Abstract
Aim
To assess the association of regular, unsupervised sports and exercise during pregnancy, by intensity level, with glycemic control in women with gestational diabetes (GDM).
Methods
Prospective cohort study of 971 women who, shortly after being diagnosed with GDM, completed a Pregnancy Physical Activity Questionnaire assessing moderate and vigorous intensity sports and exercise in the past 3 months. Self-monitored capillary glucose values were obtained for the 6 week period following the questionnaire, with optimal glycemic control defined ≥80% values meeting the targets <5.3 mmol/l for fasting and <7.8 mmol/l 1-hour after meals. Logistic regression estimated the odds of achieving optimal control; linear regression estimated activity level-specific least square mean glucose, as well as between-level mean glucose differences.
Results
For volume of moderate intensity sports and exercise [(MET · hours)/week], the highest quartile, compared to the lowest, had significantly increased odds of optimal control [OR= 1.82 (95% CI 1.06–3.14) P= 0.03]. There were significant trends for decreasing mean 1-hour post breakfast, lunch and dinner glycemia with increasing quartile of moderate activity (all P < 0.05). Any participation in vigorous intensity sports and exercise was associated with decreased mean 1-hour post breakfast and lunch glycemia (both P < 0.05). No associations were observed for fasting.
Conclusion
Higher volumes of moderate intensity sports and exercise, reported shortly after GDM diagnosis, were significantly associated with increased odds of achieving glycemic control. Clinicians should be aware that unsupervised moderate intensity sports and exercise performed in mid-pregnancy aids in subsequent glycemic control among women with GDM.
Keywords: Pregnancy, physical activity, exercise, gestational diabetes, glycemic control, capillary glucose
Introduction
Gestational diabetes mellitus (GDM), defined as carbohydrate intolerance first recognized during pregnancy, is associated with an increased risk of adverse perinatal outcomes [1], including fetal overgrowth [2, 3]. Achieving optimal glycemic control reduces the risk of these adverse outcomes [4, 5], and when achieved without supplementary insulin, reduces women’s risk of diabetes following delivery [6].
Nutritional and exercise counseling constitute first line therapy to stabilize pregnancy glucose levels [7], but consensus regarding the impact of exercise on glycemic control in women with GDM is lacking [8]. The American College of Obstetricians and Gynecologists recommends 20–30 minutes of moderate intensity exercise a day on most or all days of the week during pregnancy and advises that exercise during pregnancy can lower glucose levels in women with GDM [9].
Randomized controlled trials evaluating the effects of structured, mostly supervised exercise interventions on glucose levels in women with GDM have been conducted among small samples (ranging from 19 to 64) [10–16]. Yet the impact of non-prescribed, unsupervised, regular exercise during pregnancy on glycemic control in women with GDM remains largely unknown. This cohort study sought to fill gaps in the literature by examining the association of several types and intensities of physical activity during pregnancy with glycemic control in a large, diverse cohort of women with GDM. We hypothesized that intentional activity, specifically sports and exercise activity, would be associated with better glycemic control. Given the scarcity of studies investigating vigorous intensity activity during pregnancy, we estimate the association of sports and exercise activity with glycemic control separately by intensity level.
Material and Methods
Study Setting
This study utilizes baseline physical activity data obtained shortly after GDM diagnosis and subsequent self-monitored capillary glucose data for women included in the GDM’s Effects on Moms (GEM) study, a cluster randomized trial of the comparative effectiveness of diabetes prevention strategies for women with GDM implemented during the early postpartum period [17]. Briefly, 44 medical facilities were randomized to two distinct postpartum diabetes prevention strategies addressing postpartum weight retention for women with GDM delivered at the health system level: postpartum mailed recommendations (usual care) or usual care plus one prenatal newsletter and a 13 session telephone-based counseling program for weight management to be completed during the 6 months postpartum. Prenatal care and GDM management were identical across treatment groups. GEM took place at Kaiser Permanente Northern California, a large integrated health system. Kaiser Permanente Northern California’s membership includes approximately 30% of the geographic area served and is representative of the surrounding population in regards to sociodemographic characteristics, except the lower extremes of income and education [18]. The study was approved by the Kaiser Permanente Northern California institutional review board.
During the study period, all pregnant women at Kaiser Permanente Northern California received a random 50g 1-hour glucose screening test at 24–28 weeks gestation, followed by a fasting 100g 3-hour oral glucose tolerance test (OGTT) if the screening test was abnormal (i.e., 1-hour glucose ≥ 140 mg/dl). Women with two or more values during the 100-g, 3-h OGTT at or above the Carpenter and Coustan criteria thresholds (i.e., 5.3 mmol/l for fasting, 10.1 mmol/l for 1-hour, 8.7 mmol/l for 2-hour, and 7.8 mmol/l for 3-hour) were diagnosed with GDM, as recommended by the American College of Obstetricians and Gynecologists during the study period [19, 20]. From March 2011 to March 2012, all women with a diagnosis of GDM by the Carpenter and Coustan criteria who were 18 years of age or older were identified in the Kaiser Permanente Northern California electronic health record system. Women were invited by telephone to participate in the baseline (i.e., pregnancy) GEM survey soon after GDM diagnosis to obtain data not available in the electronic heath records [17].
Physical Activity
The baseline GEM survey included a self-administered Pregnancy Physical Activity Questionnaire [17, 21, 22] that assessed key components of physical activity (i.e., type, frequency, duration and intensity) and is recommended for use in pregnancy and the postpartum [23]. The GEM Pregnancy Physical Activity Questionnaire asked women to report time spent in 36 population-appropriate activities over the past three months (questions on yoga/Pilates, use of cardiovascular exercise machines, aerobic exercise classes, weight lifting/resistance exercises and team sports were added to the original Pregnancy Physical Activity Questionnaire). Activities not addressed by the questionnaire were captured by an open-ended question. Participants selected one of six response options for the amount of time spent in each activity (e.g., none, < ½ hour per day, ½ to almost 1 hour per day, 1 to almost 2 hours per day, 2 to almost 3 hours per day, or ≥ 3 hours per day). The mid-point of the time category selected for each activity (representing duration and frequency) was multiplied by the intensity, measured in METs (metabolic equivalent), assigned to that activity to arrive at an estimate of the volume of physical activity [(MET · min)/week]. MET values for walking and light to moderate intensity household tasks came from field-based measurements of pregnant women [24]; otherwise Compendium-based MET values were used [25].
Volume of physical activity was estimated for five activity constructs, including moderate and vigorous intensity sports and exercise activity, transportation activity (i.e., walking specifically to go places), household/caregiving activity, sedentary activity and total physical activity. Volume from 10 items (3.2–6 MET) were combined for moderate intensity sports and exercise activity; 2 items (6.5 and 7 MET) for vigorous intensity sports and exercise activity; 2 items (2.5 and 4 MET) for transportation activity; 12 items (2–4.4 MET) household/caregiving activity; 5 items (1–1.6 MET) for sedentary activity; and 31 items (≥ 2 MET) were combined for total physical activity. All activities were categorized as quartiles, except for vigorous intensity sports and exercise activity which was categorized as any versus none because few women reported any participation in activity of that intensity. The lowest activity level (i.e., the first quartile, or none for vigorous intensity sports and exercise activity) served as the reference.
Glycemic Control
The outcome of interest was glycemic control in the 6 week period following completion of the Pregnancy Physical Activity Questionnaire. Capillary glucose data were obtained from a clinical database maintained by the Kaiser Permanente Northern California Regional Perinatal Service Center, which provides telephone-based case management to nearly all women with GDM in this setting [17, 26]. Women with GDM are provided a glucometer and instructed to self-monitor capillary glucose in the morning in the fasting state, as well as 1-hour after breakfast, lunch, and dinner [27, 28]. Glycemic control was defined as ≥80% of all capillary glucose measurements meeting the targets recommended across the clinics of Kaiser Permanente Northern California: <5.3 mmol/l for fasting and <7.8 mmol/l for 1-hour after meals [27, 28]. All GEM participants took part in the case management program, which instructs women to record glucose measurements in tracking booklets provided by the Center. Glucose measurements are then reported to the Center staff during weekly telephone counseling calls and data recorded in the Center’s Patient Reported Capillary Glucose Clinical Database. Fasting, 1-hour post breakfast, 1-hour post lunch and 1-hour post dinner glucose values reported in the 6 week period following completion of the Pregnancy Physical Activity Questionnaire were individually averaged.
Covariates
Prepregnancy weight was available in the electronic health record system for the majority of the cohort (95%); otherwise self-reported prepregnancy weight was obtained from the GEM survey. Height measured by Kaiser Permanente Northern California clinical staff was obtained from the electronic health record system for 99% of the analytic cohort; otherwise self-reported height was obtained from the GEM survey. Prepregnancy body mass index (BMI) was calculated as the prepregnancy weight (kg) divided by the height (m) squared.
Data on maternal age, race-ethnicity, and household income were obtained from the GEM survey. The GEM survey also included a semi-quantitative food frequency questionnaire, the Block 2005 [29], which provided an estimate of average daily glycemic index [30]. Fasting glucose on the diagnostic 100g, 3-hr oral glucose tolerance test (a proxy of GDM severity) was obtained from the electronic health record system. Gestational age, contraindications to physical activity in pregnancy [9] and prescriptions for diabetes medications were also obtained from the electronic health record system.
Participants
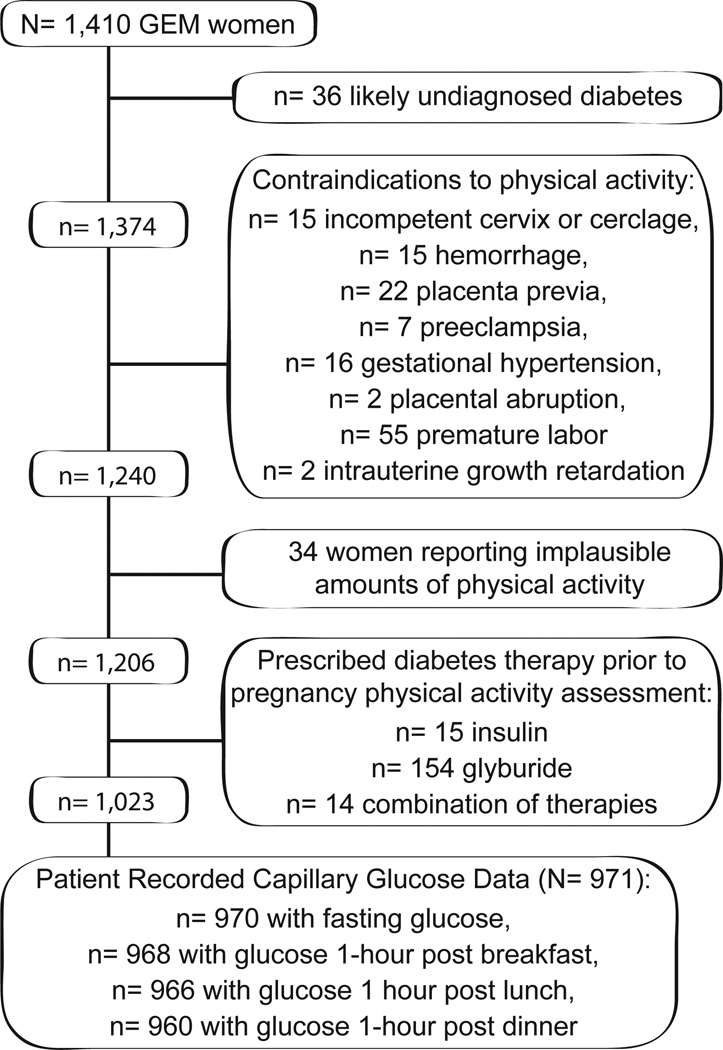
In total, 1,580 GEM women had Pregnancy Physical Activity Questionnaire data available. There were 1,410 (89%) GEM women with complete Pregnancy Physical Activity Questionnaire data who delivered singleton neonates that were eligible for the current study. We excluded 36 women likely to have undiagnosed diabetes prior to pregnancy (i.e., fasting glucose >7.0 mmol/l or any glucose > 11.1 mmol/l on two or more occasions during pregnancy); 134 women with contraindications to physical activity in pregnancy [9] diagnosed prior to completing the Pregnancy Physical Activity Questionnaire; and 34 women reporting implausibly high levels of physical activity (e.g., > 24 hours per day of total activity, > 13.8 hours per day of total moderate intensity activity). We then excluded 183 women prescribed diabetes medication prior to completing the Pregnancy Physical Activity Questionnaire (n= 15 were prescribed insulin, n= 154 glyburide, and n= 14 a combination of therapies; none were prescribed metformin) to avoid potential bias (i.e., disease severity inducing both increases in physical activity and improved glycemic control due to medication). Of the remaining 1,023 women not using any hyperglycemic medication at the time of the physical activity assessment, 971 (95%) had fasting, 1-hour post breakfast, 1-hour post lunch and/or 1-hour post dinner capillary glucose data available in the 6 week observation period and thus comprised the analytic cohort (Figure 1).
Figure 1.
Cohort assembly, the Gestational Diabetes’ Effects on Moms Study, Kaiser Permanente Northern California, 2011–2012
Statistical Methods
Logistic regression models (PROC LOGISTIC in SAS) were used to estimate the association of activity level (lowest level serving as the reference) with the odds of achieving optimal glycemic control and corresponding 95% confidence intervals, in addition to assessing trends with increasing activity level. Separate multiple linear regression models were used to test for changes in mean glucose values with increasing level of activity by time point (PROC GLM in SAS): fasting glucose and glucose measured 1-hour post breakfast, lunch and dinner were examined separately. Activity level-specific least square means and between-level mean differences were calculated (LSMEANS option; lowest activity level serving as the reference), along with their 95% confidence intervals. Mean MET minutes per week were calculated for each quartile and then modeled as a continuous variable to test for trend.
All models were adjusted for the following covariates, selected a priori based on previously observed associations with pregnancy hyperglycemia: age, prepregnancy BMI, race-ethnicity, education, household income, average daily dietary glycemic index, fasting glucose on the 100g, 3-hr oral glucose tolerance test. In addition, we included gestational age at the 100g, 3-hr oral glucose tolerance test, number of glucose values reported for that outcome in the 6-week observation period, and GEM randomization condition. The initiation of diabetes medications in the 6 week follow up period was hypothesized to be on the causal pathway between physical activity and glycemic control, and thus was not included as an adjustment variable. The cut point for statistical significance was set at P < 0.05.
Assessment of Potential Biases
We assessed potential selection bias by comparing the baseline characteristics of GEM women included in the study vs. excluded. Among women included in the study, we assessed potential reporting bias by comparing the baseline characteristics of those in the lowest quartile for total number of capillary glucose measurements reported in the observation period vs. the remainder of the study cohort.
As compared to GEM women excluded from this study, women included differed by education and race-ethnicity (both P < 0.05): women included were more highly educated (22% vs. 15% had postgraduate education), more likely to be Asian (45% vs. 34%) and less likely to be Hispanic (19% vs. 24%). Among those included, women in the lowest quartile for total number of capillary glucose measurements reported in the observation period did not differ significantly from the remainder of the study cohort in terms of baseline characteristics (all P > 0.05).
Results
The mean age of the study cohort was 31.9 years (4.9 SD) and the median volume of moderate intensity sports and exercise activity reported was 575 (MET ∙ min)/week (861 IQR). The individually averaged cohort mean capillary glucose values were 4.7 mmol/l (SD 0.4) for fasting, 6.5 mmol/l (SD 0.5) for 1-hour post breakfast, 6.7 mmol/l (SD 0.5) for 1-hour post lunch and 6.7 mmol/l (SD 0.5) for 1-hour post dinner.
The characteristics of the cohort by quartile of moderate intensity sports and exercise activity are presented in Table 1. Women in the highest quartile of moderate intensity sports and exercise activity were more highly educated than those in the lowest quartile (P= 0.04). Fasting glucose on the 100g, 3-hr oral glucose tolerance test (i.e., at baseline) was higher for women in the highest quartile of moderate intensity sports and exercise activity than those in the lower quartiles (P= 0.02). As compared to women in the lowest (first) and highest (fourth) quartiles of moderate intensity sports and exercise activity, those in the middle (second and third) quartiles had lower fasting glucose on the 100g, 3-hour OGTT (P= 0.02). There was the suggestion of a difference in prepregnancy BMI by quartile: more normal weight women were in the highest than the lowest quartile of moderate intensity sports and exercise activity (P= 0.05). No other baseline characteristic differed between quartiles (Table 1).
Table 1.
Cohort characteristics by quartile of moderate intensity sports and exercise activity; the Gestational Diabetes’ Effects on Moms Study, Kaiser Permanente Northern California, 2011–2012.
| Moderate Intensity Sports and Exercise Activity |
|||||
|---|---|---|---|---|---|
| Quartile 1 N=241 |
Quartile 2 N=241 |
Quartile 3 N=246 |
Quartile 4 N=243 |
||
| n (%) | n (%) | n (%) | n (%) | P* | |
| Age (years) | 0.32 | ||||
| 18 – 24 | 12 (5.0) | 21 (8.7) | 8 (3.3) | 14 (5.8) | |
| 25 – 29 | 64 (26.6) | 57 (23.7) | 65 (26.4) | 66 (27.2) | |
| 30 – 34 | 93 (38.6) | 86 (35.7) | 94 (38.2) | 100 (41.2) | |
| ≥ 35 | 72 (29.9) | 77 (32.0) | 79 (32.1) | 63 (25.9) | |
| Race-ethnicity | 0.19 | ||||
| Non-Hispanic White | 45 (18.7) | 54 (22.4) | 64 (26.0) | 63 (25.9) | |
| African American | 5 (2.1) | 4 (1.7) | 5 (2.0) | 9 (3.7) | |
| Hispanic | 56 (23.2) | 41 (17.0) | 42 (17.1) | 41 (16.9) | |
| Asian | 102 (42.3) | 123 (51.0) | 112 (45.5) | 99 (40.7) | |
| Multiracial | 26 (10.8) | 15 (6.2) | 19 (7.7) | 27 (11.2) | |
| Other | 7 (2.9) | 4 (1.7) | 4 (1.6) | 4 (1.7) | |
| Household Income | 0.21 | ||||
| < $50,000 | 68 (30.2) | 56 (25.2) | 63 (27.0) | 49 (21.5) | |
| $50,000 – $79,999 | 57 (25.3) | 40 (18.0) | 55 (23.6) | 54 (23.7) | |
| $80,000 – $149,999 | 74 (32.9) | 88 (39.6) | 76 (32.6) | 86 (37.7) | |
| ≥ $150,000 | 26 (11.6) | 38 (17.1) | 39 (16.7) | 39 (17.1) | |
| Pre-pregnancy BMI, kg/m2 | 0.05 | ||||
| < 18.5 | 2 (0.8) | 2 (0.8) | 6 (2.4) | 3 (1.2) | |
| 18.5 – 24.9 | 74 (31.0) | 92 (38.2) | 95 (38.6) | 91 (37.6) | |
| 25.0 – 29.9 | 86 (36.0) | 70 (29.1) | 76 (30.9) | 61 (25.2) | |
| 30.0 – 34.9 | 37 (15.5) | 46 (19.1) | 44 (17.9) | 38 (15.7) | |
| ≥ 35.0 | 40 (16.7) | 31 (12.9) | 25 (10.2) | 49 (20.3) | |
| Education | 0.04 | ||||
| High school or less | 40 (16.7) | 31 (12.9) | 34 (13.9) | 31 (12.8) | |
| Some college | 91 (37.9) | 69 (28.6) | 65 (26.6) | 75 (30.9) | |
| 4 years of college | 72 (30.0) | 88 (36.5) | 84 (34.4) | 72 (29.6) | |
| Postgraduate | 37 (15.4) | 53 (22.0) | 61 (25.0) | 65 (26.8) | |
| GEM Treatment Group | 0.58 | ||||
| Intervention | 118 (49.0) | 125 (51.9) | 136 (55.3) | 127 (52.3) | |
| Usual Care | 123 (51.0) | 116 (48.1) | 110 (44.7) | 116 (47.7) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | P* | |
| Daily Glycemic Index | 49.3 (4.6) | 49.1 (4.0) | 48.7 (3.7) | 48.5 (3.8) | 0.13 |
| 3-hr Oral Glucose Tolerance Test | |||||
| Fasting value (mmol/l) | 5.0 (0.5) | 4.9 (0.5) | 4.9 (0.5) | 5.0 (0.5) | 0.02 |
| 1-hr value (mmol/l) | 10.9 (1.1) | 10.9 (1.3) | 10.8 (1.4) | 11.0 (1.3) | 0.32 |
| 2-hr value (mmol/l) | 9.8 (1.3) | 9.7 (1.2) | 9.7 (1.2) | 9.7 (1.3) | 0.77 |
| 3-hr value (mmol/l) | 7.2 (1.6) | 7.1 (1.7) | 6.9 (1.9) | 7.1 (1.8) | 0.11 |
P excludes missing, Chi-square or one way ANOVA for group differences by quartile
Adjusted odds ratio (OR) estimates for the association of moderate and vigorous intensity sports and exercise activity with achieving optimal glycemic control are presented in Table 2. As compared with women in the lowest (first) quartile for volume of moderate intensity sports and exercise, women in the highest (fourth) quartile had 82% increased odds of achieving optimal glycemic control [OR= 1.82 (95% CI 1.06, 3.14)]. There was the suggestion of a 65% increased odds of achieving optimal glycemic control [OR= 1.65 (95% CI 0.97, 2.82)] for women in the highest (fourth) quartile compared to the lowest (first) quartile, but the estimate did not attain statistical significance. There was a significant trend for increasing odds of achieving optimal glycemic control with increasing quartile of volume of moderate intensity sports and exercise (P= 0.02). Vigorous intensity sports and exercise was not statistically significantly associated with achieving optimal glycemic control (Table 2), nor were any of the other domains of physical activity investigated (see e-supplement Table 1).
Table 2.
Adjusteda Odds Ratiosb (95% Confidence Intervals) for Achieving Optimal Glycemic Controlc by Quartile of Sports and Exercise Activity: the Gestational Diabetes’ Effects on Moms Study, Kaiser Permanente Northern California.
| Achieving Optimal Glycemic Controlc | ||
|---|---|---|
| Prevalence % | ORab (95% Cl) | |
|
Moderate Intensity Sports and Exercise Activity |
||
| Quartile 1 | 81.3 | 1.00 (referent) |
| Quartile 2 | 82.6 | 1.04 (0.63–1.73) |
| Quartile 3 | 87.0 | 1.65 (0.97–2.82) |
| Quartile 4 | 87.2 | 1.82* (1.06–3.14) |
| P Trend | .02 | |
|
Vigorous Intensity Sports and Exercise Activity |
||
| None (= 0) | 83.3 | 1.00 (referent) |
| Any (≥ 0) | 85.8 | 1.25 (0.81, 1.92) |
| P Trend | 0.32 | |
Adjusted for maternal age, prepregnancy BMI, race-ethnicity, education, household income, fasting glucose value and gestational age at the 100g 3-hr oral glucose tolerance test, dietary glycemic index, number of glucose values reported in the 6-week observation period, and randomized treatment group.
Quartile 1 serving as the reference; none as the reference for vigorous intensity sports and exercise.
Optimal Glycemic Control: ≥80% of all capillary glucose measurements meeting institutional targets (below 5.3 mmol/l for fasting and below 7.8 mmol/l 1-hour after meals)
P < 0.05
Adjusted group mean capillary glucose values by level of moderate and vigorous intensity sports and exercise activity, as well as the mean differences between activity levels, are presented in Table 3. Women in the fourth quartile of moderate intensity sports and exercise activity had significantly lower adjusted mean 1-hour postprandial glucose, as compared to the first quartile, for breakfast [mean difference −0.16 mmol/l (95% CI −0.26, −0.06), P= 0.002] and lunch [mean difference −0.14 mmol/l (95% CI −0.23, −0.05), P= 0.003], with significant trends for decreasing mean 1-hour post breakfast, lunch and dinner glucose with increasing quartile of volume moderate intensity sports and exercise (all P< 0.05). Compared with the first quartile of volume of moderate intensity sports and exercise, the third quartile had significantly lower adjusted mean 1-hour post breakfast [mean difference −0.10 mmol/l (95% CI −0.20, −0.003), P= 0.04] and the second quartile had significantly lower adjusted mean 1-hour post lunch glucose [mean difference −0.10 mmol/l (95% CI −0.20, −0.01), P=0.02].
Table 3.
Adjusteda Mean Capillary Glucose Values and Mean Differences in Capillary Glucose between Levels of Moderate and Vigorous Intensity Sports and Exercise Activityb the Gestational Diabetes’ Effects on Moms Study, Kaiser Permanente Northern California.
| Fasting Glucose, mmol/l N=898 |
1-h post Breakfast Glucose, mmol/l N=896 |
1-h post Lunch Glucose, mmol/l N=895 |
1-h post Dinner Glucose, mmol/l N=889 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean Capillary Glucose (95% CI) |
Mean Difference in Capillary Glucose (95% CI) |
N | Mean Capillary Glucose (95% CI) |
Mean Difference in Capillary Glucose (95% CI) |
N | Mean Capillary Glucose (95% CI) |
Mean Difference in Capillary Glucose (95% CI) |
N | Mean Capillary Glucose (95% CI) |
Mean Difference in Capillary Glucose (95% CI) |
|
|
Moderate Intensity Sports and Exercise Activity |
||||||||||||
| Quartile 1 | 220 | 4.67 (4.62, 4.73) |
- | 220 | 6.57 (6.48, 6.66) |
- | 220 | 6.69 (6.60, 6.77) |
- | 216 | 6.80 (6.71, 6.89) |
- |
| Quartile 2 | 220 | 4.66 (4.61, 4.72) |
−0.01 (−0.07, 0.05) |
221 | 6.53 (6.43, 6.62) |
−0.05 (−0.14, 0.05) |
221 | 6.58 (6.50, 6.67) |
−0.10* (−0.20,−0.01) |
221 | 6.78 (6.69, 6.87) |
−0.02 (−0.11, 0.08) |
| Quartile 3 | 232 | 4.6 (4.62, 4.72) |
−0.001 (−0.06, 0.06) |
231 | 6.47 (6.38, 6.56) |
−0.10* (−0.20,−0.003) |
230 | 6.65 (6.57, 6.74) |
−0.04 (−0.13, 0.05) |
229 | 6.80 (6.71, 6.88) |
−0.002 (−0.09, 0.09) |
| Quartile 4 | 226 | 4.66 4.61, 4.71) |
−0.02 (−0.07, 0.04) |
224 | 6.41 (6.33, 6.50) |
−0.16* (−0.26,−0.06) |
224 | 6.55 (6.47, 6.63) |
−0.14* (−0.23,−0.05) |
223 | 6.71 (6.62, 6.79) |
−0.09 (−0.18, 0.00) |
| P Trend | 0.65 | <.001 | 0.01 | 0.04 | ||||||||
|
Vigorous Intensity Sports and Exercise Activity |
||||||||||||
| None (= 0) | 644 | 4.68 (4.63, 4.72) |
- | 643 | 6.51 (6.44, 6.59) |
- | 642 | 6.64 (6.57, 6.70) |
- | 638 | 6.78 (6.72, 6.85) |
- |
| Any (≥ 0) | 254 | 4.64 (4.59, 4.69) |
−0.03 (−0.08, 0.01) |
253 | 6.43 (6.34, 6.52) |
−0.08* (−0.16,−0.01) |
253 | 6.56 (6.48, 6.64) |
−0.08* (−0.15,−0.01) |
251 | 6.72 (6.64, 6.81) |
−0.06 (−0.13, 0.01) |
| P Trend | 0.14 | 0.04 | 0.04 | 0.11 | ||||||||
Adjusted for maternal age, prepregnancy BMI, race-ethnicity, education, household income, fasting glucose value and gestational age at the 100g 3-hr oral glucose tolerance test, dietary glycemic index, number of glucose values reported in the 6-week observation period, and randomized treatment group.
Quartile 1 serving as the reference; none as the reference for vigorous intensity sports and exercise.
P < 0.05
About one quarter of the women (n= 277) participated in any vigorous intensity sports and exercise; these women had significantly lower adjusted mean 1-hour postprandial glucose, as compared to those participating in none, for breakfast [mean difference −0.08 mmol/l (95% CI −0.16, −0.01), P= 0.04] and lunch [mean difference −0.08 mmol/l (95%CI −0.15, −0.01), P= 0.04] (Table 3).
See e-supplement Table 2 for the other types of physical activity investigated.
Discussion
In this large, prospective cohort study estimating the impact of regular, unsupervised physical activity during pregnancy on subsequent glycemic control in women with GDM, moderate intensity sports and exercise demonstrated a positive association with optimal glycemic control. Women in the highest quartile for volume of moderate intensity sports and exercise [e.g., those who reported ‘walking quickly for fun or exercise’ (at 4.6 METs) for 35 minutes per day or more] had almost twice the odds of achieving optimal glycemic control as compared to women in the lowest quartile. This finding is consistent with the observed trends of decreasing 1-hour postprandial glucose at breakfast, lunch, and dinner with increasing moderate intensity spots and exercise activity.
Activity in the sports and exercise domain has long been recognized for its beneficial effects on insulin sensitivity outside of pregnancy [31]. Exercise stimulates glucose uptake in the muscle in both insulin dependent and insulin independent ways. Insulin independent mechanisms are less well characterized, but evident from the increased glucose uptake by muscle tissue in response to exercise among insulin resistant individuals [31].
The few randomized controlled trials of structured, mostly supervised exercise interventions in women with GDM have had small sample sizes, ranging from 19 to 64 [10–16], and thus limited power and generalizability [28]. Taken together, the results of these trials suggest that structured, supervised interventions of moderate intensity exercise likely improve glucose levels in women with GDM. The current study corroborates these findings and extends them to the domain of unsupervised moderate intensity sports and exercise in an unselected population.
In the current study, while moderate in intensity sports and exercise activity was associated with optimal glycemic control and postprandial capillary glucose levels, we did not observe associations with fasting glucose levels. This is consistent with the majority of prior randomized trials examining exercise interventions and glycemic control in GDM, only one of which found a significant reduction in fasting glucose [10]. Our finding is also consistent with the results of a recent meta-analysis of studies among adults with type 2 diabetes which found that exercise was associated with reduced postprandial glucose but not fasting glucose [32]. This lack of association may be explained, in part, by the increased hepatic insulin resistance in the overnight-fasting state—and resulting reduction in the suppression of hepatic gluconeogenesis—observed in women with GDM [33], which may not be impacted by physical activity. While the cellular determinants of insulin resistance during pregnancy are not completely understood, defects in the insulin signaling cascade of skeletal muscle are believed to play a role [33] and may be where physical activity exerts its effects. Although previous studies have shown that elevated fasting glucose during the oral glucose tolerance test used to diagnose GDM is a stronger predictor of fetal macrosomia than post challenge glucose levels [2, 3], once GDM is diagnosed, elevated postprandial glucose is more predictive of fetal macrosomia and morbidity than fasting values [28]. Therefore it is plausible that increasing participation in moderate intensity sports and exercise among women with GDM may result in more favorable infant outcomes.
Strengths of the current study include the large and racially and ethnically diverse cohort of women with GDM; the wide range of activities assessed; the ability to control for potential confounders, particularly diet; and outcome data that were collected in conjunction with clinical care. We found no difference in baseline characteristics between women with the fewest capillary glucose measurements and the rest of the cohort, thereby ruling out potential bias due to differentially missing outcome data. As such, the current study provides useful and practical information for providers in regards to recommending moderate intensity sports and exercise activity to patients with GDM.
The study also has limitations. The Pregnancy Physical Activity Questionnaire utilized in this study was designed as a ranking tool (i.e., leas to most active) [21], not an absolute measure of energy expenditure. Therefore, future studies with objective physical activity measures will be better able to describe the shape of the relationship with pregnancy glycemia and identify the ideal dose of activity needed for achieving optimal glycemic control. Self-report of physical activity may have resulted in over-reporting, although it is likely to have been non-differential by pregnancy glycemia due to the prospective design, and therefore would result in appropriate ranking of women in quartiles from least to most active. Physical activity was assessed only once during pregnancy: we were unable to account for changes in physical activity prompted by the diagnosis and clinical management of GDM, as well as reductions in physical activity with advancing gestation. Therefore, it should be emphasized that our findings only pertain to physical activity performed before or near the GDM diagnosis. The study’s use of self-reported blood glucose values is also a limitation. Misclassification of pregnancy hyperglycemia, as well as potential departures from the postprandial glucose measurement protocol (i.e., postprandial measurements made too early or too late), are likely to have been non-differential across activity quartiles, thus our study’s estimates would underestimate the ‘true’ associations. Only fasting and 1-hour postprandial glucose measurements were available for this study (i.e., no 2-hour measurements), although 1-hour OGTT values have been shown to correlate well with 2-hour OGTT values [34], so we would expect similar findings for 2-hour postprandial glucose. In addition, as with any observational study, there is the potential for bias due to unmeasured confounding.
Conclusions
In a large, diverse cohort of women with GDM, unsupervised moderate intensity sports and exercise activity was associated with an eighty percent increased odds of achieving optimal glycemic control, largely driven by its impact on postprandial glycemic control. The findings of this study support the utility of regular, unsupervised, moderate intensity sports and exercise activity for controlling pregnancy glycemia, as has been reported for structured exercise interventions assessed in controlled experiments among women with GDM. Clinicians should thus be aware that unsupervised moderate intensity sports and exercise activity aids in the control of pregnancy glycemia among women with GDM. Additional research is needed to determine the ideal dose of moderate intensity sports and exercise for achieving optimal glycemic control, elucidate the mechanisms by which moderate intensity sports and exercise activity impact glycemic control and examine the impact of this type of activity on perinatal outcomes in women with GDM.
Supplementary Material
Acknowledgments
We thank the participants and staff of the Gestational Diabetes’ Effects on Moms (GEM) study for their contributions.
Funding:
This research was funded by a Kaiser Permanente Community Benefit Research Grant to Dr. Ehrlich, grant R01 HS019367 from the Agency for Healthcare Research and Quality to Dr. Ferrara and grant R18 DK067334 from the National Institute of Diabetes and Digestive and Kidney Diseases to Dr. Ferrara. Dr. Ferrara is also supported by grant P30 DK092924 from the National Institute of Diabetes Digestive and Kidney Diseases, Dr. Ehrlich by grant K01DK105106 from the National Institute of Diabetes Digestive and Kidney Diseases and Dr. Brown by grant K01 DK099404 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- GDM
gestational diabetes
- GEM
GDM’s Effects on Moms study
- MET
metabolic equivalent
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Presentation Information: This work was presented as a poster presentation at the American Diabetes Association’s 75th Scientific Sessions in Boston, Massachusetts (June 5–9, 2015).
References
- 1.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286(20):2516–2518. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara A, Weiss NS, Hedderson MM, Quesenberry CP, Jr, Selby JV, Ergas IJ, et al. Pregnancy plasma glucose levels exceeding the American Diabetes Association thresholds, but below the National Diabetes Data Group thresholds for gestational diabetes mellitus, are related to the risk of neonatal macrosomia, hypoglycaemia and hyperbilirubinaemia. Diabetologia. 2007;50(2):298–306. doi: 10.1007/s00125-006-0517-8. [DOI] [PubMed] [Google Scholar]
- 3.Ehrlich SF, Crites YM, Hedderson MM, Darbinian JA, Ferrara A. The risk of large for gestational age across increasing categories of pregnancy glycemia. Am J Obstet Gynecol. 2011;204(3):240.e1–246.e1. doi: 10.1016/j.ajog.2010.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 5.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baptiste-Roberts K, Barone BB, Gary TL, Golden SH, Wilson LM, Bass EB, et al. Risk factors for type 2 diabetes among women with gestational diabetes: a systematic review. Am J Med. 2009;122(3):207.e4–214.e4. doi: 10.1016/j.amjmed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 8.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33(12):2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol. 2015;126(6):e135–e142. doi: 10.1097/AOG.0000000000001214. [DOI] [PubMed] [Google Scholar]
- 10.Jovanovic-Peterson L, Durak EP, Peterson CM. Randomized trial of diet versus diet plus cardiovascular conditioning on glucose levels in gestational diabetes. Am J Obstet Gynecol. 1989;161(2):415–419. doi: 10.1016/0002-9378(89)90534-6. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Patterson A, Martin E, Ubeda J, Maria MA, de Leiva A, Corcoy R. Evaluation of light exercise in the treatment of gestational diabetes. Diabetes Care. 2001;24(11):2006–2007. doi: 10.2337/diacare.24.11.2006. [DOI] [PubMed] [Google Scholar]
- 12.de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2010;203(6):556.e1–556.e1. doi: 10.1016/j.ajog.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Davenport MH, Mottola MF, McManus R, Gratton R. A walking intervention improves capillary glucose control in women with gestational diabetes mellitus: a pilot study. Appl Physiol Nutr Metab. 2008;33(3):511–517. doi: 10.1139/H08-018. [DOI] [PubMed] [Google Scholar]
- 14.Avery MD, Leon AS, Kopher RA. Effects of a partially home-based exercise program for women with gestational diabetes. Obstet Gynecol. 1997;89(1):10–15. doi: 10.1016/s0029-7844(97)84256-1. [DOI] [PubMed] [Google Scholar]
- 15.Halse RE, Wallman KE, Newnham JP, Guelfi KJ. Home-based exercise training improves capillary glucose profile in women with gestational diabetes. Med Sci Sports Exerc. 2014;46(9):1702–1709. doi: 10.1249/MSS.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 16.Bung P, Artal R, Khodiguian N, Kjos S. Exercise in gestational diabetes. An optional therapeutic approach? Diabetes. 1991;40(Suppl 2):182–185. doi: 10.2337/diab.40.2.s182. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara A, Hedderson MM, Brown SD, Albright CL, Ehrlich SF, Tsai AL, et al. The Comparative Effectiveness of Diabetes Prevention Strategies to Reduce Postpartum Weight Retention in Women With Gestational Diabetes Mellitus: The Gestational Diabetes' Effects on Moms (GEM) Cluster Randomized Controlled Trial. Diabetes Care. 2016;39(1):65–74. doi: 10.2337/dc15-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 19.Committee opinion no. 504: Screening and diagnosis of gestational diabetes mellitus. Obstet Gynecol. 2011;118(3):751–753. doi: 10.1097/AOG.0b013e3182310cc3. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 21.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and Validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004;36(10):1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich SF, Sternfeld B, Krefman AE, Hedderson MM, Brown SD, Mevi A, et al. Moderate and Vigorous Intensity Exercise During Pregnancy and Gestational Weight Gain in Women with Gestational Diabetes. Matern Child Health J. 2016;20(6):1247–1257. doi: 10.1007/s10995-016-1926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evenson KR, Chasan-Taber L, Symons Downs D, Pearce EE. Review of self-reported physical activity assessments for pregnancy: summary of the evidence for validity and reliability. Paediatr Perinat Epidemiol. 2012;26(5):479–494. doi: 10.1111/j.1365-3016.2012.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chasan-Taber L, Freedson PS, Roberts DE, Schmidt MD, Fragala MS. Energy Expenditure of Selected Household Activities during Pregnancy [Research Note] Res Q Exerc Sport. 2007;78(1):133–137. doi: 10.1080/02701367.2007.10599410. [DOI] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara A, Hedderson MM, Ching J, Kim C, Peng T, Crites YM. Referral to telephonic nurse management improves outcomes in women with gestational diabetes. Am J Obstet Gynecol. 2012;206(6):491.e1–495.e1. doi: 10.1016/j.ajog.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.13. Management of Diabetes in Pregnancy. Diabetes Care. 2017;40(Suppl 1):S114–s119. doi: 10.2337/dc17-S016. [DOI] [PubMed] [Google Scholar]
- 28.Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 31.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 32.van Dijk JW, van Loon LJ. Exercise strategies to optimize glycemic control in type 2 diabetes: a continuing glucose monitoring perspective. Diabetes Spectr. 2015;28(1):24–31. doi: 10.2337/diaspect.28.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–948. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 34.Joshipura KJ, Andriankaja MO, Hu FB, Ritchie CS. Relative utility of 1-h Oral Glucose Tolerance Test as a measure of abnormal glucose homeostasis. Diabetes Res Clin Pract. 2011;93(2):268–275. doi: 10.1016/j.diabres.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.