Abstract
Background
A Seasonal Asthma Exacerbation Predictive Index (saEPI) was previously reported based on two prior NIAID Inner City Asthma Consortium trials.
Objective
We sought to validate the saEPI in a separate trial designed to prevent fall exacerbations with omalizumab therapy.
Methods
The saEPI and its components were analyzed to characterize those who had an asthma exacerbation during the PROSE (Preventative Omalizumab or Step-Up Therapy for Fall Exacerbations) study. We characterized those inner-city children with and without asthma exacerbations in the fall periods treated with guidelines based therapy (GBT) in the absence and presence of omalizumab.
Results
A higher saEPI was associated with an exacerbation in both the GBT alone (p<0.001, AUC 0.76) and the GBT + omalizumab group (p<0.01, AUC 0.65). In the GBT group, younger age at recruitment, higher total IgE, higher blood eosinophil percent and number, and higher treatment step were associated with those who had an exacerbation compared to those who did not. In the GBT + omalizumab group, younger age at recruitment, increased eosinophil number, recent exacerbation, and higher treatment step were also associated with those who had an exacerbation. The saEPI was associated with a high negative predictive value in both groups.
Conclusions
An exacerbation in children treated with GBT with or without omalizumab was associated with a higher saEPI along with higher markers of allergic inflammation, treatment step, and a recent exacerbation. Those that exacerbated on omalizumab had similar features, indicating a need to develop better markers to predict poor response to omalizumab therapy and alternative treatment strategies for children with these risk factors. The saEPI was able to reliably predict those children unlikely to have an asthma exacerbation in both groups.
Keywords: Fall asthma exacerbation, omalizumab, guidelines-based therapy, asthma exacerbation predictors, Seasonal Asthma Exacerbation Predictive Index (saEPI)
Introduction
Asthma is a chronic disease with widespread impact, affecting approximately 6.8 million children in the US in 2012, which is about 9.3% of the US population of children.1 Asthma exacerbations are an increasingly important outcome in the determination of efficacy of asthma therapy, due to the high burden of disease, as well as significantly increased healthcare costs in patients who exacerbate2. Children in the inner city are at higher risk for asthma related morbidity and mortality, for a variety of reasons.3
The National Institute of Allergy and Infectious Diseases (NIAID) has sponsored the Inner City Asthma Consortium (ICAC) since 1991, with a focus on reducing disparities for children with asthma residing in the inner-city.4 A previous retrospective ICAC analysis used data from two previous trials (Asthma Control Evaluation [ACE]5) and Inner City Anti-IgE Therapy for Asthma [ICATA]6) to identify season-specific risk factors for asthma exacerbations and to develop a seasonal asthma exacerbation predictive index (saEPI) (Table E1 in the Online Repository).7 This index consisted of 8 variables, which were each given a low, medium or high point value. The composite index score was then used to determine exacerbation risk during each season (see Methods section for more detail). The fall season is a time of particular risk of exacerbation for children with asthma,8–12 beginning about 2 weeks after the start of the school year.13 The ICAC’s Preventative Omalizumab or Step-up Therapy for Fall Exacerbations, or PROSE study14 included a run-in period prior to the beginning of school, with a treatment period initiated at school start dates, affording a unique opportunity to re-examine the saEPI specifically for this fall period.
Our primary objective was to test the reliability of the saEPI in a population of children treated with the consensus Expert Panel Recommendations (EPR-3)15 (which we have called Guidelines Based Therapy or GBT), with and without the addition of omalizumab. Our hypothesis was that those with a high saEPI would be more likely to have an asthma exacerbation on GBT. Our secondary objectives were 1) to determine if these predictors might change in the presence of anti-IgE, and 2) to determine if providers with access to varying amounts of data would be able to use portions of the index to effectively predict the risk of an asthma exacerbation.
Methods
Study group
The PROSE study randomized 486 children with an asthma diagnosis (or asthma symptoms) for >1 year, an exacerbation requiring systemic corticosteroids or hospitalization within the prior 14–19 months, at least one positive skin test to a perennial allergen in the last year, residence in a low-income census tract, body weight and IgE appropriate for omalizumab dosing, and insurance coverage for asthma medications. Participants were randomized at a ratio of 3:3:1 to GBT +omalizumab arm (n=223), a GBT + inhaled corticosteroid boost arm (n=155) or GBT only arm (n=89) (see PROSE study for further details).14 The following data were collected during the run-in period: spirometry, FeNO, total IgE, blood eosinophils (percent and total number). Response predictors were collected at randomization. For this analysis, participants from the GBT and GBT +omalizumab groups were analyzed post-hoc to determine the characteristics of those that had an exacerbation during the fall treatment period (starting 4–6 weeks from fall school start plus 90 days) in these two treatment groups. The ICS boost arm was not included in this analysis as the goal was to validate the results of previous evaluations of GBT only, as well as determine if characteristics were different in the group receiving biologic therapy (omalizumab). Those in the ICS boost arm were limited to participants receiving Step 2 through 4 treatment, while participants in the other two groups could exceed these limits, thus the ICS boost arm was not felt to accurately reflect the target population under study.
Seasonal Asthma Exacerbation Predictive Index (saEPI)
The saEPI was developed by assigning cutoff values to 8 risk variables, and assigning point values to the risk variable range (low risk=0 points, medium risk=1 point, high risk=2 points) with a composite score ranging from 0 to16 (Supplement Table 1). Variables included age, allergic propensity (total IgE and allergen skin test positivity), percent blood eosinophils, exacerbation in the prior season, ICS step, FEV1/FVC, and FeNO. An additional parameter tested separately included total eosinophil count.
Statistical Analysis
The primary objective of these analyses was the validation of the previously derived predictors of future asthma exacerbations7 for both the GBT and GBT + Omalizumab arm. For the comparison between treatment groups (Table 1) and between exacerbation within treatment groups (Table 2A & 2B) we used the Mann-Whitney U, and the Chi-square test for continuous and categorical variables respectively to test for independence. The graphical relation between the saEPI and the dichotomous exacerbation (Figure 1), measured during the 90 days double-blind phase of the study, was constructed using a univariate logistic regression model, and a Chi-square test was used to test their association.
Table 1.
Demographics of the study population*
| Overall | Guidelines Based Therapy | Guidelines Based Therapy + Omalizumab | P | |
|---|---|---|---|---|
|
| ||||
| N=478 | N=89 | N=259 | ||
| Study Cohort - no. (%): | ||||
| 2012 | 229 (47.9%) | 43 (48.3%) | 127 (49.0%) | 0.99 |
| 2013 | 249 (52.1%) | 46 (51.7%) | 132 (51.0%) | |
| Injection schedule - no. (%): | ||||
| Once per 2 weeks | 183 (38.3%) | 36 (40.4%) | 100 (38.6%) | 0.86 |
| Once per 4 weeks ¶ | 295 (61.7%) | 53 (59.6%) | 159 (61.4%) | |
| Race or ethnic group - no. (%): | ||||
| African American | 279 (58.4%) | 54 (60.7%) | 145 (56.0%) | 0.43 |
| Hispanic | 161 (33.7%) | 30 (33.7%) | 88 (34.0%) | |
| White, mixed or other | 38 (7.95%) | 5 (5.62%) | 26 (10.0%) | |
| Caretaker completed high school - no. (%) | 340 (71.3%) | 55 (61.8%) | 188 (72.9%) | 0.07 |
| 1+ household member employed - no. (%) | 330 (69.0%) | 59 (66.3%) | 175 (67.6%) | 0.93 |
| Annual household income <$15,000 - no. (%) | 262 (55.4%) | 51 (58.6%) | 145 (56.6%) | 0.84 |
| Age – yr. | 10.0 [8.0–12.0] | 9.00 [8.0–12.0] | 10.0 [8.0–12.0] | 0.48 |
| Male sex - no. (%) | 303 (63.4%) | 59 (66.3%) | 174 (67.2%) | 0.98 |
| Duration of asthma – yr. | 7.25 [4.85–9.90] | 6.75 [4.92–9.50] | 7.67 [4.96–10.1] | 0.15 |
| C-ACT score in the previous month, age 4 to11 yr. (n=358) † | 21.6 (3.63) | 21.3 (3.52) | 21.3 (3.70) | 0.91 |
| ACT score in the previous month, age 12 yr. or older (n=119) † | 21.5 (3.18) | 21.2 (3.87) | 21.4 (3.05) | 0.84 |
| Asthma-related symptoms - days in prior 2 weeks ‡ | 2.34 (3.13) | 2.56 (2.95) | 2.51 (3.25) | 0.89 |
| Wheezing | 1.79 (2.65) | 1.98 (2.37) | 1.89 (2.70) | 0.77 |
| Interference with activity | 1.39 (2.61) | 1.72 (2.82) | 1.56 (2.84) | 0.65 |
| Nighttime sleep disruption | 0.77 (1.80) | 0.90 (1.98) | 0.88 (1.84) | 0.93 |
| FEV1 - % of predicted value | 90.8 [79.7–101] | 89.6 [77.4–102] | 88.7 [78.9–98.9] | 0.81 |
| FEV1:FVC ×100 | 78.3 [72.0–84.6] | 78.2 [70.0–84.8] | 77.3 [71.1–84.4] | 0.97 |
| Medication - no. (%) § | 0.89 | |||
| Step level 2 to 4 | 294 (61.5%) | 43 (48.3%) | 121 (46.7%) | |
| Step level 5 | 184 (38.5%) | 46 (51.7%) | 138 (53.3%) | |
| 1+ Asthma Exacerbation** | 168 (35.1%) | 35 (39.3%) | 106 (40.9%) | 0.89 |
Values are counts (percentages), means (SDs) or medians [IQR]. Calculation of the p-values for the independence test between groups was calculated using the Mann-Whitney U test, and the chi-square test for continuous and categorical variables respectively.
Scores on the Childhood Asthma Control Test (C-ACT) and the Asthma Control Test (ACT) were measured on scales of 0 to 27 and 5 to 25, respectively. A score of 19 or less on either test indicates that asthma is not well controlled. The minimally important difference for ACT equals 3 points; for the C-ACT, a 3-point increase suggests a clinically relevant improvement in asthma control, whereas a 2-point decrease suggests a clinically relevant worsening.
The number of days with symptoms was calculated as the largest of the following variables during the previous 2 weeks: number of days with wheezing, chest tightness, or cough; number of nights of sleep disturbance; and number of days when activities were affected. This symptom scale ranges from 0 to 14 days per 2-week period.
Six treatment steps were established, which is consistent with report 3 of the National Asthma Education and Prevention Program guidelines to standardize prescribing patterns according to levels of asthma severity summarized here. Steps 1 and 2 apply to mild asthma, step 3 to moderate asthma, and steps 4 through 5 to severe asthma. At step 0, the recommendation is for no asthma control medication or albuterol as needed; at step 1, the recommendation is for 50 μg of fluticasone twice a day; at step 2, the recommendation is for 100 μg of fluticasone twice a day; at step 3, the recommendation is for 250 μg of fluticasone twice a day; at step 4, the recommendation is for 250 μg of fluticasone and 50 μg of salmeterol twice a day (Advair, GlaxoSmithKline); and at step 5, the recommendation is for 500 μg of fluticasone and 50 μg of salmeterol twice a day (Advair, GlaxoSmithKline).
Injections once every 2 or 4 weeks based on bodyweight and IgE , see APPENDIX 1: XOLAIR® (OMALIZUMAB) DOSING AND INJECTIONS, Table A1a Xolair® (omalizumab) Dosing Table in original protocol.
One or more asthma-related exacerbations, requiring treatment with a systemic corticosteroid course, during the double blind phase of the study (90 days period).
Table 2.
| A. Guidelines Based Therapy Group*
| |||
|---|---|---|---|
| Exacerbations | P | ||
| No N=66 | Yes N=23 | ||
| Seasonal Asthma Exacerbation Predictive Index (saEPI) | 7.00 [6.00–9.00] | 9.00 [8.50–11.0] | <0.001 |
|
| |||
| Variables included in the saEPI | |||
| Age (years) | 10.0 [8.00–13.0] | 8.00 [7.00–9.50] | 0.01 |
| Total IgE (kU/l) | 242 [107–417] | 410 [262–531] | 0.01 |
| # of Positive Skin Tests | 4.00 [3.00–5.75] | 4.00 [3.00–5.50] | 0.88 |
| Eosinophil % | 4.20 [2.47–6.95] | 7.30 [4.95–8.35] | 0.01 |
| Prior 90 days Exacerbations: | |||
| No | 55 (83.3%) | 14 (60.9%) | 0.05 |
| Yes | 11 (16.7%) | 9 (39.1%) | |
| FEV1/FVC Ratio | 78.3 [73.5–84.5] | 75.3 [67.1–85.1] | 0.28 |
| Exhaled Nitric Oxide (ppb) | 21.7 [11.0–34.5] | 34.0 [17.0–48.5] | 0.09 |
| Treatment Step | 4.00 [2.00–5.00] | 5.00 [4.00–5.00] | 0.01 |
| Variables not included in the saEPI | |||
| Eosinophils (cells/uL) | 265 [162–430] | 380 [350–600] | 0.01 |
| B. Guidelines Based Therapy + Omalizumab Group *
| |||
|---|---|---|---|
| Exacerbations | P | ||
| No N=223 | Yes N=36 | ||
| Seasonal Asthma Exacerbation Predictive Index (saEPI) | 8.00 [6.00–9.50] | 9.00 [7.00–10.2] | 0.01 |
|
| |||
| Variables included in the saEPI | |||
| Age (years) | 10.0 [8.00–13.0] | 9.50 [7.00–11.0] | 0.02 |
| Total IgE (kU/l) | 233 [132–446] | 282 [151–522] | 0.42 |
| # of Positive Skin Tests | 4.00 [2.00–6.00] | 4.00 [3.00–6.00] | 0.47 |
| Eosinophil % | 4.40 [2.80–6.90] | 5.55 [3.97–7.00] | 0.06 |
| Prior 90 days Exacerbations: | 0.03 | ||
| No | 195 (87.4%) | 26 (72.2%) | |
| Yes | 28 (12.6%) | 10 (27.8%) | |
| FEV1/FVC Ratio | 77.4 [71.2–84.5] | 76.8 [69.0–83.2] | 0.29 |
| Exhaled Nitric Oxide (ppb) | 23.5 [14.0–44.8] | 28.7 [16.2–54.5] | 0.27 |
| Treatment Step | 4.00 [2.00–5.00] | 5.00 [3.75–5.00] | 0.01 |
| Variables not included in the saEPI | |||
| Eosinophils (cells/uL) | 290 [180–445] | 400 [250–508] | 0.02 |
Values are counts (percentages), and medians [IQR]. Calculation of the p-values for the independence test between groups was calculated using the Mann-Whitney U test, and the chi-square test for continuous and categorical variables respectively. All variables were obtained at the time of randomization.
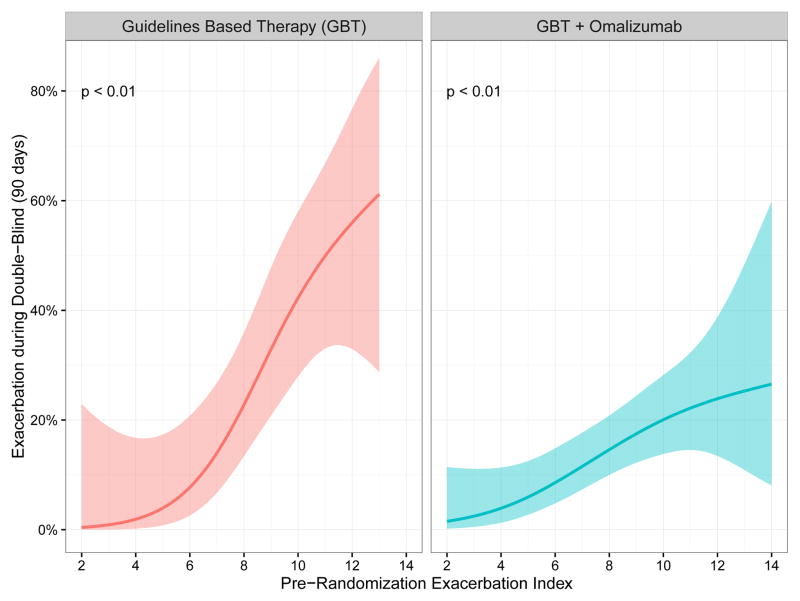
Figure 1.
Association between saEPI and the probability of an asthma exacerbation. Risk score on the x-axis is composite saEPI score, y-axis represents the probability of having an exacerbation in the 90 day PROSE treatment period. The shaded areas represent the 95% confidence interval. The saEPI successfully predicts exacerbations in the fall treatment period.
Multivariate logistic regression analyses were conducted to quantify the risk factors and saEPI associations with exacerbations during the double-blind period. Likelihood-ratio Chi-square tests were used to compare the fit of nested models and to provide a test of significance for the added variables to the model (Supplement Table 2). The order in which the variables were entered into the analyses was determined a priori, according to ease and cost of obtaining the clinical measurements.
The purpose of relative importance16 is to quantify the relative contribution of an individual variable to the model’s total explanatory value by considering averaging over all possible orderings of variables in the model. These are computer-intensive methods that have become achievable17 as a result of recent advances in computational capabilities.
Discrimination was calculated by Receiver Operating Characteristic (ROC) curves and area under the curve (AUC or c statistic), and optimal cutpoint18 for the score were derived from the ROC.
Log-transformations of skewed data (FeNO, total IgE) were used for partial multivariate analyses. A p value <.05 was considered statistically significant. All statistical analyses were performed using the R system for statistical computing version 3.2.19 The calculation of relative importance was conducted using the R add-on package hier.part.20
Results
The treatment groups were similar in terms of demographic characteristics, with a slight predominance of males in each group (Table 1). Most children were African American or Hispanic. They had carried an asthma diagnosis for several years and averaged more than one asthma related symptom day in the prior 2 weeks. About half were in ICS treatment step 2–4, and more than 1/3 in each group had one or more asthma exacerbations during the run in.
Guidelines Based Therapy (GBT) Group
The previous study of children in the ACE5 and ICATA6 trials reported age at recruitment, recent exacerbation, treatment step, total IgE, allergen skin test positivity, blood eosinophils, FEV1/FVC ratio and FeNO to be important predictors for fall exacerbations, and these variables were included as predictors in the saEPI7. When applied to the GBT group, the index was significantly higher in those with compared to those without an exacerbation (Chi-square test p<0.01) (Figure 1). Those with an exacerbation were also younger at enrollment, with a higher total IgE, blood eosinophils (both count and percent), and ICS treatment step than those who did not have a fall exacerbation (Table 2A). Of note, there was not a significant association with the numbers of positive allergen skin tests, previous exacerbations, FeNO or FEV1/FVC. The AUC (area under the curve) for the GBT Receiver Operating Curve (ROC) was 0.76 with a PPV of 0.44 and NPV of 0.88 (Table 3 and supplement Figure 1).
Table 3.
Threshold values for the index for differentiation between exacerbations
| Guidelines Based Therapy (GBT) | GBT + Omalizumab | |||
|---|---|---|---|---|
|
| ||||
| Estimate | 95% CI | Estimate | 95% CI | |
| Area under the ROC curve (AUC) | 0.76 | (0.66, 0.86) | 0.65 | (0.56, 0.74) |
| Optimal Cutoff of index * | 9.0 | 9.0 | ||
| Sensitivity † | 0.74 | (0.52, 0.90) | 0.58 | (0.41, 0.74) |
| Specificity ‡ | 0.67 | (0.54, 0.78) | 0.63 | (0.56, 0.70) |
| Positive predictive value § | 0.44 | (0.31, 0.70) | 0.20 | (0.16, 0.35) |
| Negative predictive value || | 0.88 | (0.73, 0.93) | 0.90 | (0.82, 0.93) |
| False Positive | 22 | 82 | ||
| False Negative | 6 | 15 | ||
The Optimal Cutoff is the exacerbation index value at which we minimize the difference between sensitivity and specificity. An exacerbation index equal or greater than 9.0 we will predict the participant as exacerbator during the fall season.
Sensitivity = True Positive / Total Positive
Specificity = True negatives / (False positives + True negatives)
Positive predictive value = True positive / (False positives + True negatives)
Negative predictive value = True negative / (False positives + True negatives)
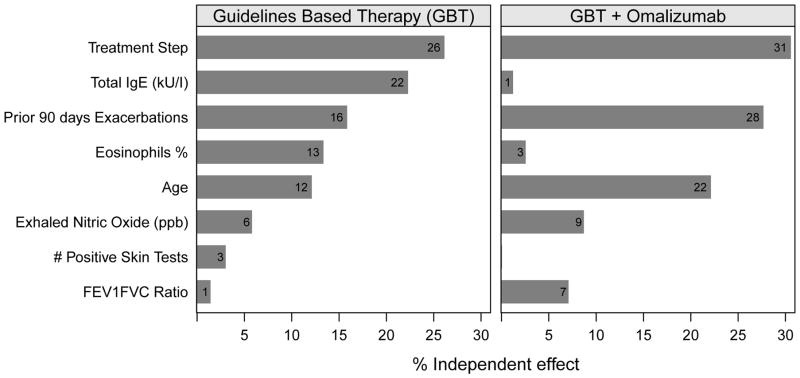
We evaluated the relative importance of the index characteristics for predicting exacerbations. In the GBT group, treatment step, IgE, exacerbation in the prior 90 days, eosinophil percentage and age at randomization were independently important in exacerbation prediction (Figure 2).
Figure 2.
Relative importance of index variables for predicting exacerbations during the intervention period. Bar length represents the independent % contribution of the variable in explaining exacerbations. The numbers at the end of each bar represent the independent effect of each variable.
Guidelines based Therapy plus Omalizumab Group
When the index was applied to the GBT+omalizumab group, its predictive ability remained (Chi-square test p<0.01), though at a lower magnitude than the GBT group (Figure 1). Those with an exacerbation were again younger in age at enrollment, with higher blood eosinophils and ICS treatment step, and more likely to have had an exacerbation in the prior season as compared to those without an exacerbation (Table 2B). Total IgE, the numbers of positive skin tests, eosinophil percentage, FEV1/FVC ratio and FeNO were not significantly different in those with or without an exacerbation. The AUC for the GBT + omalizumab ROC was 0.65 with a PPV of 0.20 and NPV of 0.90 (Table 3 and Supplement Figure 1).
In the GBT + omalizumab group, ICS treatment step, exacerbation in the prior 90 days and age at randomization and were most important independent factors of the probability of exacerbations, while total IgE and blood eosinophil % were no longer important (Figure 2).
Group Predictors
To determine the relative importance of the index components to provider screening questions in evaluating exacerbation risk, we evaluated Model 1 (M1), including age, history of previous exacerbation and treatment step. We then added the saEPI laboratory variables (IgE and blood eosinophils) to Model 2 (M2). The third model (M3) included more specialized testing including allergen skin testing, spirometry (FEV1/FVC ratio) and FeNO.
In the GBT group, the model M2 (including questionnaire data and laboratory testing) was the most parsimonious model to retain significant predictive ability (Supplement Table 2). In the GBT + omalizumab group, model M1 (questionnaire data only) was the most parsimonious model to achieve statistical significance.
Discussion
Our primary objective was to reassess and validate the reliability of the saEPI in the fall season. Developing predictive models for asthma have been historically difficult as asthma is a complex, heterogenous disease21 although previous indices have been utilized to predict the development of asthma in children,22,23 attempts to correlate asthma phenotypes to asthma exacerbations have shown promise in adult and adolescent populations, but not previously in children.24 Prior studies evaluated symptom scores in the days prior to an exacerbation,25 however, scores to predict exacerbations over the long term have remained more challenging. Based on our prior analysis, we hypothesized that the saEPI applied would reliably predict an exacerbation in the fall season. We found that the saEPI correlated well with exacerbations in both groups (see Figure 1). While the positive predictive value was statistically reasonable at 0.44 for the GBT group, what is more striking is the NPV of 0.88 and 0.90 for the GBT and GBT + omalizumab groups respectively. This indicates a good ability of the index to predict those children who are unlikely to have an asthma exacerbation during the fall time frame. This is similar to the Asthma Predictive Index introduced by Castro-Rodrigues et al, which had a low PPV of only 26.2 in the 6-year-old population, but a striking NPV of 93.926.
We were able to demonstrate that the majority of the fall-specific risk factors for asthma exacerbations that were determined previously7 continued to be associated with those who had an exacerbation in the GBT population (Table 2A). Younger age has been shown to be a risk factor for asthma exacerbation27, and this was confirmed by our data. Additionally, markers of allergic disease including IgE and blood eosinophils were higher in those with an exacerbation, consistent with prior studies.28–30 A history of an exacerbation in the previous 90 days was more likely in those with an exacerbation during the study period, consistent with prior studies,8,31,32 however this association did not reach statistical significance. As a marker of asthma severity, the ICS treatment step was also higher in the group with an exacerbation. Very poorly controlled asthma has been demonstrated previously as a risk factor for future exacerbations,33,34 although it should be noted that the predictors of asthma exacerbations and asthma control are not always directly correlated.27
Studies have demonstrated that poor asthma control leads to worsened asthma quality of life measures.35 Some patients remain difficult to control despite being treated with multiple controller medications, and these patients are high utilizers of healthcare resources,36 in part due to their increased rates of asthma exacerbations. Of interest, FeNO and FEV1/FVC ratio were not significantly different in those with and without an exacerbation. We previously projected that those with a higher FeNO and lower FEV1/FVC ratio would be more likely to have an exacerbation.
A proportion of participants had an exacerbation despite the addition of omalizumab (Figure 1). When the risk factors were evaluated in the population of children receiving GBT + omalizumab, there were some notable differences (Table 2B). Specifically, the allergic sensitization markers were largely no longer significant in comparing those with and without an exacerbation. This observation suggests attenuation of these risk factors by omalizumab. Previous studies have shown a greater omalizumab effect in allergen-sensitized patients6. This highlights the need for further elucidation of high fidelity biomarkers for predicting exacerbations in this group that continues to exacerbate despite omalizumab therapy.
In the GBT group, total IgE, age, recent exacerbation history, eosinophil percent, and treatment step were comparable in predicting exacerbations, with FEV1/FVC ratio, FeNO, and skin test positivity being less important (Figure 2). For the group receiving GBT + omalizumab, total IgE, and eosinophil percent were significantly less important relative to the rest of the predictors. In this group, we again see that omalizumab dampens the previous association of total IgE and eosinophils on predicting asthma exacerbations (Figure 2). Although the blood eosinophil percentage did not reach statistical significance in comparing those who exacerbated and those who did not exacerbate with omalizumab therapy, the total eosinophil count was statistically different. Therefore, a high total eosinophil count, perhaps approaching 400 cells/uL and above might be associated with a compromised effect of omalizumab, and an indicator to select another alternative immunomodulator, such as anti-IL-5 specific treatment.
Data derived from the receiver operating curves for the GBT group demonstrated an AUC of 0.76, consistent with fair ability to distinguish between those who exacerbated and those who did not with a low PPV and high NPV. The AUC for the GBT + omalizumab group was even lower, 0.65, with a similar low PPV and high NPV. While the cutoff score of 9 on the saEPI could be a useful point of discrimination, especially for the GBT group, this index requires further refinement. In addition, utilizing a risk score that is validated for omalizumab specifically will help to determine those most likely to fail this treatment, and should prompt the evaluation of other treatment strategies for those who fall into this risk category.
Finally, we utilized multivariate modeling to evaluate the utility of various grouped portions of the saEPI in relation to clinical access. The first model (which includes questionnaire data that could be easily obtained in any clinical setting) demonstrated good utility for exacerbation prediction in both groups. The model continued to maintain efficacy when easily obtainable blood markers including IgE and blood eosinophils were added for those in the guidelines based therapy group but not in the omalizumab group. However, this finding must be interpreted with caution in the context of the population studied. All of the patients in this study population had allergen skin test positivity, as well as moderate to severe asthma, which may have affected the utility of spirometry and skin testing for this population.
The question of when to step down asthma therapy is a difficult decision. Though the GINA asthma strategies recommend step down of therapy after 3 months of well-controlled asthma ,37 this can be difficult to assess, as children may have fewer triggers during the summer (and therefore have good control), and then have exacerbations during the fall return to school. While we did not evaluate this specifically in our studies, perhaps those with high blood eosinophils, high total IgE and an exacerbation in the prior year, should be approached cautiously, despite recent evidence of control. However, specific evaluation of the saEPI and its various components in this setting is needed.
There are several limitations to our study in regards to validation of the saEPI. We conducted a post-hoc analysis of the data in a smaller population than previously studied, leading to some loss of power to detect significant differences. The population studied consisted largely of urban and minority children and did not include children with intermittent or mild persistent asthma, which may limit generalizability to other groups. In addition, the inclusion criteria for the PROSE study restricted our patient population (limited by total IgE level, weight and allergen skin test positivity). Both groups had good adherence to guidelines based asthma therapy, which may have modified some of the risk factors that would be observed in a less adherent group. Treatment changes made during the summer run-in period (including systemic corticosteroids for exacerbations) may have modified some of the risk factors as measured at the beginning of the intervention period. Moreover, the number of exacerbations in the GBT + omalizumab group was relatively low, tempering conclusions about this group. There were a small number of children in the placebo group for comparison as well. Finally, the time period of the PROSE study limited our ability to validate risk factors outside of the fall season.
In conclusion, fall asthma exacerbations occurred in those receiving guidelines-based therapy in the absence and presence of additional omalizumab therapy in inner city children. In both groups, those who suffered an asthma exacerbation were more likely to be at higher ICS treatment steps, have higher blood eosinophils, and be at a younger age, than those who did not. The saEPI appears to be a good tool to evaluate which children are unlikely to have an asthma exacerbation in the fall. Further studies are needed to enhance the ability to predict an asthma exacerbation in the general population of children with asthma as well as alternative strategies, including the continued search for better biomarkers to predict exacerbations for those who continue to do so, despite omalizumab therapy.
Supplementary Material
Clinical Implications.
Fall seasonal exacerbations, despite guidelines-based asthma therapy including omalizumab, are associated with a higher saEPI and markers of allergic inflammation along with the history of a recent asthma exacerbation. The saEPI can reliably predict children unlikely to have an asthma exacerbation
Acknowledgments
Sources of Funding: This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract numbers HHSN272200900052C, HHSN272201000052I, 1UM1AI114271-01 and UM2AI117870. Additional support was provided by the National Center for Research Resources, and National Center for Advancing Translational Sciences, National Institutes of Health, under grants NCRR/NIH UL1TR000451, 1UL1RR025780, UL1TR000075 and NCATS/NIH UL1 TR000154, UL1TR001082, UL1 TR000077-04, NCATS/NIH UL1TR000040, UL1TR000150, and UL1TR001105, NIH NIAID 5R01AI098077, and UM1AI109565. The following were donated: omalizumab and matching placebo by Novartis, and fluticasone and matching placebo by Glaxo Smith Kline, under a clinical trial agreement with the University of Wisconsin-Madison; EpiPens by Mylan; and Ayr nasal rinse by B.F. Ascher & Company, Inc. None of these companies had a role in the development or approval of the protocol, conduct of the trial, data analysis, manuscript preparation, or the decision to submit for publication.
ABBREVIATIONS
- ACE
Asthma Control Evaluation
- CASI
Composite Asthma Severity Index
- EPR-3
Expert Panel Recommendations-3
- FeNO
Fractional Exhaled Nitric Oxide
- GBT
Guidelines Based Therapy
- ICAC
Inner City Asthma Consortium
- ICATA
The Inner-City Anti-IgE Therapy for Asthma
- ICS
Inhaled corticosteroids
- NIAID
National Institute of Allergy and Infectious Diseases
- NPV
Negative Predictive Value
- PPV
Positive Predictive Value
- PROSE
Preventative Omalizumab or Step-Up Therapy for Fall Exacerbations
- saEPI
Seasonal Asthma Exacerbation Predictive Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloom B, Cohen RA, Freeman G. Summary health statistics for u.s. Children: national health interview survey, 2011. Vital and health statistics Series 10, Data from the National Health Survey. 2012:1–88. [PubMed] [Google Scholar]
- 2.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. The Journal of allergy and clinical immunology. 2012;129:1229–35. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Gergen PJ, Togias A. Inner city asthma. Immunol Allergy Clin North Am. 2015;35:101–14. doi: 10.1016/j.iac.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busse WW. The National Institutes of Allergy and Infectious Diseases networks on asthma in inner-city children: an approach to improved care. The Journal of allergy and clinical immunology. 2010;125:529–37. doi: 10.1016/j.jaci.2010.01.036. quiz 38–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. The New England journal of medicine. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teach SJ, Gergen PJ, Szefler SJ, et al. Seasonal risk factors for asthma exacerbations among inner-city children. The Journal of allergy and clinical immunology. 2015;135:1465–73. e5. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covar RA, Szefler SJ, Zeiger RS, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. The Journal of allergy and clinical immunology. 2008;122:741–7. e4. doi: 10.1016/j.jaci.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gergen PJ, Mitchell H, Lynn H. Understanding the seasonal pattern of childhood asthma: results from the National Cooperative Inner-City Asthma Study (NCICAS) The Journal of pediatrics. 2002;141:631–6. doi: 10.1067/mpd.2002.127510. [DOI] [PubMed] [Google Scholar]
- 10.Reeves MJ, Lyon-Callo S, Brown MD, Rosenman K, Wasilevich E, Williams SG. Using billing data to describe patterns in asthma-related emergency department visits in children. Pediatrics. 2006;117:S106–17. doi: 10.1542/peds.2005-2000H. [DOI] [PubMed] [Google Scholar]
- 11.Silverman RA, Stevenson L, Hastings HM. Age-related seasonal patterns of emergency department visits for acute asthma in an urban environment. Annals of emergency medicine. 2003;42:577–86. doi: 10.1067/s0196-0644(03)00410-4. [DOI] [PubMed] [Google Scholar]
- 12.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: school children as disease vectors. The Journal of allergy and clinical immunology. 2006;117:557–62. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Silverman RA, Ito K, Stevenson L, Hastings HM. The relationship of fall school opening and emergency department asthma visits in a large metropolitan area. Archives of pediatrics & adolescent medicine. 2005;159:818–23. doi: 10.1001/archpedi.159.9.818. [DOI] [PubMed] [Google Scholar]
- 14.Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. The Journal of allergy and clinical immunology. 2015;136:1476–85. doi: 10.1016/j.jaci.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. The Journal of allergy and clinical immunology. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Lindeman RH, Merenda PF, Gold RZ. Introduction to bivariate and multivariate analysis. Scott, Foresman; 1980. [Google Scholar]
- 17.Walsh C, Nally RM. hier.part: Hierarchical Partitioning. R package version 1.0–4. 2013 https://CRAN.R-project.org/package=hier.part.
- 18.Lopez-Raton MR-AM, Cadarso-Suarez C, Gude-Sampedro F. Optimal Cutpoints : An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. J Stat Softw. 2014;61:1–36. [Google Scholar]
- 19.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- 20.Chevan A, Sutherland M. Hierarchical Partitioning. The American Statistician. 1991;45:90–6. [Google Scholar]
- 21.Fuhlbrigge AL. Predicting asthma exacerbations: peak expiratory flow revisited. The Journal of allergy and clinical immunology. 2011;127:1503–4. doi: 10.1016/j.jaci.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Chang TS, Lemanske RF, Jr, Guilbert TW, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. The journal of allergy and clinical immunology In practice. 2013;1:152–6. doi: 10.1016/j.jaip.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pescatore AM, Dogaru CM, Duembgen L, et al. A simple asthma prediction tool for preschool children with wheeze or cough. The Journal of allergy and clinical immunology. 2014;133:111–8. e1–13. doi: 10.1016/j.jaci.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Schatz M, Hsu JW, Zeiger RS, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology. 2014;133:1549–56. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Swern AS, Tozzi CA, Knorr B, Bisgaard H. Predicting an asthma exacerbation in children 2 to 5 years of age. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2008;101:626–30. doi: 10.1016/S1081-1206(10)60226-8. [DOI] [PubMed] [Google Scholar]
- 26.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. American journal of respiratory and critical care medicine. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 27.Wu AC, Tantisira K, Li L, et al. Predictors of symptoms are different from predictors of severe exacerbations from asthma in children. Chest. 2011;140:100–7. doi: 10.1378/chest.10-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chipps BE, Zeiger RS, Borish L, et al. Key findings and clinical implications from The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. The Journal of allergy and clinical immunology. 2012;130:332–42. e10. doi: 10.1016/j.jaci.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chipps BE, Zeiger RS, Dorenbaum A, et al. Assessment of asthma control and asthma exacerbations in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) observational cohort. Current respiratory care reports. 2012;1:259–69. doi: 10.1007/s13665-012-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haselkorn T, Szefler SJ, Simons FE, et al. Allergy, total serum immunoglobulin E, and airflow in children and adolescents in TENOR. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2010;21:1157–65. doi: 10.1111/j.1399-3038.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- 31.Haselkorn T, Zeiger RS, Chipps BE, et al. Recent asthma exacerbations predict future exacerbations in children with severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology. 2009;124:921–7. doi: 10.1016/j.jaci.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Miller MK, Lee JH, Miller DP, Wenzel SE, Group TS. Recent asthma exacerbations: a key predictor of future exacerbations. Respiratory medicine. 2007;101:481–9. doi: 10.1016/j.rmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Haselkorn T, Fish JE, Zeiger RS, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. The Journal of allergy and clinical immunology. 2009;124:895–902. e1–4. doi: 10.1016/j.jaci.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan SD, Wenzel SE, Bresnahan BW, et al. Association of control and risk of severe asthma-related events in severe or difficult-to-treat asthma patients. Allergy. 2007;62:655–60. doi: 10.1111/j.1398-9995.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Gould MK, Blanc PD, et al. Asthma control, severity, and quality of life: quantifying the effect of uncontrolled disease. The Journal of allergy and clinical immunology. 2007;120:396–402. doi: 10.1016/j.jaci.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Chipps BE, Szefler SJ, Simons FE, et al. Demographic and clinical characteristics of children and adolescents with severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology. 2007;119:1156–63. doi: 10.1016/j.jaci.2006.12.668. [DOI] [PubMed] [Google Scholar]
- 37. [Accessed 07/20/2016. 2016];Global Strategy for Asthma Management and Prevention-Updated. 2016 http://ginasthma.org/2016-gina-report-global-strategy-for-asthma-management-and-prevention/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.