Abstract
Purpose
Calcific aortic valve disease (CAVD) is a major cause of morbidity in the aging population, but underlying mechanisms of its progression remain poorly understood. Aortic valve calcification preferentially occurs on the fibrosa, which is subjected to disturbed flow. The side-specific progression of the disease is characterized by inflammation, calcific lesions, and extracellular matrix (ECM) degradation. Here, we explored the role of mechanosensitive microRNA-181b and its downstream targets in human aortic valve endothelial cells (HAVECs).
Methods and Results
Mechanistically, miR-181b is upregulated in OS and fibrosa, and it targets TIMP3, SIRT1, and GATA6, correlated with increased gelatinase/MMP activity. Overexpression of miR-181b led to decreased TIMP3 and exacerbated MMP activity as shown by gelatinase assay, and miR-181b inhibition decreased gelatinase activity through the repression of TIMP3 levels. Luciferase assay showed specific binding of miR-181b to the TIMP3 gene. Overexpression of miR-181b in HAVECs subjected to either LS or OS increased MMP activity, and miR-181b inhibition abrogated shear-sensitive MMP activity.
Conclusions
These studies suggest that targeting this shear-dependent miRNA may provide a novel non-invasive treatment for CAVD.
Introduction
Calcific aortic valve disease (CAVD) is a major cause of morbidity among the aging population and a major risk factor for myocardial infarction and other cardiovascular complications 1–3. Once diagnosed, the only available treatment is valve repair or replacement. Stenosis, the endpoint of CAVD, is characterized by inflammation, sclerotic and calcific lesions, and extracellular matrix (ECM) degradation 4,5. In addition, many genetic factors (e.g., bicuspid aortic valve) and environmental factors (e.g., left ventricular hypertrophy) lead to increased risk for CAVD. Although CAVD poses a major medical problem for the aging population, the mechanisms underlying its initiation and progression are not well-defined, and few advancements have been made in therapeutic options for the disease.
The sclerosis and calcification of the aortic valve is a side-specific phenomenon, correlated with the differential shear stresses observed during the cardiac cycle. Each side of the valve experiences a distinct shear profile sensed by the overlying endothelium. The ventricularis layer faces the ventricle and experiences a high-magnitude and pulsatile, unidirectional, laminar shear stress (LS) and rarely calcifies. The fibrosa layer facing the aorta experiences low magnitude and oscillatory shear stress (OS). This endothelial layer exhibits a highly inflammatory phenotype, expressing abundant adhesion molecules and other inflammatory markers, and downregulating anti-inflammatory signals such as Klf2. The fibrosa preferentially exhibits sclerosis and calcification, with large nodules and macrophage infiltration mimicking atherosclerosis observed in the spongiosa and interstitium near this valve face. In vitro, LS conditions applied to aortic valve endothelial cells upregulate CAVD-protective genes such as KLF2, KLF4 and eNOS, whereas OS conditions upregulate pro-CAVD genes and paracrine mediators such as BMP4, Cathepsin K, and matrix metalloproteinases MMP-2 and MMP-9 6,7.
Notably, the matrix metalloproteinases (MMPs) have been correlated with valve disease in various studies of human and porcine valves. MMP levels are known to be higher at sites of calcification in both the endothelium and the interstitial cells. However, the mechanism by which MMPs are upregulated in CAVD conditions has not yet been discerned, and while their causative role in cancers and atherosclerosis has been shown, their contribution to CAVD remains undefined 8–10. MMPs are known to degrade extracellular matrix (ECM) in order to allow for cellular growth, migration, or infiltration as observed in tumor metastasis. The remodeling of the ECM has been previously studied in the context of aortic valve stenosis and calcification 11–14, but the activity of MMPs in that phenomenon are still unknown. In addition, the potentially important mechanism by which low and oscillatory shear-stress may mediate MMP activity and extracellular matrix degradation in CAVD have not been characterized.
In order to find novel pathways regulating CAVD in the aortic valve, our group has recently performed a microarray exploring side- and shear-dependent RNA expression in aortic valve endothelium15. We isolated endothelial enriched RNA from fresh porcine valves to determine which RNA is uniquely expressed on each side of the valve. In addition, we subjected human aortic valve endothelial cells (HAVECs) to LS or OS for 24 hours to determine mechanosensitive RNA expression. Our array revealed many side- and shear-dependent microRNAs (miRNAs). miRNAs are short (18–22) nucleotide sequences which bind to the 3′-untranslated region (3′UTR) of mRNA thereby regulating protein expression by degradation of mRNA targets or by suppression of translation 16. Despite their characterization in other cardiovascular systems, miRNAs have not been thoroughly explored in the AV endothelium. In the interstitium, these mediators have proven to be critical for regulation of osteogenic transformation: miRNA-30b was shown to prevent interstitial cells transforming into calcifying cells 17, and miRNA-141 targets TGF-β and BMP-2 signaling, which are critical for valvular interstitial osteoblastic differentiation leading to tissue calcification18. From our array analyses, we discovered one particular microRNA, miR-181b, that is abundant in HAVECs and consistently upregulated in OS in vitro. This microRNA was also predicted to regulate the previously undefined TIMP3/MMP pathway, and thus was chosen as a potential novel therapeutic candidate for further study.
MiRNA-181b has been shown to be shear-sensitive in aortic valve endothelial cells 6 as well as side-specific in porcine aortic valves 19 however, its potential role in CAVD has yet to be discovered. In order to determine the mechanistic role of miR-181b in HAVECs, we conducted an in silico analysis to determine its predicted and partially validated target genes. From this analysis we found that miRNA-181b targets TIMP3, a known inhibitor of MMPs that has been previously shown to be shear-sensitive and critical in preventing matrix remodeling in atherosclerosis 20. Via overexpression and inhibition of miR-181b along with TIMP3 3′UTR luciferase reporter studies, we found that TIMP3 is a direct target of miR-181b. Through a series of gelatinase assays showing HAVEC MMP activity, we found that miR-181b suppresses TIMP3 in OS conditions leading to increased ECM degradation and remodeling. These findings provide a novel therapeutic target for CAVD by targeting miR-181b or TIMP3 to prevent ECM remodeling or degradation characteristic of CAVD pathology.
Materials and methods
Quantitative real-time PCR
Total RNA was reverse transcribed for use in a two-step quantitative reverse transcribed–PCR using the qRT-PCR kits (Invitrogen). For measurement of mRNA targets, qPCR was performed on selected genes using VeriQuest Fast SYBR QPCR Master Mix (Affimetrix) with custom designed primers using 18S as house-keeping control. The PCR conditions were 2 min at 50 °C, 5 min at 95 °C, followed by 40 cycles of 95 °C for 4 s and 60 °C for 30 s. Fold changes between LS and OS were determined for all targets using the ΔΔCt method. Sequences for primers used for mRNA expression studies have been listed in Supplementary Tables S1.
Mature miRNA assays were performed using miRNA quantification and was performed by SYBR green qPCR assay using miScript reverse transcription kit (Qiagen) according to the manufacturer’s instructions. qPCR was performed using miScript SYBR Green PCR kit (Affimetrix) with miScript universal primer (U6B) and the miRNA-specific forward primers and relative fold change was calculated1. The specific mature primers were purchased from Qiagen. The amplification profile was denaturation at 95 °C, 15 min, followed by 40 cycles of 94 °C, 15 s; 55 °C, 30 s; and 70 °C, 30 s.
Cell culture and in vitro shear stress system
Side-specific HAVECs from both the fibrosa (fHAVECs) and the ventricularis (vHAVECs) endothelium were isolated from noncalcified AVs obtained from heart transplant recipient surgeries (n = 6) (according to an Insititutional Review Board-approved protocol at Emory University and Georgia Institute of Technology) as previously described6 and detailed in the Supplemental Material.
Upon confluency, HAVECs were exposed to steady shear stress (LS) using the cone-and-plate viscometer, as has been previously reported6,21,22 and described in further detail in the Supplemental Material. Oscillatory shear stress (OS) was applied using the cone-and-plate viscometer. For LS, we used a unidirectional shear stress of 20 dyn/cm2; for OS, we used a bidirectional shear stress of ±5 dyn/cm2 at 1 Hz to approximate the complex shear stress conditions surrounding AVECs in vivo23. While the shear conditions used in this study do not exactly mimic the conditions surrounding the valve in vivo, we have previously shown LS and OS at these magnitudes to induce anti-inflammatory Klf2 expression or inflammatory signaling at levels similar to those observed in vivo6.
Immunofluorescent staining of human aortic valves
Calcified human AVs were obtained immediately following valve replacement surgeries in 16 patients at Emory University Hospital Midtown according to the IRB-approved study at Emory University with written informed consent. Fifteen patients had trileaflet valves, while one patient had a bicuspid AV. Patient demographics are noted in our group’s previous publication using this cohort22. Briefly, the patient characteristics are: average 55 years old; 16% female; 50% diabetic patients; and, 33% of hypertensive patients. Immediately following harvesting, the AVs were photographed, washed in ice-cold phosphate buffered saline, and cusps were individually snap-frozen in optimal cutting temperature (O.C.T.) compound (Tissue-Tek). Valves were then sectioned (7 μm) in the radial direction to include the base and free edge (tip), stored at −80°C and used for immunohistochemical staining studies.
Staining was performed as we have described previously22, using specific antibody against TIMP3 (Abcam) and CD31 (BD Biosciences), and appropriate secondary antibody conjugated to fluorophore at A488 or A568 (Life Technologies). Hoechst 33342 (Life Technologies) was used to stain nuclei. All measurements were performed using ImageJ (NIH) and they are represented as intensity of TIMP3 staining normalized to number of DAPI positive cells.
Transient transfection with oligonucleotides
For the functional tests of miR-181b in vitro, cells were transiently transfected with miRNA-181b-5p mimic (Life Technologies), mimic control, anti-miR-181b-5p (Exiqon), mismatched anti-miR control (Exiqon) using Oligofectamine (Invitrogen), following manufacturer’s protocol as described previously24. For static conditions cells were collected 48h after transfection. For shear experiments, cells were sheared 24h after transfection.
Dual-luciferase activity assays
Firefly luciferase activity measurement was obtained at room temperature using Luc-Pair miR Luciferase Assay Kit (GeneCopoeia) and a single tube luminometer (Model TD 20/20 Turner Designs, Sunnyvale, CA). Dual-luciferase reporter constructs containing 3′UTR of TIMP3 with miR-181b binding sites (GeneCopoeia) were transfected into HAVECs using a Nucleofection Kit (Lonza). HAVECs were transfected first with wild-type TIMP3 gene 3′-UTR using HUVEC Nucleofector™Kit (Lonza) and were allowed to recover for 24 h. Second transfection was performed using increasing concentrations of miR-181b mimics or mimic control with Oligofectamine 2000 (Invitrogen). Firefly and Renilla luciferase activities were measured using a Luc-Pair™miR Luciferase Assay (GeneCopoeia) as per manufacturer’s recommendations.
Gelatinase assay
In order to determine the activity of MMPs in HAVECs under different shear conditions, we used the EnzChek Gelatinase/Collagenase Assay kit (Thermo Fisher). The kit utilizes a gelatin substrate from porcine skin, labeled with fluorescein, which can be degraded by gelatinases and collagenases such as MMPs. The level of substrate degradation is reflected by fluorescent signal, absorption 495 nm/emission 515 nm, read via Biotek plate reader. We incubated 100 ul HAVEC lysate suspended in kit buffer with 20 ug DQ gelatin substrate for 2 hours, and then recorded fluorescent signal for each sample. Values were adjusted by subtracting fluorescent signal of blank wells, and normalizing to total sample protein content.
In vitro MMP inhibition
We analyzed the specific contribution of MMPs to the gelatinase fluorescence using MMP chemical inhibitor GM6001 (Millipore), which efficiently inhibits many MMPs, including MMP-1, 2, 3, 8, and 9. After transfection with miR-181b mimic, we treated HAVECs with 30 nM for 24 hours. The cell lysate was collected and gelatinase assay was performed as described above.
Statistical analysis
Statistical analyses were performed using Excel and Graphpad Prism software. Error bars are reported as the standard deviation value. Pairwise comparisons were performed using 2-tailed Student’s t test and one-way ANOVA, where appropriate. Details of statistics within datasets are included in figure legends and Results.
Results
miR-181b is a shear-sensitive miRNA upregulated in OS conditions and it targets TIMP3 which is shear-sensitive in HAVECs and side-specific in human aortic valves
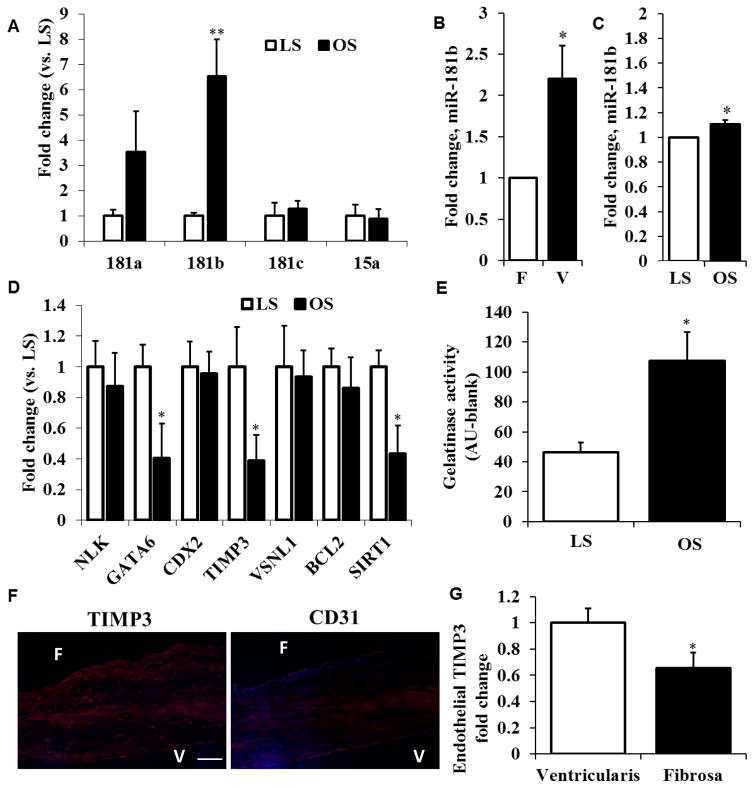
From our previous microarray studies, we chose 5 of the top side- and shear-sensitive microRNAs to validate using real-time PCR. In HAVECs in vitro, miR-181b was significantly higher in OS compared to LS (Figure 1A). This was consistent with the porcine side-specific endothelial array and the previous HAVEC array, both of which showed increased miR-181b at the fibrosa and under disturbed flow (Figure 1B and 1C)19. We then proceeded to conduct an in silico analysis on miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/) and miRwalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) to find predicted targets of miR-181b from previous arrays and published studies. The top predicted and published target genes at that time were NLK, GATA6, CDX2, TIMP3, VSNL1, BCL2 and SIRT1. We probed for these genes in HAVECs sheared in LS or OS for 24h, and we found that GATA6, TIMP3 and SIRT1 were highly shear-sensitive, showing a decrease in OS compared to LS (Figure 1D). Because of its known role in matrix degradation and our group’s previous work in this area, we focused on TIMP3 for further study. TIMP3 is an inhibitor of extracellular matrix degradation through MMP inhibition that potentially links previous studies describing increased ECM degradation in stenotic or calcific valves compared to healthy valves9,10. As a functional readout of matrix remodeling in HAVECs, we used the DQ gelatin substrate in the gelatinase assay25, which has been shown to correlate with MMP activity and matrix degradation15. Correlating with the loss of TIMP3 in OS, we observed a 2.5-fold increase in gelatinase activity in HAVECs sheared in OS conditions compared to LS (Figure 1E).
Figure 1. Mechanosensitive miRNA-181b is upregulated in OS, correlated with decreased TIMP3 and increased MMP activity.
A) HAVECs were sheared for 24h under high-magnitude unidirectional shear-stress (LS, 20dyn/cm2) or under low-magnitude bidirectional shear-stress (OS, ±5dyn/cm2 @1Hz). miRNA-181b was significantly upregulated in OS conditions compared to LS. n=4, **p<0.01. B,C) Endothelial enriched-RNA from the fibrosa layer (F) and the ventricularis layer (V) from fresh porcine valves as well as RNA from HAVECs sheared in OS or LS conditions was harvested and a miRNA array was conducted. In both cases miR-181b was upregulated in disturbed flow conditions compared to steady flow (F/V and OS/LS) indicating side- and shear-dependence. D) qPCR quantification of predicted miR-181b gene targets from HAVECs sheared under LS or OS conditions. n=4, *p<0.05. E) MMP activity quantification from HAVECs sheared for 24h under LS or OS. n=3, *p<0.05. F,G) Immunohistochemistry was conducted on noncalcified human aortic valves to quantify TIMP3 expression on each layer of the aortic valve as well as the endothelial marker CD31. We have normalized TIMP3 intensity to the number of DAPI positive cells in the endothelial layer. n=6, *p<0.05. Scale bar=100 μm.
In order to determine the expression of TIMP3 in valve tissue, we sectioned and stained noncalcified human aortic valves with antibody specific for TIMP3 as well as with antibody specific for CD31, an endothelial cell marker. We found that TIMP3 was significantly downregulated in the fibrosa endothelium compared to the ventricularis, confirming our analyses in vitro (Figure 1F). After quantification of TIMP3 staining in 6 valves using ImageJ software and normalizing to the number of DAPI positive cells, we observed a 35% decrease of TIMP3 in the fibrosa compared to the ventricularis layer (Figure 1G). This data suggests that miR-181b is upregulated in the fibrosa under disturbed flow and it may be targeting TIMP3 to increase matrix degradation.
miR-181b targets TIMP3 and increases ECM degradation in HAVECs
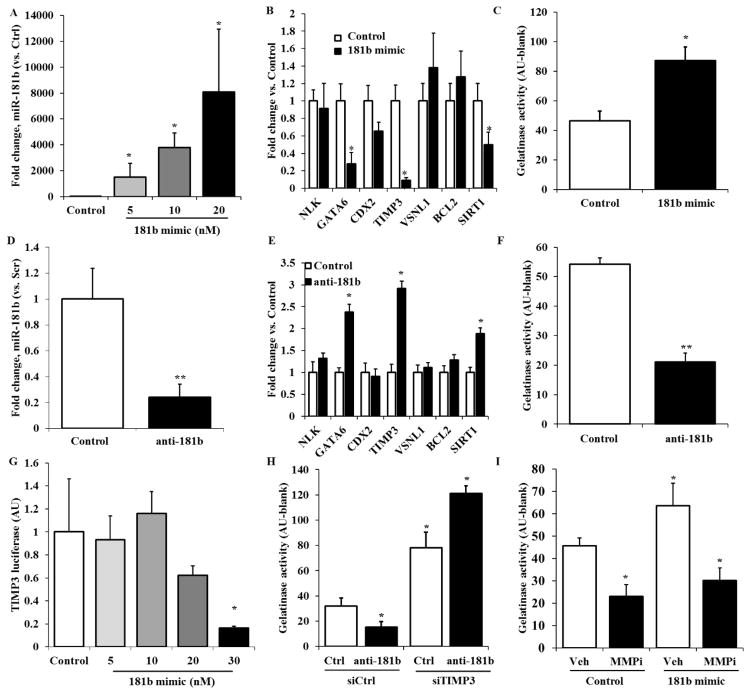
In order to determine if miR-181b directly regulates TIMP3 at the gene level, we treated HAVECs with miR-181b-mimic at a concentration of 20nM for 48h and we quantified the expression of miR-181b, miR-181a and miR-181c. The mimic for miR-181b was specific, only showing an increase in miR-181b and not its other family members (Figure 2A). Quantification of target genes of miR-181b by qPCR conducted on HAVECs treated with miR-181b mimic showed TIMP3 (along with the two other target genes we observed earlier, GATA6 and SIRT1) was downregulated when miR-181b was overexpressed (Figure 2B). Gelatinase assay was performed on HAVECs treated with miR-181b mimic to determine altered MMP activity, and we found that there was two-fold increase in MMP activity when miR-181b was upregulated (Figure 1C). Thus, miR-181b overexpression leads to downregulation of TIMP3 and an increase in gelatinase/MMP activity.
Figure 2. miR-181b targets TIMP3 and increases matrix degradation in HAVECs.
A) HAVECS were treated with miR-181b mimic at 20nM for 48h and expression of miR-181 miRNAs was quantified by qPCR. B) Expression of predicted gene targets of miR-181b was measured in HAVECs treated with miR-181b mimic or control 48h after transfection. C) MMP activity was measured via gelatinase assay in HAVECs transfected with miR-181b mimic. D) HAVECs were treated with anti-miR-181b at 100nM for 48h and expression of miR-181b was quantified by qPCR. E) Expression of predicted gene targets of miR-181b were measured in HAVECs treated with anti-miR-181b or anti-miR control 48h after transfection. F) MMP activity was measured via gelatinase assay in HAVECs transfected with anti-miR-181b G) HAVECs were transfected with wild-type TIMP3 3′-UTR firefly luciferase construct for 24h and with serial concentrations of miR-181b mimic for 24h. A dual-luciferase reporter assay was then used to measure transcription of the TIMP3 3′-UTR construct. H) HAVECs were transfected with miR-181b mimic or mimic control at 20nM and treated with MMP inhibitor GM6001 or vehicle control (DMSO) for 48h. MMP activity was measured using the gelatinase assay. I) HAVECs were first transfected with anti-miR-181b or anti-miR-control for 24h before being transfected with siRNA-TIMP3 or siRNA control at 50nM for 24h. We then measured MMP activity on these samples. n=3, *p<0.05
We performed the same set of experiments using antimiR-181b at a concentration of 100 nM, which resulted in a decrease in miR-181b by 75% after 48 hours of treatment (Figure 2D). The TIMP3, GATA6, and SIRT1 genes were all significantly upregulated when miR-181b was silenced (Figure 2E). MMP activity in HAVECs treated with anti-miR-181b was decreased by 2.5 fold (Figure 2F). These results strengthened the hypothesis that miR-181b targets TIMP3 to increase matrix remodeling, and decreasing miR-181b may rescue this effect.
To test if miR-181b binds directly to the 3′ UTR of TIMP3 to decrease its expression, we employed a dual-luciferase reporter assay in HAVECs transfected with wild-type TIMP3 3′-UTR firefly luciferase construct, as we have described previously15. Treatment of cells with miR-181b mimic inhibited luciferase activity in a dose-dependent manner, while control pre-miR had no effect (Figure 2G), demonstrating that miR-181b indeed binds to the TIMP3 3′-UTR and inhibits its expression in HAVECs. In order to confirm the gelatinase assay was primarily showing MMP activity, we used MMP inhibitor GM6001 to decrease MMP activity in HAVECs treated with miR-181b mimic. The gelatinase activity normally observed in HAVECs, treated with either control or miR-181b mimic, was decreased after MMP inhibition (Figure 2H), suggesting that the MMPs are responsible for the majority of the gelatinase activity changes observed in HAVECs. To finally confirm that miR-181b is increasing gelatinase activity via its targeting of TIMP3, we transfected HAVECs with both anti-miR-181b and siRNA for TIMP3. The HAVECs with knockdown of both miR-181b and TIMP3 showed higher levels of MMP activity compared to anti-miR alone (Figure 2I). This result clearly shows that the decreased matrix degradation resulting from anti-miR-181b treatment works through specific rescue of TIMP3.
miR-181b is responsible of the increase in matrix degradation observed in OS conditions
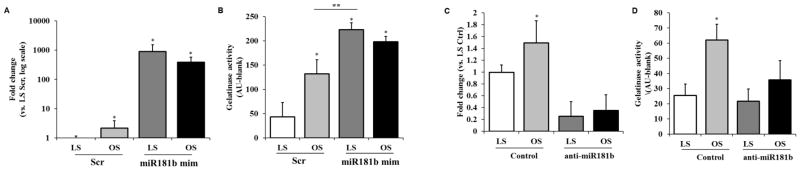
To test the hypothesis that matrix remodeling is increased by mechanosensitive miR-181b under disturbed flow, we subjected HAVECs treated with miR-181b mimic to either LS or OS conditions for 24h. We quantified the levels of miR-181b after shear was applied and we found that miR-181b levels were increased dramatically in both LS and OS (Figure 3A), and this correlated with increased gelatinase/MMP activity in both shear stress conditions (Figure 3B). Notably, the usually low levels of gelatinase activity observed in LS were increased 4-fold after treatment with miR-181b mimic. We performed the same assays using HAVECs treated with anti-miR-181b under LS and OS. Anti-miR treatment decreased miR-181b by 80% (Figure 3C), and the normally high levels of MMP activity observed in OS were abrogated upon anti-miR-181b treatment (Figure 3D). These data suggest that the shear-dependent change in matrix degradation we observe in HAVECs is dependent on miR-181b, which may be a master microRNA regulator of shear-dependent matrix changes in the aortic valve endothelium, as shown in Figure 4.
Figure 3. miR-181b is responsible for shear-dependent ECM degradation.
A–B) HAVECs were transfected with miR-181 mimic (181b mimic) or mimic control at 20nM for 24h previous to application of 24h shear-stress conditions either LS or OS. Expression of mir-181b was quantified via qPCR (A), and MMP activity was measured via gelatinase activity (B). n=4, *p<0.05,**p<0.01. C–D) HAVECs were transfected with anti-miR-181b (anti-181b) or anti-miR control at 100nM for 24h previous to application either LS or OS. Expression of mir-181b was quantified via qPCR (C) and MMP activity was measured via gelatinase activity (D).
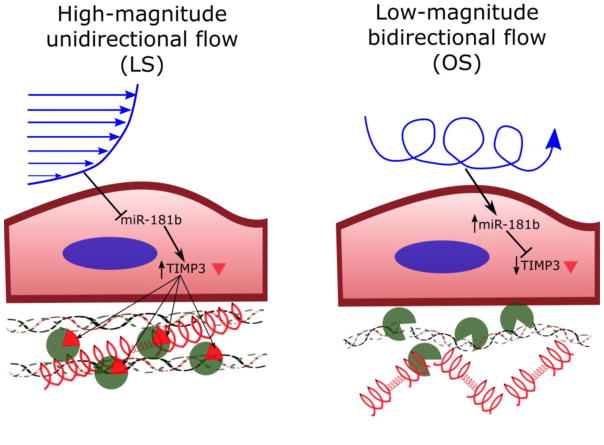
Figure 4. Schematic: miR-181b induces endothelial ECM degradation by targeting TIMP3.
High-magnitude laminar shear stress at the ventricularis suppresses endothelial expression of miR-181b, leading to an increase in TIMP3 expression. TIMP3 inhibits MMP activation and extracellular matrix degradation, protecting the ventricularis from further events leading to valve calcification, as described by Chen and Simmons35. Low-magnitude oscillatory shear stress at the fibrosa stimulates endothelial expression of miR-181b, which downregulates TIMP3, leading to increased MMP activity and extracellular matrix degradation. This remodeling of the ECM may lead to aortic valve sclerosis and calcification through multiple mechanisms35.
Discussion
These studies are the first to show a direct effect of mechanosensitive microRNAs on matrix degradation in the aortic valve endothelium. While the levels and activity of MMPs have been suggested in previous studies to play a role in the degradation of the valvular extracellular matrix contributing to calcification13,26,27, our work suggests a causative endothelial mechanosensitive pathway responsible for this phenomenon. MMPs are critical for the progression of cardiovascular disease in various tissues, including atherosclerosis28–30 and ischemia reperfusion injury31,32. However, MMP activity has not been shown to participate directly in valve calcification, and MMP-mediated ECM degradation may participate in the progression of inflammatory cell infiltration12,33. Here, we have highlighted a novel pathway, showing TIMP3 inhibition of MMP activity is vital for maintenance of valve endothelial cell homeostasis, and disturbed flow leads to downregulation of TIMP3 via mechanosensitive microRNA-181b. This causes increased MMP activity and leads to increased matrix degradation, known to lead to worsened valve sclerosis and calcification34,35.
While TIMP3 has previously been identified as a major regulator of matrix remodeling36, our current work reveals its previously unknown role in the valve endothelium. In separate studies, our group has shown that TIMP3 is downregulated by disturbed flow in atherosclerosis, which leads to exacerbated MMP activity and lesion formation15. The study highlighted here shows a similar role for TIMP3 in the valvular endothelium, although under the control of a different mechanosensitive microRNA, miR-181b. We have performed a microarray to determine microRNAs that are mechanosensitive in HAVECs and porcine valves19, which proved a powerful tool in determining microRNAs which may be regulated by differential flow in the valve. The array revealed miR-181b to be significantly upregulated in disturbed flow and the porcine fibrosa, which was confirmed by quantitative PCR shown in these studies. Future work from our group will continue to utilize the dataset from the microarray to further analyze other shear-sensitive microRNAs and their downstream targets, which may be critical in the initialization and progression of valve sclerosis and calcification.
The targeting of TIMP3 by microRNA-181b has been described in the context of cancers, including gastric cancer37 and hepatocellular carcinoma38. In these diseases, miR-181b suppresses TIMP3 and increases MMP activity, allowing for tumor growth and infiltration into the surrounding tissue environment. These studies correlate with our observations that miR-181b downregulates TIMP3 in the valve endothelium, which increases matrix remodeling critical for valve sclerosis and calcification. The analysis of this pathway in previous studies may also point toward a regulatory pathway upstream: Wang et al. found that miR-181b can be induced by alteration of transforming growth factor-beta and its Smad targets38. This pathway and its relevance to the aortic valve warrant further study in the valve endothelium and is a focus of our future work.
Our current studies demonstrate the pathological role of microRNA-181b induced by disturbed flow in valve endothelium. Our work clearly shows that mechanosensitive microRNA-181b targets and downregulates TIMP3 via binding to the TIMP3 3′UTR, leading to increased MMP-mediated degradation of extracellular matrix. This microRNA in particular has been studied at length in various endothelial cells and diseases39–42. It seems to play a conflicting role in disease progression or protection depending on tissue and disease context. In elegant and thorough studies, microRNA-181b was shown to protect against atherosclerosis by inhibiting endothelial inflammation41. Circulating miR-181b was found to be reduced in the blood of patients with coronary artery disease and ApoE−/− mice fed with high-fat diet also presented lower levels of miR-181b in the aortic intima layer. Systemic delivery of miR-181b in ApoE−/− fed with high-fat diet inhibited atherosclerotic lesion formation, proinflammatory gene expression and the influx of lesional macrophages and CD4+ T cells in the vessel wall. While our current work shows an opposing role for this microRNA in the valve, this incongruity may indicate a complex system of regulation of microRNA-181b that is context-dependent. In certain cancers, miR-181b is tumorigenic43, while it may regulate glucose levels in adipose tissue42. Thus, our work combined with the previously defined pathways of miR-181b regulation indicate a tissue-specific role for this microRNA. While more work is required to determine the reasons for the differences observed between our studies and others, we have clearly observed a pathological role for miR-181b in the valve endothelium.
We have also shown here that microRNA-181b targets other genes in HAVECs, including SIRT1 and GATA6. These studies are the first to demonstrate the mechanosensitivity of both of these genes, and their targeting by microRNA181b. In fact, the role of SIRT1 and GATA6 in the valve has not yet been described. Thus, these represent novel targets for future studies by our group. Both of these genes are known to play a protective role in atherosclerosis: in an angiotensin-induced model of atherosclerosis, SIRT1 induces endothelial relaxation and may inhibit the formation of foam cells in the vasculature44. GATA6 protects against intimal hyperplasia by controlling smooth muscle cell differentiation as well as inhibiting VCAM-1 expression in treated endothelial cells45. Both of these targets present novel avenues of study in the aortic valve, and prove to be as important as TIMP3 in maintaining valvular homeostasis and tissue integrity. When taken together, the wide array of functions controlled by target genes of miR-181b suggest that this microRNA may be a master regulator of aortic valve endothelial cells under disturbed flow, orchestrating a complex a multi-faceted change in the phenotype of the cells and the structure of the whole valve. The pathway we have highlighted in this study may be just one of many important for this change, and thus further analysis is necessary to obtain a better overall picture of miR-181b network regulation.
Supplementary Material
Supplementary Figure I. List of quantitative PCR primers used in analysis of gene targets of miRNA-181b.
Footnotes
Compliance with ethical standards
Funding: This study was funded by NIH (R01HL114772) as well as NHLBI grants (HL119798, HL113451, HL095070 and HL124879).
Conflict of Interest: All the authors declare that they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Hsu SY, Hsieh IC, Chang SH, Wen MS, Hung KC. Aortic valve sclerosis is an echocardiographic indicator of significant coronary disease in patients undergoing diagnostic coronary angiography. International Journal of Clinical Practice. 2005;59:72–77. doi: 10.1111/j.1742-1241.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. New England Journal of Medicine. 1999;341:142–147. doi: 10.1056/nejm199907153410302. [DOI] [PubMed] [Google Scholar]
- 3.Mohler ER, Sheridan MJ, Nichols R, Harvey WP, Waller BF. DEVELOPMENT AND PROGRESSION OF AORTIC-VALVE STENOSIS - ATHEROSCLEROSIS RISK-FACTORS - A CAUSAL RELATIONSHIP - A CLINICAL MORPHOLOGICAL-STUDY. Clinical Cardiology. 1991;14:995–999. doi: 10.1002/clc.4960141210. [DOI] [PubMed] [Google Scholar]
- 4.Mohler ER, et al. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, Obrien KD. CHARACTERIZATION OF THE EARLY LESION OF DEGENERATIVE VALVULAR AORTIC-STENOSIS - HISTOLOGICAL AND IMMUNOHISTOCHEMICAL STUDIES. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 6.Holliday CJ, Ankeny RF, Jo H, Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. American journal of physiology. Heart and circulatory physiology. 2011;301:H856–867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Rajamannan NM, Sucosky P. Defining the role of fluid shear stress in the expression of early signaling markers for calcific aortic valve disease. PloS one. 2013;8:e84433. doi: 10.1371/journal.pone.0084433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaden JJ, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovascular Pathology. 2005;14:80–87. doi: 10.1016/j.carpath.2005.01.002. http://dx.doi.org/10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Cappelli S, et al. Aortic valve disease and gamma-glutamyltransferase: Accumulation in tissue and relationships with calcific degeneration. Atherosclerosis. 2010;213:385–391. doi: 10.1016/j.atherosclerosis.2010.08.063. http://dx.doi.org/10.1016/j.atherosclerosis.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 10.Nagy E, et al. Valvular osteoclasts in calcification and aortic valve stenosis severity. International Journal of Cardiology. 2013;168:2264–2271. doi: 10.1016/j.ijcard.2013.01.207. http://dx.doi.org/10.1016/j.ijcard.2013.01.207. [DOI] [PubMed] [Google Scholar]
- 11.Jung J-J, et al. Multimodality and Molecular Imaging of Matrix Metalloproteinase Activation in Calcific Aortic Valve Disease. Journal of Nuclear Medicine. 2015;56:933–938. doi: 10.2967/jnumed.114.152355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrotta I, Sciangula A, Aquila S, Mazzulla S. Matrix Metalloproteinase-9 Expression in Calcified Human Aortic Valves: A Histopathologic, Immunohistochemical, and Ultrastructural Study. Applied Immunohistochemistry & Molecular Morphology. 2016;24:128–137. doi: 10.1097/pai.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Circulating matrix metalloproteinase patterns in association with aortic dilatation in bicuspid aortic valve patients with isolated severe aortic stenosis. Heart and vessels. 2016;31:189–197. doi: 10.1007/s00380-014-0593-5. [DOI] [PubMed] [Google Scholar]
- 14.Platt MO, Xing Y, Jo H, Yoganathan AP. Cyclic pressure and shear stress regulate matrix metalloproteinases and cathepsin activity in porcine aortic valves. The Journal of heart valve disease. 2006;15:622–629. [PubMed] [Google Scholar]
- 15.Kumar S, Kim CW, Simmons RD, Jo H. Role of Flow-Sensitive microRNAs in Endothelial Dysfunction and Atherosclerosis: Mechanosensitive Athero-miRs. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2206–2216. doi: 10.1161/atvbaha.114.303425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo HL, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–U866. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, et al. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. The Journal of Thoracic and Cardiovascular Surgery. 2014;147:1073–1080.e1072. doi: 10.1016/j.jtcvs.2013.05.011. http://dx.doi.org/10.1016/j.jtcvs.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Yanagawa B, et al. miRNA-141 is a novel regulator of BMP-2–mediated calcification in aortic stenosis. The Journal of Thoracic and Cardiovascular Surgery. 2012;144:256–262.e252. doi: 10.1016/j.jtcvs.2011.10.097. http://dx.doi.org/10.1016/j.jtcvs.2011.10.097. [DOI] [PubMed] [Google Scholar]
- 19.Rathan S, et al. Identification of side- and shear-dependent microRNAs regulating porcine aortic valve pathogenesis. Sci Rep. 2016;6:25397. doi: 10.1038/srep25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son DJ, et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CW, et al. Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1412–1421. doi: 10.1161/ATVBAHA.113.303134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankeny RF, et al. Preferential Activation of SMAD1/5/8 on the Fibrosa Endothelium in Calcified Human Aortic Valves - Association with Low BMP Antagonists and SMAD6. PloS one. 2011;6:e20969. doi: 10.1371/journal.pone.0020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered Shear Stress Stimulates Upregulation of Endothelial VCAM-1 and ICAM-1 in a BMP-4– and TGF-β1–Dependent Pathway. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:254–260. doi: 10.1161/atvbaha.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. American journal of physiology. Heart and circulatory physiology. 2011;300:H1762–1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandooren J, et al. Gelatin degradation assay reveals MMP-9 inhibitors and function of O-glycosylated domain. World J Biol Chem. 2011;2:14–24. doi: 10.4331/wjbc.v2.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. American journal of physiology. Heart and circulatory physiology. 2009;296:H756–764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PubMed] [Google Scholar]
- 27.Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. The Journal of heart valve disease. 2007;16:672–682. [PubMed] [Google Scholar]
- 28.Schonbeck U, et al. Expression of stromelysin-3 in atherosclerotic lesions: regulation via CD40-CD40 ligand signaling in vitro and in vivo. The Journal of experimental medicine. 1999;189:843–853. doi: 10.1084/jem.189.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soumyarani VS, Jayakumari N. Oxidatively modified high density lipoprotein promotes inflammatory response in human monocytes-macrophages by enhanced production of ROS, TNF-alpha, MMP-9, and MMP-2. Molecular and cellular biochemistry. 2012;366:277–285. doi: 10.1007/s11010-012-1306-y. [DOI] [PubMed] [Google Scholar]
- 30.Uzui H, et al. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: role of activated macrophages and inflammatory cytokines. Circulation. 2002;106:3024–3030. doi: 10.1161/01.cir.0000041433.94868.12. [DOI] [PubMed] [Google Scholar]
- 31.Cheung PY, et al. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 32.Alfonso-Jaume MA, et al. Cardiac ischemia-reperfusion injury induces matrix metalloproteinase-2 expression through the AP-1 components FosB and JunB. American journal of physiology. Heart and circulatory physiology. 2006;291:H1838–1846. doi: 10.1152/ajpheart.00026.2006. [DOI] [PubMed] [Google Scholar]
- 33.Perrotta I, et al. New evidence for a critical role of elastin in calcification of native heart valves: immunohistochemical and ultrastructural study with literature review. Histopathology. 2011;59:504–513. doi: 10.1111/j.1365-2559.2011.03977.x. [DOI] [PubMed] [Google Scholar]
- 34.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 35.Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circulation research. 2011;108:1510–1524. doi: 10.1161/CIRCRESAHA.110.234237. [DOI] [PubMed] [Google Scholar]
- 36.Fan D, et al. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovascular research. 2014;103:268–280. doi: 10.1093/cvr/cvu072. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, et al. Smad2/3/4 Pathway Contributes to TGF-beta-Induced MiRNA-181b Expression to Promote Gastric Cancer Metastasis by Targeting Timp3. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2016;39:453–466. doi: 10.1159/000445638. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, et al. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene. 2010;29:1787–1797. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinberg MW, Moore KJ. MicroRNA Regulation of Atherosclerosis. Circulation research. 2016;118:703–720. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J, et al. MicroRNA-181b inhibits thrombin-mediated endothelial activation and arterial thrombosis by targeting caspase recruitment domain family member 10. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016 doi: 10.1096/fj.201500163R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X, et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. The Journal of clinical investigation. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X, et al. MicroRNA-181b Improves Glucose Homeostasis and Insulin Sensitivity by Regulating Endothelial Function in White Adipose Tissue. Circulation research. 2016;118:810–821. doi: 10.1161/CIRCRESAHA.115.308166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao Q, et al. Interplay between microRNAs and the STAT3 signaling pathway in human cancers. Physiological genomics. 2013;45:1206–1214. doi: 10.1152/physiolgenomics.00122.2013. [DOI] [PubMed] [Google Scholar]
- 44.Chen YX, Zhang M, Cai Y, Zhao Q, Dai W. The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE(-)/(-) mice through inhibiting vascular inflammatory response. Biochemical and biophysical research communications. 2015;465:732–738. doi: 10.1016/j.bbrc.2015.08.066. [DOI] [PubMed] [Google Scholar]
- 45.Tsoyi K, et al. PTEN differentially regulates expressions of ICAM-1 and VCAM-1 through PI3K/Akt/GSK-3beta/GATA-6 signaling pathways in TNF-alpha-activated human endothelial cells. Atherosclerosis. 2010;213:115–121. doi: 10.1016/j.atherosclerosis.2010.07.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure I. List of quantitative PCR primers used in analysis of gene targets of miRNA-181b.