ASD across racial and ethnic groups; although one previous study found that Caucasian children diagnosed with autistic disorder had significantly lower levels of whole blood 5-HT than African-American or Hispanic/Latino children (McBride et al., 1998). The presence of sex differences on rates of hyperserotonemia in ASD has never been examined in detail, likely because the strong male bias in ASD leaves studies substantially underpowered to evaluate this biomarker in females.
Heritability estimates suggest that whole blood 5-HT levels are under strong genetic control (Abney, McPeek, & Ober, 2000; Weiss, Abney, Cook, & Ober, 2005). A quantitative trait genome-wide linkage and association study of whole blood 5-HT in a large founder population revealed significant association with ITGB3, which encodes the SERT binding partner, integrin β3 (Carneiro, Cook, Murphy, & Blakely, 2008; Weiss et al., 2004). A follow-up association study in the same population revealed that whole blood 5-HT was significantly associated with ITGB3, as well as with the SERT-encoding gene SLC6A4, in males but not in females (Weiss et al., 2005). Interestingly, linkage studies in ASD have implicated both the 17q11 region containing SLC6A4 and the 17q21 region containing ITGB3 in males, but not in females (Stone et al. 2004; Cantor et al. 2005; Sutcliffe et al. 2005). In light of the association signals underlying genetic control of whole blood 5-HT levels exclusively in males, we were struck by the low rate of hyperserotonemia observed in a population of female children with ASD recruited at the University of Illinois at Chicago (UIC). To assess whether this rate was actually significantly lower, we combined this population with a sample recruited at Vanderbilt University (VU), thereby creating, to our knowledge, the largest sample of children with ASD with measured whole blood 5-HT levels. We focused specifically on children less than 12 years of age because of the previous work showing a more pronounced rate of hyperserotonemia before puberty (McBride et al., 1998).
Methods
Participants
This study was approved by the Institutional Review Boards at the University of Illinois at Chicago and at Vanderbilt University. In total, 292 participants with a diagnosis of Autism Spectrum Disorder (ASD) were recruited. Demographic information, including race and ethnicity, was obtained from all participants by parental self-report. Inclusion criteria included diagnosis of Autistic Disorder, Asperger’s Disorder, or Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS) based on the DSM-IV-TR criteria (American Psychiatric Association, 2000). ASD classification was confirmed using the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994). Social and repetitive behavior phenotypes were evaluated using the ADOS (Lord et al., 2000). Language function was examined using the Peabody Picture Vocabulary Test (PPVT-4) (Dunn & Dunn, 2007) and the Expressive One-Word Picture Vocabulary Test (EOWPVT-4) (Martin & Brownell, 2000). Cognitive ability was examined using the Differential Ability Scales–Second Edition (DAS-II) (Elliot, 2007). Finally, adaptive functioning was assessed using the Vineland Adaptive Behavior Scale (VABS-2) (Sparrow, Cicchetti & Balla, 2005). Exclusionary criteria included participants who were taking medications that could affect 5-HT, such as serotonin reuptake inhibitors, stimulants, and atypical antipsychotic medications. Two hundred sixty-four participants were included in the analysis, 182 from University of Illinois at Chicago Autism Center of Excellence (UIC-ACE) and 82 from the Vanderbilt University Simons Simplex Collection (SSC) project.
Pubertal status was determined based on participants’ Tanner scale or chronological age (Marshall & Tanner, 1969, 1970). Subjects were classified as pre-pubertal if their Tanner scale was either I or II and post-pubertal if their Tanner scale was greater than or equal to III. In the absence of a Tanner scale, chronological age was used to classify individuals as pre- or post-pubertal. Subjects with a chronological age less than 144 months (12 years) were classified as pre-pubertal, whereas subjects whose chronological age was greater than or equal to 144 months were classified as post-pubertal.
Whole Blood Serotonin
Fasting whole blood was obtained by venipuncture using BD Vacutainer tubes containing EDTA. The samples were stored at −70°C until assayed. Whole blood 5-HT was measured by high-performance liquid chromatography, as previously described (Anderson et al. 1987; McBride et al. 1998).
Data Analysis
One-way analyses of variance and independent sample t-tests were used to examine the effect of puberty, race, and ethnicity on whole blood 5-HT levels. Categorical analyses were implemented to compare participants with hyperserotonemia versus participants with normoserotonemia using Pearson’s chi-square test of association. All analyses were conducted using SPSS Version 23. All tables and plots were generated using R version 3.1.2.
Results
In the current study, 264 participants were included in data analysis. Pubertal status had a significant effect on whole blood 5-HT levels, with higher values observed before puberty (F(1, 262)=12.81, p<0.0001). Participants were divided into pre- and post-pubertal groups for subsequent analysis. Neither race nor ethnicity was significantly associated with whole blood 5-HT levels in pre-pubertal children with ASD (Race F(5, 176)=1.33, p=0.25), Ethnicity F(1, 180)=0.07, p=0.79). When each sex was considered separately, there remained no significant race or ethnicity effects (Male Race F(5,153)=0.81 p=0.54 and Ethnicity F(1,157)=0.02, p=0.88; Female Race F(4, 18)=0.91, p=0.47) and Ethnicity F(1, 21)=0.13, p=0.72).
Hyperserotonemia has been defined using various different criteria including 1.5 – 2.0 standard deviations above the whole blood 5-HT mean or the 95th percentile of whole blood 5-HT in typically developing individuals. The current sample collection was designed to evaluate potential relationships between whole blood 5-HT levels and genetic variation, neuropsychological function, neuroimaging results, ASD symptoms, and associated medical disorders, and therefore no separate control group was collected. Instead, we relied upon published norms to define the hyperserotonemia range. The overall distribution of whole blood 5-HT levels for pre-pubertal male children showed a rightward shift from a normal distribution (Figure 1). Kolmogorov-Smirnov tests of normality indicated that whole blood 5-HT levels for pre- and post-pubertal females did not differ significantly from a normal distribution (D(23)=0.15, p=.16; D(14)=0.13, p=.20); whereas distributions for pre- and post-pubertal males were significantly non-normal (D(157)=0.08, p=.005; D(70)=0.14, p=.001, respectively). Based upon the published literature (Leventhal et al., 1990; McBride et al., 1998; Cook et al., 2000; and Anderson et al., 2012), hyperserotonemia was defined as whole blood 5-HT level above 270 ng/mL, expected to correspond to the 95th percentile of the typically developing population; whereas those with whole blood 5-HT levels below 270 ng/mL were classified as normoserotonemic. In the current study, 42% of pre-pubertal participants were within the hyperserotonemia range (see Table 1). The mean whole blood 5-HT levels were not significantly different for the male and female pre-pubertal populations (t (180)=1.29, p=0.19). Among pre-pubertal children with ASD, however, males were more likely to have hyperserotonemia compared to females (x2=4.338, p=0.03).
Figure 1.
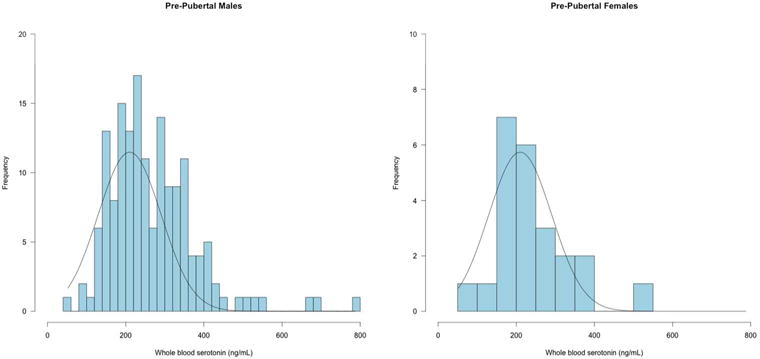
Distribution of whole blood serotonin (5-HT) in pre-pubertal males and females with an expected normal distribution curve. Frequency distribution of 5-HT levels, in absolute values (ng/mL). N = 159 males; N = 23 females.
Table 1.
Pre-Pubertal Whole Blood Serotonin Levels in Males and Females
| Male (N=159) | Female (N=23) | Total (N=182) | |
|---|---|---|---|
| Hyperserotonemia | 71 | 5 | 76 |
| Hyperserotonemia (%) | 44% | 21% | 42% |
| Normoserotonemia | 88 | 18 | 106 |
| WB5-HT Mean (ng/mL) | 269 | 237 | 264 |
| Standard Deviation | 112 | 96 | 110 |
| Std. Error of Mean | 9 | 20 | 8 |
| Skewness | 1.47 | 1.40 | 1.47 |
| Age: Mean (SD) | 7.60 (2.38) | 7.34 (2.64) | 7.56 (2.41) |
| Race: (WB5-HT Mean) | |||
| African-American | 20 (310) | 2 (348) | 22 (313) |
| Asian-American | 6 (264) | 1 (257) | 7 (263) |
| Caucasian | 106 (263) | 16 (226) | 122 (258) |
| Caucasian/Hispanic | 24 (261) | 3 (243) | 27 (260) |
| Mixed Race | 2 (211) | 1 (154) | 3 (192) |
| Native American/Alaskan | 1 (341) | 0 | 1 (341) |
| Ethnicity: (WB5-HT Mean) | |||
| Hispanic/Latino | 26 (266) | 4 (221) | 30 (260) |
| Non Hispanic Latino | 133 (269) | 19 (240) | 152 (265) |
In the post-pubertal portion of the sample, there was also no significant influence of race (F(5, 76)=1.67, p=0.15) or ethnicity (F(1, 80)=0.04, p=0.83) on whole blood 5-HT levels. The rate of hyperserotonemia in post-pubertal participants was 24%, putting the overall rate at 36% across the full sample. In post-pubertal individuals with autism, males were no more likely to have hyperserotonemia compared to females (x2(N 82)=0.08, p=0.77). In the subset of participants that had behavioral and cognitive data available for analysis (n = 182), there were no significant correlations between whole blood 5-HT levels and ADOS, DAS, PPVT-4, EOWPVT-4, or VABS-2 subscale scores, either in the overall sample or when analyzed separately in the pre-pubertal and post-pubertal subgroups.
DISCUSSION
This represents the largest sample of children with ASD for whom whole blood 5-HT levels were measured. Similar to previous studies, we found that a substantial subset of children with ASD had whole blood 5-HT levels in the hyperserotonemia range, with higher values more likely before puberty (Gabriele et al., 2014). In the pre-pubertal sample, we found that males with ASD are more likely to have hyperserotonemia than females. This is consistent with the genetic literature identifying sexually dimorphic genetic associations with whole blood 5-HT levels and the SLC6A4 and ITGB3 in a large founder population, as well as sexually dimorphic ASD linkage signals in the 17q11 and 17q21 chromosomal regions containing these two genes (Cantor et al., 2005; Stone et al., 2004; Sutcliffe et al., 2005).
This study has important limitations that constrain our confidence in the observed sexual dimorphism. The primary limit is one of statistical power. Since the ratio of males to females with ASD is 5:1 in the current sample, as is typical in ASD, we could only analyze 23 pre- and 14 post-pubertal females with ASD, even within the largest study of hyperserotonemia to date. This left us significantly underpowered to compare mean 5-HT levels between males and females with ASD. Future, prospective studies to examine this apparent sexual dimorphism would ideally enrich their recruitment to achieve similar number of females to males. If other groups were to publish their sex-specific data, this could allow for a meta-analysis to further examine the presence of a sex difference on hyperserotonemia within ASD. The second substantial limitation is that without typically developing, age-matched controls, we had to rely on previously published 5-HT ranges to define hyperserotonemia in this population. Although our pre- and post-pubertal male distribution was non-normal, it was not bi-modal; therefore, we were unable to identify a biochemically defined hyperserotonemia subgroup within our own sample as was previously found by Mulder and colleagues (2004) in a Dutch sample. Further, while we did not observe race or ethnicity effects in our affected individuals, it is possible that such effects would have been observed if we were able to compare to a matched control population, potentially changing the threshold for hyperserotonemia for some participants. Although we clearly observed an effect of puberty in our population, some participants did not have Tanner staging available, forcing us to rely on age alone in a subset of the sample. Lastly, it is possible that the lower rate of hyperserotonemia in the post-pubertal sample is driven, in part, by the necessary exclusion of participants taking medications that could affect blood 5-HT levels. If individuals with hyperserotonemia are more likely to be prescribed these medications, their exclusion would show an apparent lack of hyperserotonemia at the time point when medications are typically prescribed. Unfortunately, many of these medications result in a dramatic reduction of blood 5-HT levels, precluding our ability to assess this possibility. Longitudinal follow up of our sample to assess rate of medication use would be necessary to evaluate this idea. An even more rigorous approach would be a prospective, longitudinal sample beginning prior to the diagnostic window for ASD.
While these data point to a sex effect on hyperserotonemia that was not previously detected in smaller samples, we cannot elucidate the potential underlying mechanisms in this sample. One potential interpretation of these data is that elevated whole blood 5-HT levels may be caused by a common factor or factors that contribute to ASD risk to a larger degree in males than in females. The developmental hyperserotonemia model of autism hypothesizes that during the early stages of fetal development before the BBB is fully formed, elevated levels of maternal blood 5-HT could enter the fetal brain, resulting in a loss of 5-HT terminals via negative feedback (Hadjikhani, 2010). Studies attempting to elucidate the association between selective serotonin reuptake inhibitors (SSRIs) and risk of ASD in offspring have yielded inconsistent findings (Croen et al., 2011; Gidaya et al., 2014; Boukhris et al., 2015). However, one study demonstrated pre-natal exposure to SSRIs increased susceptibility to ASD or developmental disability in males only (Harrington et al., 2014). Reciprocally, an ASD risk factor or factors could have a larger effect on whole blood 5-HT levels in males than in females. A number of proteins and genes within the 5-HT system are affected by sex hormones, suggesting possible avenues for this sexual dimorphism. For example, 5-HT plays a role in the development of the medial and lateral paraventrical nucleus (PVN) in rats in a sexually dimporphic manner (Madden & Zup, 2014). Administration of 5-methoxytryptamine lead to alterations in oxytocinergic cells in the PVN in females, but not males (Madden & Zup, 2014). Additionally, there are sexual differences in the effect of 5-HT on luteinizing hormone (LH) secretion in pre-pubertal rats. During administration of a 5-HT neurotoxin that depletes 5-HT levels in the brain, female rats demonstrated a significant increase in LH release, while the neurotoxin had no effect in males (Moguilevsky et al., 1985). Additionally, during the estrous cycle in female rats, serotonin receptors undergo changes in receptor affinity leading to a reduction in 5-HT binding (Biegon, Bercovitz, & Samuel, 1980). Central 5-HT release has also been known to vary across the menstrual cycle (Yang, Sampson, Senturk, & Andrews, 2015). Additional work will be needed to replicate this intriguing finding, and to understand whether it could potentially explain differences in patterns of ASD risk between males and females.
Table 2.
Post-Pubertal Whole Blood Serotonin Levels in Males and Females
| Male (N=68) | Female (N=14) | Total (N=82) | |
|---|---|---|---|
| Hyperserotonemia | 17 | 3 | 20 |
| Hyperserotonemia (%) | 25% | 21% | 24% |
| Normoserotonemia | 51 | 11 | 62 |
| WB5-HT Mean (ng/mL) | 213 | 222 | 215 |
| Standard Deviation | 92 | 77 | 89 |
| Std. Error of Mean | 11 | 21 | 10 |
| Skewness | .93 | .76 | .89 |
| Age: Mean (SD) | 16.46 (4.72) | 17.99 (4.71) | 16.72 (4.72) |
| Race: (WB5-HT Mean) | |||
| African-American | 8 (251) | 3 (263) | 11 (254) |
| Asian-American | 2 (87) | 0 | 2 (87) |
| Caucasian | 50 (211) | 9 (216) | 59 (212) |
| Caucasian/Hispanic | 7 (194) | 2 (194) | 9 (194) |
| Mixed Race | 1 (292) | 0 | 1 (292) |
| Native American/Alaskan | 1 (288) | 0 | 1 (288) |
| Ethnicity: (WB5-HT Mean) | |||
| Hispanic/Latino | 7 (213) | 2 (194) | 9 (209) |
| Non Hispanic Latino | 61 (213) | 12 (228) | 73 (216) |
Lay Abstract.
Elevated whole blood serotonin (5-HT) was the first biomarker established in autism and is found in approximately 30% of individuals with autism spectrum disorder (ASD). Few studies have examined the presence of a sex difference on elevated whole blood serotonin, also known as hyperserotonemia, within ASD. Serotonin plays an important role in gut function and immune regulation, with potential downstream effects on the brain. To assess whether the rate of hyperserotonemia is different in males than in females with ASD, we measured whole blood 5-HT levels in 292 children and adolescents with ASD, the largest sample in which this biomarker has been assessed. Based upon previous work suggesting that hyperserotonemia is more common prior to puberty, we focused our analysis on the 182 pre-pubertal children with ASD. 42% of pre-pubertal participants were within the hyperserotonemia range. In this population, we found that males were significantly more likely to manifest hyperserotonemia than females. As expected, no significant difference was found in the post-pubertal population. Additional work will be needed to replicate this intriguing finding and to understand whether it could potentially explain differences in patterns of ASD risk between males and females.
Acknowledgments
Funding: NIH HD055751, NIH MH094604, NIH MH016434, the Simons Foundation Autism Research Initiative Simplex Project, NCRR/NIH Vanderbilt CTSA grant 5UL1 RR024975, the New York State Psychiatric Institute, and the Mortimer D. Sackler, M.D., Foundation.
Footnotes
JV has consulted or served on advisory boards for Roche, Novartis, and SynapDx; has received research funding from Roche Pharmaceuticals, Novartis, SynapDx, Seaside Therapeutics, and Forest; and has received stipends for editorial work from Springer and Wiley. EHC has consulted with and received research funding from Seaside Therapeutics. SJ has received funding from Roche Pharmaceuticals.
References
- Abney M, McPeek M, Ober C. Estimation of variance components of quantitative traits in inbred populations. Am J Hum Genet. 2000;66(2):629–650. doi: 10.1086/302759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-Text Revision (DSM-IV-TR) 4th. Washington, D.C.: American Psychiatric Association Press, Inc.; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed, DSM-5) Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anderson GM, Feibel FC, Cohen DJ. Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultrafiltrate. Life Sci. 1987;40(11):1063–1070. doi: 10.1016/0024-3205(87)90568-6. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Freedman DX, Cohen DJ, Volkmar FR, Hoder EL, McPhedran P, Young JG. Whole blood serotonin in autistic and normal subjects. Journal of Child Psychology, Psychiatry and Allied Disciplines. 1987;28:885–900. doi: 10.1111/j.1469-7610.1987.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Hertzig ME, McBride PA. Brief report: Platelet-poor plasma serotonin in autism. J Autism Dev Disord. 2012;42(7):1510–1514. doi: 10.1007/s10803-011-1371-1. [DOI] [PubMed] [Google Scholar]
- Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez MA, Garcés-Alvarez ME, Hurtado-Alvarado G, Quintero-Fabian S, Pavón L. Immunomodulatory effects mediated by serotonin. Journal of Immunology Research. 2015;2015:354957. doi: 10.1155/2015/354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chemical Neuroscience. 2013;4:48–63. doi: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Bercovitz H, Samuel D. Serotonin receptor concentration during the estrous cycle of the rat. Brain Res. 1980;187(1):221–225. doi: 10.1016/0006-8993(80)90509-0. [DOI] [PubMed] [Google Scholar]
- Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA pediatrics. 2016;170(2):117–124. doi: 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
- Cantor RM, Kono N, Duvall JA, Alvarez-Retuerto A, Stone JL, Alarcon M, Geschwind DH. Replication of Autism Linkage: Fine-Mapping Peak at 17q21. Am J Hum Genet. 2005;76(6) doi: 10.1086/430278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118(4):1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook E, Leventhal B. The serotonin system in autism. Current Opinion in Pediatrics. 1996;8(4):348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Cook EH, Leventhal BL, Heller W, Metz J, Wainwright M, Freedman DX. Autistic children and their first-degree relatives: relationships between serotonin and norepinephrine levels and intelligence. J Neuropsychiatry Clin Neurosci. 1990;2:268–274. doi: 10.1176/jnp.2.3.268. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Archives of general psychiatry. 2011;68(11):1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 4th. Bloomington, MN: NCS Pearson; 2007. [Google Scholar]
- Elliot CD. Differential Ability Scales-II (DAS-II) San Antonio. TX: Pearson; 2007. [Google Scholar]
- Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(6):919–929. doi: 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20(1):14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. Journal of autism and developmental disorders. 2014;44(10):2558–2567. doi: 10.1007/s10803-014-2128-4. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N. Serotonin, pregnancy and increased autism prevalence: is there a link? Medical Hypotheses. 2010;74(5):880–883. doi: 10.1016/j.mehy.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics. 2014;133(5):e1241–e1248. doi: 10.1542/peds.2013-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal BL, Cook EH, Morford M, Ravitz A, Freedman DX. Relationships of whole blood serotonin and plasma norepinephrine within families. Journal of autism and developmental disorders. 1990;20(4):499–511. doi: 10.1007/BF02216055. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview - Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Madden AM, Zup SL. Effects of developmental hyperserotonemia on juvenile play behavior, oxytocin and serotonin receptor expression in the hypothalamus are age and sex dependent. Physiology & behavior. 2014;128:260–269. doi: 10.1016/j.physbeh.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson … GM, Gershon MD. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. Journal of Clinical Investigation. 2016 doi: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NA, Brownell R. Expressive One-Word Picture Vocabulary Test. 4th. Novato, CA: Academic Therapy Publications; 2010. [Google Scholar]
- McBride PA, Anderson GM, Hertzig ME, Snow ME, Thompson SM, Khait VD, Cohen DJ. Effects of diagnosis, race, and puberty on platelet serotonin levels in autism and mental retardation. J Am Acad Child Adolesc Psychiatry. 1998;37(7):767–776. doi: 10.1097/00004583-199807000-00017. [DOI] [PubMed] [Google Scholar]
- Moguilevsky JA, Faigón MR, Rubio MC, Scacchi P, Szwarcfarb B. Sexual differences in the effect of serotonin on LH secretion in rats. Acta endocrinologica. 1985;109(3):320–325. doi: 10.1530/acta.0.1090320. [DOI] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43(4):491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Muller CL, Anacker AM, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland-II adaptive behavior scales. AGS Publishing; 2005. [Google Scholar]
- Stone JL, Merriman B, Cantor RM, Yonan AL, Gilliam TC, Geschwind DH, Nelson SF. Evidence for sex-specific risk alleles in autism spectrum disorder. Am J Hum Genet. 2004;75(6):1117–1123. doi: 10.1086/426034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77(2):265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Abney M, Cook EH, Jr, Ober C. Sex-specific genetic architecture of whole blood serotonin levels. Am J Hum Genet. 2005;76(1):33–41. doi: 10.1086/426697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Veenstra-Vanderweele J, Newman DL, Kim SJ, Dytch H, McPeek MS, Abney M. Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. Eur J Hum Genet. 2004;12(11):949–954. doi: 10.1038/sj.ejhg.5201239. [DOI] [PubMed] [Google Scholar]
- Yang H, Sampson MM, Senturk D, Andrews AM. Sex- and SERT-mediated differences in stimulated serotonin revealed by fast microdialysis. ACS Chem Neurosci. 2015;6(8):1487–1501. doi: 10.1021/acschemneuro.5b00132. [DOI] [PubMed] [Google Scholar]


