Abstract
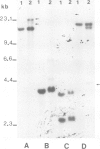
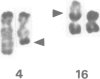
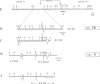
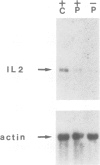
A t(4;16)(q26;p13.1) chromosome translocation found in tumour cells from a patient with a T cell lymphoma was shown to rearrange the interleukin 2 gene, normally located on chromosome band 4q26, with sequences from chromosome band 16p13.1. A cDNA library of tumour cells was screened with an interleukin 2 gene-specific probe. Three clones were isolated, which consisted, from 5' to 3', of the three first exons of the interleukin 2 gene followed by a 16p13 in-frame sequence encoding 181 amino acids. A probe derived from this sequence detected a 1.2 kb transcript in various cell lines exhibiting mature B lymphoid cell features, but this was not detected in other cell lines representative of other haematopoietic lineages, or in other organs. For this reason, the novel gene was termed BCM for B cell maturation. The open reading frame of BCM normal cDNA predicted a 184 amino acid protein with a single transmembrane domain which had no homology with any protein sequence stored in data banks. Our data indicate that BCM is a new gene whose expression coincides with B cell terminal maturation.
Full text
PDF
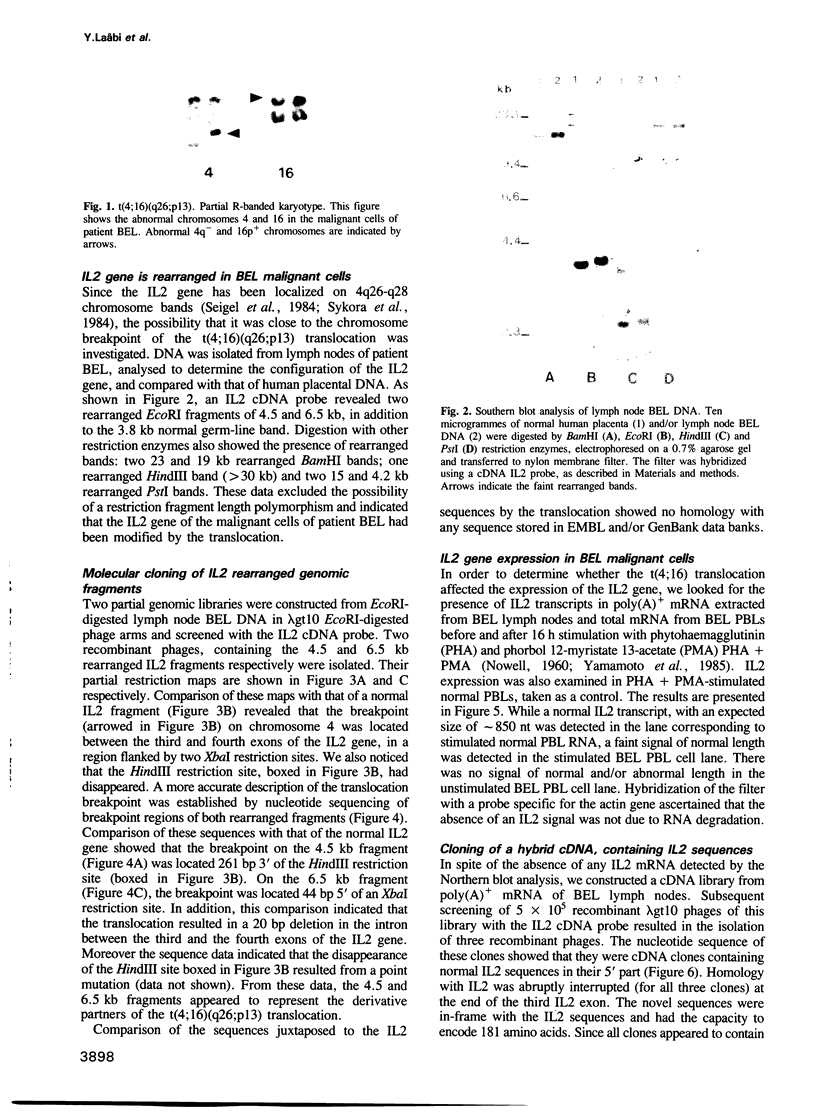


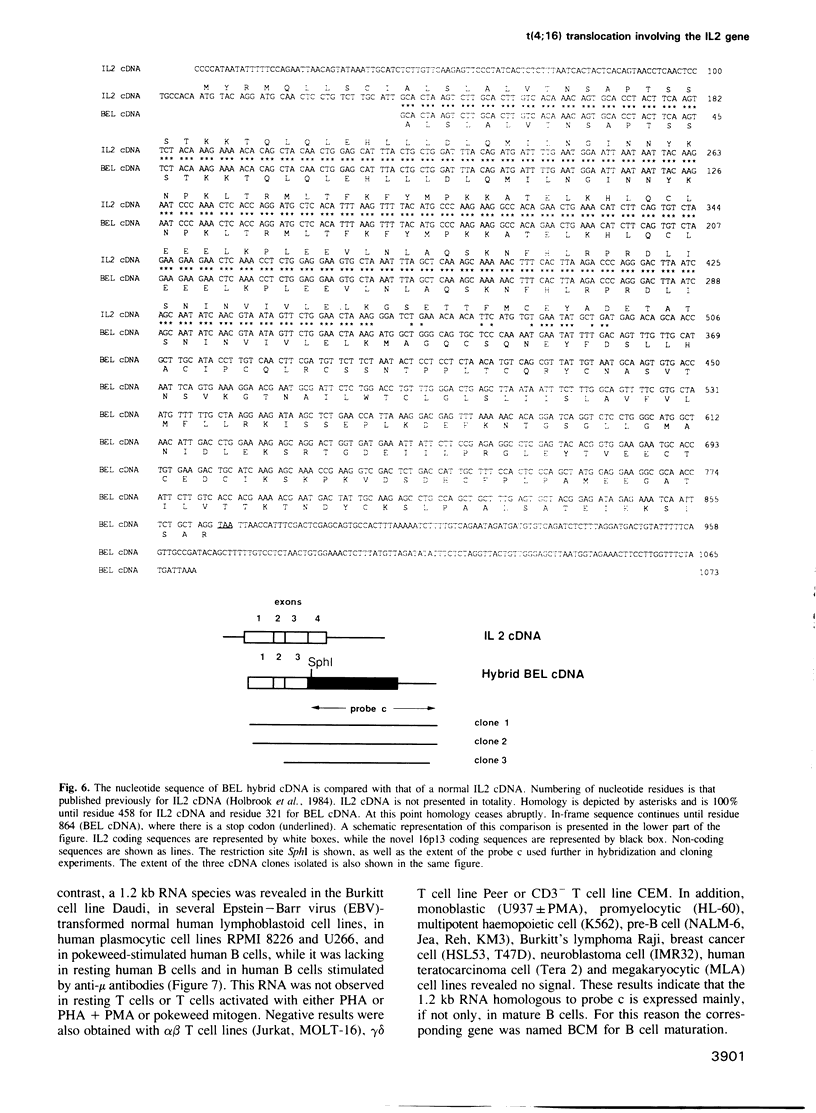
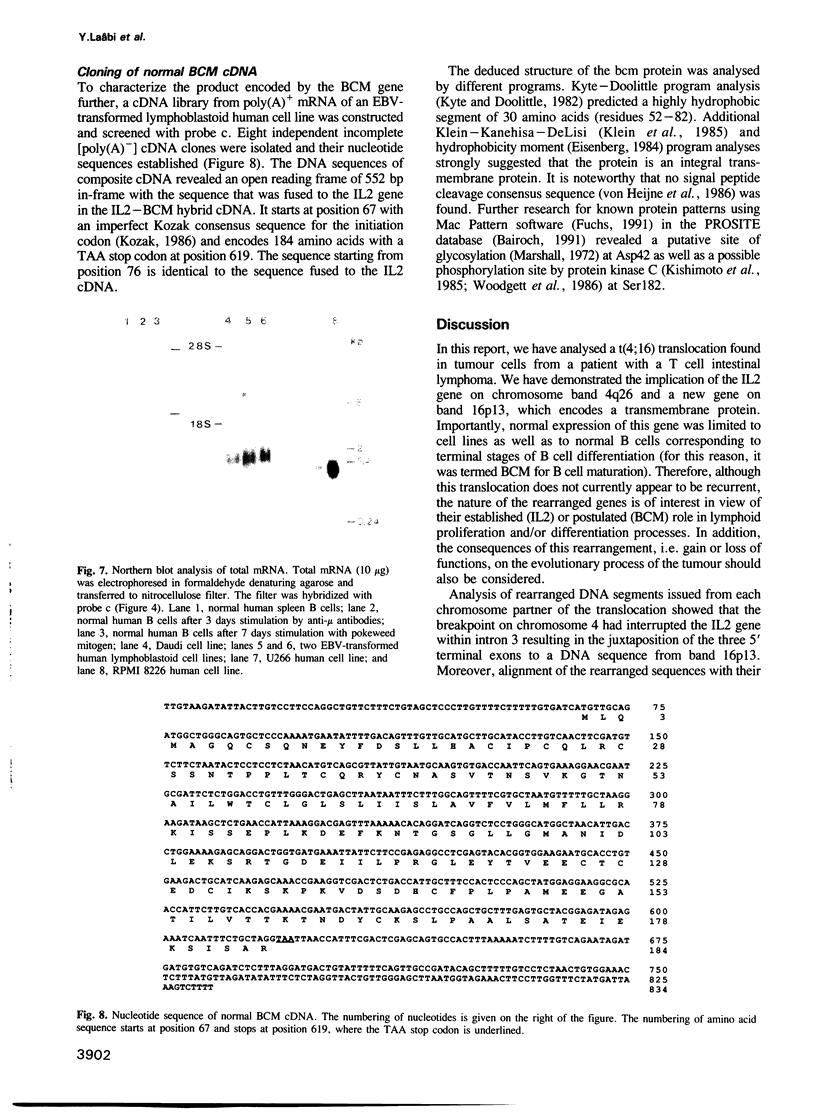


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Robberson D. L., Barrera-Saldaña H. A., Lambrou T. P., Saunders G. F. Genome instability in a region of human DNA enriched in Alu repeat sequences. Nature. 1982 Mar 18;296(5854):219–225. doi: 10.1038/296219a0. [DOI] [PubMed] [Google Scholar]
- Caubet J. F., Mathieu-Mahul D., Bernheim A., Larsen C. J., Berger R. Human proto-oncogene c-mos maps to 8q11. EMBO J. 1985 Sep;4(9):2245–2248. doi: 10.1002/j.1460-2075.1985.tb03921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrave W., Tavernier J., Duerinck F., Plaetinck G., Devos R., Fiers W. Cloning and structure of the human interleukin 2 chromosomal gene. EMBO J. 1983;2(12):2349–2353. doi: 10.1002/j.1460-2075.1983.tb01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Ellisen L. W., Bird J., West D. C., Soreng A. L., Reynolds T. C., Smith S. D., Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991 Aug 23;66(4):649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- Fuchs R. MacPattern: protein pattern searching on the Apple Macintosh. Comput Appl Biosci. 1991 Jan;7(1):105–106. doi: 10.1093/bioinformatics/7.1.105. [DOI] [PubMed] [Google Scholar]
- Fujita T., Takaoka C., Matsui H., Taniguchi T. Structure of the human interleukin 2 gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7437–7441. doi: 10.1073/pnas.80.24.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Rowley J. D., Variakojis D., Golomb H. M. Chromosome abnormalities in poorly differentiated lymphocytic lymphoma. Cancer Res. 1979 Aug;39(8):3119–3128. [PubMed] [Google Scholar]
- Grimaldi J. C., Meeker T. C. The t(5;14) chromosomal translocation in a case of acute lymphocytic leukemia joins the interleukin-3 gene to the immunoglobulin heavy chain gene. Blood. 1989 Jun;73(8):2081–2085. [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Avanzi G. C., Billström R., Kristoffersson U., Mandahl N., Bekassy A. N., Garwicz S., Wiebe T., Pegoraro L., Falda M. A new specific chromosomal rearrangement, t(8;16) (p11;p13), in acute monocytic leukaemia. Br J Haematol. 1987 Jul;66(3):323–326. doi: 10.1111/j.1365-2141.1987.tb06917.x. [DOI] [PubMed] [Google Scholar]
- Hermans A., Heisterkamp N., von Linden M., van Baal S., Meijer D., van der Plas D., Wiedemann L. M., Groffen J., Bootsma D., Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987 Oct 9;51(1):33–40. doi: 10.1016/0092-8674(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Holbrook N. J., Smith K. A., Fornace A. J., Jr, Comeau C. M., Wiskocil R. L., Crabtree G. R. T-cell growth factor: complete nucleotide sequence and organization of the gene in normal and malignant cells. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1634–1638. doi: 10.1073/pnas.81.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Nishiyama K., Nakanishi H., Uratsuji Y., Nomura H., Takeyama Y., Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1985 Oct 15;260(23):12492–12499. [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Larson R. A., Williams S. F., Le Beau M. M., Bitter M. A., Vardiman J. W., Rowley J. D. Acute myelomonocytic leukemia with abnormal eosinophils and inv(16) or t(16;16) has a favorable prognosis. Blood. 1986 Dec;68(6):1242–1249. [PubMed] [Google Scholar]
- Laï J. L., Zandecki M., Jouet J. P., Savary J. B., Lambiliotte A., Bauters F., Cosson A., Deminatti M. Three cases of translocation (8;16)(p11;p13) observed in acute myelomonocytic leukemia: a new specific subgroup? Cancer Genet Cytogenet. 1987 Jul;27(1):101–109. doi: 10.1016/0165-4608(87)90265-2. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Larson R. A., Bitter M. A., Vardiman J. W., Golomb H. M., Rowley J. D. Association of an inversion of chromosome 16 with abnormal marrow eosinophils in acute myelomonocytic leukemia. A unique cytogenetic-clinicopathological association. N Engl J Med. 1983 Sep 15;309(11):630–636. doi: 10.1056/NEJM198309153091103. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991 Oct 31;353(6347):858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Meeker T. C., Hardy D., Willman C., Hogan T., Abrams J. Activation of the interleukin-3 gene by chromosome translocation in acute lymphocytic leukemia with eosinophilia. Blood. 1990 Jul 15;76(2):285–289. [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- Perlino E., Paonessa G., Ciliberto G. Alu sequences transcription in X. laevis oocytes: nuclear-cytoplasmic partitioning and evidence for 3' end processing reactions. Nucleic Acids Res. 1985 Dec 9;13(23):8359–8377. doi: 10.1093/nar/13.23.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum J., De Smedt M. Differentiation of thymocytes in fetal organ culture: lack of evidence for the functional role of the interleukin 2 receptor expressed by prothymocytes. Eur J Immunol. 1988 May;18(5):795–799. doi: 10.1002/eji.1830180521. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H. Translocations, master genes, and differences between the origins of acute and chronic leukemias. Cell. 1991 Nov 15;67(4):641–644. doi: 10.1016/0092-8674(91)90057-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers C. L., Denny C. T., Witte O. N. Leukemia and the disruption of normal hematopoiesis. Cell. 1991 Jan 25;64(2):337–350. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- Seigel L. J., Harper M. E., Wong-Staal F., Gallo R. C., Nash W. G., O'Brien S. J. Gene for T-cell growth factor: location on human chromosome 4q and feline chromosome B1. Science. 1984 Jan 13;223(4632):175–178. doi: 10.1126/science.6318318. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Solomon E., Borrow J., Goddard A. D. Chromosome aberrations and cancer. Science. 1991 Nov 22;254(5035):1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Sykora K. W., Kolitz J., Szabo P., Grzeschik K. H., Moore M. A., Mertelsmann R. Human interleukin 2 gene is located on chromosome 4. Cancer Invest. 1984;2(4):261–265. doi: 10.3109/07357908409018440. [DOI] [PubMed] [Google Scholar]
- Tentori L., Longo D. L., Zuñiga-Pflucker J. C., Wing C., Kruisbeek A. M. Essential role of the interleukin 2-interleukin 2 receptor pathway in thymocyte maturation in vivo. J Exp Med. 1988 Nov 1;168(5):1741–1747. doi: 10.1084/jem.168.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Wessels J. W., Mollevanger P., Dauwerse J. G., Cluitmans F. H., Breuning M. H., Beverstock G. C. Two distinct loci on the short arm of chromosome 16 are involved in myeloid leukemia. Blood. 1991 Apr 1;77(7):1555–1559. [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Ohmura T., Fujimoto K., Onoue K. Interleukin 2 mRNA induction in human lymphocytes: analysis of the synergistic effect of a calcium ionophore A23187 and a phorbol ester. Eur J Immunol. 1985 Dec;15(12):1204–1208. doi: 10.1002/eji.1830151212. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Oken M. M., Kaplan M. E., Ensrud K. M., Howe R. R., Theologides A. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. N Engl J Med. 1982 Nov 11;307(20):1231–1236. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Sawyer J. R., Ball D. W. Characterization of banding patterns of metaphase-prophase G-banded chromosomes and their use in gene mapping. Cytogenet Cell Genet. 1978;22(1-6):679–683. doi: 10.1159/000131052. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]