Abstract
To investigate the effects of age at diagnosis on metastatic breast cancer and patients’ prognosis, we collected patient data from the Surveillance, Epidemiology, and End Results (SEER) database. We finally identified 4932 eligible metastatic breast cancer patients diagnosed between 2010–2013, including 850 younger patients (<50 years), 2,540 middle-aged patients (50–69 years) and 1,542 elder patients (>69 years). The results revealed that in stage IV patients, elder patients were more likely to have lung metastasis (P < 0.001) and less likely to have only distant lymphatic spread (P = 0.004). Higher proportion of younger (34.9%) and middle-aged (36.2%) patients had multiple metastatic sites than elder patients (28.3%) (P < 0.001). In survival analysis, younger patients presented the best prognosis, while elder patients had the worst both in overall survival (χ2 = 121.9, P < 0.001) and breast cancer-specific survival (χ2 = 69.8, P < 0.001). Age at diagnosis was an independent prognostic factor for metastatic breast cancer patients. Moreover, patients with bone metastasis only had superior survival compared to other metastatic patients (P < 0.001). Brain metastasis only group and multiple sites metastasis group had the poorest prognosis (P < 0.05). We hope the results will provide insights into a better understanding of distant metastatic breast cancer.
Introduction
Breast cancer is the most prevalent type of cancer among females in many countries in the past few years. It was estimated that there were 246,660 new female breast cancer patients in 2016. The overall 5-year relative survival rate is about 89% in the United States. The median age at diagnosis is 61 years, younger than many other kinds of cancer1. Although most breast cancer patients were diagnosed over 60 years, more and more patients were diagnosed at a younger age in the past decade2.
Despite the relatively high 5-year survival rate compared to other malignant tumors, distant metastasis has long been the principal cause of mortality among breast cancer patients. The most common metastatic organs were bone, lung and liver. The effective treatment involves systemic chemotherapy, endocrinotherapy and targeted therapy. However, most of the patients still have poor prognosis after metastasis. Previous study reviewed the breast cancer-specific survival (BCSS) at 10 years in primary stage IV female patients. The 10-year survival was 15.7% for ages 40 and below, 14.9% for ages 41 to 50 and 11.7% for ages 51 to 703. In order to prevent and treat cancer metastasis more precisely, we have to learn more about their clinical features. The disparities of survival time among metastatic breast cancer patients were greatly associated with various clinical indicators such as pathological subtypes4, tumor volumes5, nodal status6, etc.
Cancer metastasis has caused enormous burden on young women patients. Some researchers found that the age of diagnosis probably played important roles in the prognosis of breast cancer7, 8, but few researches focused on the roles of age on metastatic patients. Knowledge of disparities in metastatic patterns may be helpful to make diagnosis of metastasis and treatment decision. In our study, we divided the metastatic patients included into three age groups, namely the younger group (<50 years), middle-aged group (50–69 years) and elder group (>69 years). Previous investigation revealed that breast cancer in younger patients may have more aggressive biological behaviours9. Also, elderly patients with triple-negative breast cancer (TNBC) showed higher early mortality compared to younger counterparts in the first two years of diagnosis10. Therefore, we aimed to identify the clinical characteristics critical and relevant to distant metastatic breast cancer by age groups in a large population via the Surveillance, Epidemiology, and End Results (SEER) database. We hope the results will contribute to the diagnosis and prevention of breast cancer progression.
Results
Demographics and clinical characteristics of metastatic breast cancer patients by age groups
Overall, 4932 metastatic breast cancer patients were included in our study, among which 850 (5.0%) patients were diagnosed below 50 years, 2,540 (63.7%) patients between 50 and 69 years, and 1,542 (31.2%) patients over 70 years. The median age at diagnosis was 62 years and the overall median follow-up time and interquartile range (IQR) was 10 months (2–22 months). The median observation time was 14, 11 and 6 months for the three age groups respectively. In younger group, 329 patients died at the end of the study and 296 died of breast cancer directly. The numbers were 1,200 and 1,007 respectively in middle-aged group, 914 and 727 in elder group. Table 1 summarised the frequency and proportion of some characteristics of these patient groups. There were a series of significant differences among the cohorts of patient samples including race, T stage, N stage, molecular subtypes, surgery and radiation therapy, histological types, etc. (P < 0.05). Year of diagnosis and gender had no substantive differences across the three groups. Specifically, more White people tended to have metastatic breast cancer at older age (68.0% vs. 72.6% vs. 82.2% respectively in younger, middle-aged and elder group, P < 0.05). On the contrary, Black people tended to have metastatic breast cancer at younger age (21.4% vs. 18.6% vs. 13.5% respectively, P < 0.05). Generally, younger and middle-aged patients had bigger tumor size (69.6% vs. 60.6% vs. 52.0% respectively between T2 and T4, P < 0.001) and higher rate of lymph node involvement than elder group (70.6% vs. 61.5% vs. 47.8% respectively, P < 0.001). The T stage and N stage of a few patients were unknown from SEER database. Therefore, the results may need further validation. Moreover, breast cancer molecular subtypes were also important indicators for treatment and prognosis. These three age groups also showed different molecular subtype patterns. Triple negative breast cancer (TNBC) is usually more aggressive than other subtypes and there were limited therapies for TNBC so far. It was noticed that younger patients had obviously higher rate of TNBC (13.1% vs. 10.9% vs. 9.3% respectively, P < 0.05) in our study. As for treatment, younger and middle-aged patients had significantly higher rate of surgery and radiation treatment compared to elder counterparts (P < 0.001). The results may be attributed to their better physical condition to withstand the treatment. In addition, the histological types also showed differences in stage IV patients by age groups (P < 0.001). The most common histological type was infiltrating duct carcinoma (IDC) (younger vs. middle-aged vs. elder: 65.8% vs. 55.0% vs. 46.0% respectively). The second and third common histological types were carcinoma in situ (7.2%) and adenocarcinoma (6.9%) in younger group, adenocarcinoma (12.1%) and lobular carcinoma (10.8%) in middle-aged group, adenocarcinoma (15.2%) and carcinoma in situ (14.0%) in elder group.
Table 1.
Characteristics of breast cancer patients with distant metastasis from SEER 18 population-based registries by age groups.
| Age < 50 years n = 850 (100%) | 50–69 years n = 2,540 (100%) | Age > 69 years n = 1,542 (100%) | Total n = 4,932(100%) | P-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |||
| Gender | Female | 841 | 98.9% | 2,512 | 98.9% | 1,519 | 98.5% | 4,872 | 98.8% | 0.491 |
| Male | 9 | 1.1% | 28 | 1.1% | 23 | 1.5% | 60 | 1.2% | ||
| Year of diagnosis | 2010 | 205 | 24.1% | 639 | 25.2% | 357 | 23.2% | 1,201 | 24.4% | 0.432 |
| 2011 | 225 | 26.5% | 624 | 24.6% | 380 | 24.6% | 1,229 | 24.9% | ||
| 2012 | 221 | 26.0% | 634 | 25.0% | 387 | 25.1% | 1,242 | 25.2% | ||
| 2013 | 199 | 23.4% | 643 | 25.3% | 418 | 27.1% | 1,260 | 25.5% | ||
| Race | White | 578 | 68.0% | 1,843 | 72.6% | 1,267 | 82.2% | 3,688 | 74.8% | <0.001 |
| Black | 182 | 21.4% | 472 | 18.6% | 208 | 13.5% | 862 | 17.5% | ||
| Othersb | 87 | 10.2% | 207 | 8.1% | 59 | 3.8% | 353 | 7.2% | ||
| Unknown | 3 | 0.4% | 18 | 0.7% | 8 | 0.5% | 29 | 0.6% | ||
| T stage | T0 | 23 | 2.7% | 144 | 5.7% | 109 | 7.1% | 276 | 5.6% | <0.001 |
| T1 | 63 | 7.4% | 168 | 6.6% | 92 | 6.0% | 323 | 6.5% | ||
| T2 | 157 | 18.5% | 289 | 11.4% | 189 | 12.3% | 635 | 12.9% | ||
| T3 | 108 | 12.7% | 249 | 9.8% | 129 | 8.4% | 486 | 9.9% | ||
| T4 | 326 | 38.4% | 1,001 | 39.4% | 483 | 31.3% | 1,810 | 36.7% | ||
| Tx | 173 | 20.4% | 689 | 27.1% | 540 | 35.0% | 1,402 | 28.4% | ||
| N stage | N0 | 149 | 17.5% | 534 | 21.0% | 418 | 27.1% | 1,101 | 22.3% | <0.001 |
| N1 | 357 | 42.0% | 910 | 35.8% | 481 | 31.2% | 1,748 | 35.4% | ||
| N2 | 91 | 10.7% | 271 | 10.7% | 119 | 7.7% | 481 | 9.8% | ||
| N3 | 152 | 17.9% | 380 | 15.0% | 137 | 8.9% | 669 | 13.6% | ||
| Nx | 101 | 11.9% | 445 | 17.5% | 387 | 25.1% | 933 | 18.9% | ||
| Molecular subtypec | Her2−/HoR+ | 402 | 47.3% | 1,228 | 48.4% | 737 | 47.8% | 2,367 | 48.0% | <0.001 |
| Her2 + /HoR + | 148 | 17.4% | 308 | 12.1% | 130 | 8.4% | 586 | 11.9% | ||
| Her2+/HoR− | 82 | 9.6% | 185 | 7.3% | 72 | 4.7% | 339 | 6.9% | ||
| Triple Negative | 111 | 13.1% | 277 | 10.9% | 143 | 9.3% | 531 | 10.8% | ||
| Unknown | 107 | 12.6% | 541 | 21.3% | 460 | 29.8% | 1,109 | 22.5% | ||
| Surgeryd | No | 542 | 63.8% | 1,910 | 75.2% | 1,273 | 82.6% | 3,725 | 75.5% | <0.001 |
| Yes | 301 | 35.4% | 611 | 12.4% | 259 | 16.8% | 1,171 | 23.7% | ||
| Unknown | 7 | 0.8% | 19 | 0.7% | 10 | 0.6% | 36 | 0.7% | ||
| Radiation | No | 517 | 60.8% | 1,743 | 68.6% | 1,161 | 75.3% | 3,421 | 69.4% | <0.001 |
| Yes | 305 | 35.9% | 728 | 28.7% | 342 | 22.2% | 1,375 | 27.9% | ||
| Unknown | 28 | 3.3% | 69 | 2.7% | 39 | 2.5% | 136 | 2.8% | ||
| Median follow-up (months) | 14 (5–26) | 11 (3–22) | 6 (1–18) | 10 (2–22) | ||||||
aThe bold type indicates statistically significance.
bOther races includes American Indian, AK Native, Asian and Pacific Islander.
cHer2: human epidermal growth factor receptor-2; HoR: hormone receptor.
dThe surgery only included surgery at the primary site.
Different patterns of metastasis in breast cancer patients by age groups
Among the study population, we found that bone was still the most common site of metastasis for breast cancer (65.1%, including single and multiple metastatic sites), followed by lung (31.4%), liver (26.0%) and brain (8.8%) metastasis. Patients with multiple organs metastasis usually had fewer treatment options and tended to had poorer outcomes. Unfortunately, at least 33.5% of all the cases had multiple organs metastasis. The most common multiple metastatic combination was bone and lung, constituting 31.4% (n = 484) of the multiple metastatic patients.
We then identified 2,881 single site and 1,653 multiple organs metastatic patients. (Table 2). Interestingly, among patients with single metastatic site, elder patients had significantly higher rate of lung metastasis (5.9% vs. 7.6% vs. 14.2% respectively, P < 0.001) and lower rate of only distant lymphatic metastasis (7.3% vs. 5.4% vs. 4.0% respectively, P = 0.004). We also found that higher proportion of younger (34.9%) and middle-aged (36.2%) patients had multiple metastatic sites than elder patients (28.3%) (P < 0.001). However, there were no significant differences in bone, liver or brain only metastasis among the three age groups.
Table 2.
The number and proportion of breast cancer patients with single metastatic site and multiple metastatic sites.
| Age < 50 years | 50–69 years | Age > 69 years | Total | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Bone only | 307 | 36.1% | 868 | 34.2% | 581 | 37.7% | 1,756 | 35.6% | 0.072 |
| Lung only | 50 | 5.9% | 192 | 7.6% | 219 | 14.2% | 461 | 9.3% | <0.001 |
| Liver only | 69 | 8.1% | 160 | 6.3% | 88 | 5.7% | 317 | 6.4% | 0.066 |
| Brain only | 16 | 1.9% | 46 | 1.8% | 24 | 1.6% | 86 | 1.7% | 0.787 |
| Distant lymph nodes only | 62 | 7.3% | 136 | 5.4% | 63 | 4.0% | 261 | 5.3% | 0.004 |
| Multiple sites | 297 | 34.9% | 920 | 36.2% | 436 | 28.3% | 1,653 | 33.5% | <0.001 |
Comparison of overall survival (OS) and breast cancer-specific survival (BCSS) among the study population
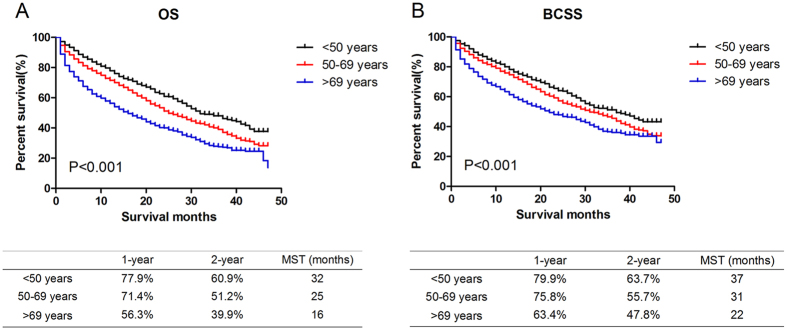
As shown in Kaplan-Meier plots (Fig. 1), there were substantive differences in OS (χ2 = 121.9, P < 0.001) and BCSS (χ2 = 69.8, P < 0.001) among the three age groups. Younger patients presented the best outcome while elder patients had the worst. The median overall survival time was 32, 25 and 16 months respectively in younger, middle-aged and elder groups.
Figure 1.
Comparison of survival in younger, middle-aged and elder metastatic breast cancer patients. Kaplan Meier analysis for overall survival (OS, χ2 = 121.9, P < 0.001, Fig. 1A) and breast cancer-specific survival (BCSS, χ2 = 69.8, P < 0.001, Fig. 1B) were shown in the graph. The prognosis became worse with the increase of age. The 1-year, 2-year survival rate and median survival time (MST) were listed respectively in the table below the graph.
Moreover, we conducted univariate analysis and multivariate analysis (Table 3) with Cox hazard regression model to evaluate the effects of baseline characteristics on OS and BCSS in the whole study population. In the multivariate analysis, we found that age at diagnosis, race, T stage, molecular subtypes, surgery, radiation therapy, and distant organ metastasis were all significantly associated with BCSS (P < 0.05). All of the factors above were associated with OS (P < 0.05) except bone metastasis (P = 0.299). However, gender, year of diagnosis and N stage were not distinctly correlated with prognosis in this model. As was shown, age at diagnosis was an independent prognostic factor for metastatic breast cancer patients. Compared to middle-aged patients, younger patients had better OS (HR: 0.77, 95% CI: 0.68–0.87, P < 0.001) and BCSS (HR: 0.81, 95% CI: 0.71–0.92, P = 0.002). The elder group had the worst OS (HR: 1.56, 95% CI: 1.43–1.70, P < 0.001) and BCSS (HR:1.52, 95% CI: 1.38–1.68, P < 0.001). The results were consistent with Kaplan-Meier plots. We also found that patients underwent primary site surgery or radiotherapy had better survival, indicating potential benefits from regional treatment in metastatic patients.
Table 3.
Univariate and multivariate analysis of overall survival (OS) and breast cancer-specific survival (BCSS) of the study population.
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OS | BCSS | OS | BCSS | ||||||
| HRs (95% CI)a | P-value | HRs (95% CI) | P-value | HRs (95% CI) | P-value | HRs (95% CI) | P-value | ||
| Age | <50 years | 0.72 (0.64–0.82) | P < 0.001 | 0.78 (0.68–0.88) | P < 0.001 | 0.77 (0.68–0.87) | P < 0.001 | 0.81 (0.71–0.92) | 0.002 |
| 50–69 years | reference | — | reference | — | reference | — | reference | — | |
| >69 years | 1.50 (1.38–1.64) | P < 0.001 | 1.43 (1.30–1.57) | P < 0.001 | 1.56 (1.43–1.70) | P < 0.001 | 1.52 (1.38–1.68) | P < 0.001 | |
| Gender | Female | reference | — | reference | — | reference | — | reference | — |
| Male | 1.04 (0.71–1.51) | 0.850 | 1.03 (0.68–1.55) | 0.907 | 0.96 (0.66–1.40) | 0.962 | 0.99 (0.65–1.50) | 0.954 | |
| Year of diagnosis | 2010 | reference | — | reference | — | reference | — | reference | — |
| 2011 | 0.93 (0.84–1.03) | 0.175 | 0.91 (0.82–1.02) | 0.913 | 0.94 (0.85–1.04) | 0.214 | 0.91 (0.82–1.02) | 0.111 | |
| 2012 | 0.97 (0.86–1.08) | 0.532 | 0.96 (0.85–1.02) | 0.481 | 0.98 (0.88–1.10) | 0.714 | 0.97 (0.86–1.10) | 0.605 | |
| 2013 | 0.94 (0.82–1.08) | 0.402 | 0.94 (0.81–1.10) | 0.440 | 0.92 (0.80–1.06) | 0.230 | 0.91 (0.78–1.06) | 0.241 | |
| Race | White | reference | — | reference | — | reference | — | reference | — |
| Black | 1.20 (1.09–1.33) | P < 0.001 | 1.17 (1.05–1.31) | 0.006 | 1.22 (1.10–1.36) | P < 0.001 | 1.15 (1.03–1.29) | 0.015 | |
| Others | 0.83 (0.70–0.98) | 0.023 | 0.87 (0.73–1.04) | 0.116 | 0.91 (0.77–1.08) | 0.270 | 0.93 (0.78–1.11) | 0.419 | |
| Unknown | 0.35 (0.13–0.95) | 0.038 | 0.43 (0.16–1.13) | 0.088 | 0.44 (0.17–1.19) | 0.443 | 0.56 (0.21–1.49) | 0.244 | |
| T stage | T0 | reference | — | reference | — | reference | — | reference | — |
| T1 | 0.67 (0.52–0.87) | 0.003 | 0.79 (0.58–1.07) | 0.121 | 0.99 (0.76–1.29) | 0.934 | 1.13 (0.83–1.54) | 0.426 | |
| T2 | 0.85 (0.69–1.06) | 0.146 | 1.07 (0.83–1.39) | 0.588 | 1.27 (1.02–1.59) | 0.035 | 1.55 (1.19–2.01) | 0.001 | |
| T3 | 0.94 (0.75–1.18) | 0.617 | 1.28 (0.99–1.67) | 0.061 | 1.34 (1.06–1.70) | 0.014 | 1.77 (1.35–2.31) | P < 0.001 | |
| T4 | 1.28 (1.06–1.55) | 0.011 | 1.66 (1.32–2.09) | P < 0.001 | 1.62 (1.33–1.98) | P < 0.001 | 2.02 (1.60–2.57) | P < 0.001 | |
| Tx | 1.44 (1.19–1.75) | P < 0.001 | 1.69 (1.34–2.14) | P < 0.001 | 1.44 (1.18–1.75) | P < 0.001 | 1.68 (1.32–2.13) | P < 0.001 | |
| N stage | N0 | reference | — | reference | — | reference | — | reference | — |
| N1 | 0.93 (0.84-1.04) | 0.208 | 1.03 (0.92–1.17) | 0.592 | 0.91 (0.81–1.02) | 0.088 | 0.96 (0.85–1.09) | 0.503 | |
| N2 | 0.78 (0.66–0.91) | 0.001 | 0.85 (0.71–1.01) | 0.060 | 0.87 (0.73–1.03) | 0.095 | 0.90 (0.75–1.08) | 0.241 | |
| N3 | 0.92 (0.80–1.06) | 0.241 | 1.02 (0.88–1.19) | 0.757 | 1.00 (0.87-1.16) | 0.975 | 1.05 (0.90–1.24) | 0.5336 | |
| Nx | 1.34 (1.19–1.50) | P < 0.001 | 1.35 (1.18–1.54) | P < 0.001 | 1.07 (0.95–1.21) | 0.285 | 1.08 (0.94–1.24) | 0.293 | |
| Molecular subtype | Her2–/HoR + | reference | — | reference | — | reference | — | reference | — |
| Her2 + /HoR+ | 0.88 (0.76-1.02) | 0.085 | 0.86 (0.73–1.01) | 0.061 | 0.86 (0.74–1.00) | 0.035 | 0.81 (0.69–0.95) | 0.011 | |
| Her2+/HoR– | 1.17 (0.99–1.38) | 0.073 | 1.18 (0.99–1.42) | 0.066 | 1.08 (0.91–1.28) | 0.368 | 1.08 (0.89–1.29) | 0.439 | |
| Triple Negative | 2.29 (2.03–2.58) | P < 0.001 | 2.33 (2.05-0.65) | P < 0.001 | 2.26 (2.00–2.56) | P < 0.001 | 2.31 (2.02–2.64) | P < 0.001 | |
| Unknown | 1.78 (1.61–1.96) | P < 0.001 | 1.64 (1.47–1.83) | P < 0.001 | 1.53 (1.38–1.70) | P < 0.001 | 1.48 (1.32–1.66) | P < 0.001 | |
| Surgery | No | reference | — | reference | — | reference | — | reference | — |
| Yes | 0.49 (0.44-0.55) | P < 0.001 | 0.51 (0.45–0.57) | P < 0.001 | 0.61 (0.55–0.69) | P < 0.001 | 0.62 (0.55–0.70) | P < 0.001 | |
| Unknown | 1.21 (0.77–1.90) | 0.414 | 1.31 (0.81-2.11) | 0.270 | 1.54 (0.96–2.45) | 0.071 | 1.64 (1.00–2.69) | 0.051 | |
| Radiation | No | reference | — | reference | — | reference | — | reference | — |
| Yes | 0.72 (0.66–0.79) | P < 0.001 | 0.75 (0.68–0.83) | P < 0.001 | 0.79 (0.72–0.87) | P < 0.001 | 0.80 (0.72–0.89) | P < 0.001 | |
| Unknown | 0.65 (0.49-0.86) | 0.003 | 0.67 (0.49–0.91) | 0.010 | 0.77 (0.58–1.03) | 0.080 | 0.77 (0.56–1.06) | 0.109 | |
| Bone metastasis | No | reference | — | reference | — | reference | — | reference | — |
| Yes | 0.92 (0.85-1.00) | 0.040 | 0.97 (0.87–1.06) | 0.970 | 1.05 (0.96–1.15) | 0.267 | 1.11 (1.01–1.22) | 0.035 | |
| Lung metastasis | No | reference | — | reference | — | reference | — | reference | — |
| Yes | 1.47 (1.36-1.60) | P < 0.001 | 1.52 (1.39–1.67) | P < 0.001 | 1.22 (1.12–1.33) | P < 0.001 | 1.24 (1.13-1.36) | P < 0.001 | |
| Liver metastasis | No | reference | — | reference | — | reference | — | reference | — |
| Yes | 1.79 (1.64–1.94) | P < 0.001 | 1.90 (1.73–2.09) | P < 0.001 | 1.80 (1.65-1.97) | P < 0.001 | 1.90 (1.72–2.09) | P < 0.001 | |
| Brain metastasis | No | reference | — | reference | — | reference | — | reference | — |
| Yes | 1.81 (1.60–2.05) | P < 0.001 | 1.87 (1.64-2.14) | P < 0.001 | 1.74 (1.53–1.98) | P < 0.001 | 1.77 (1.54–2.04) | P < 0.001 | |
aHRs: hazard ratios; CI: confidence interval.
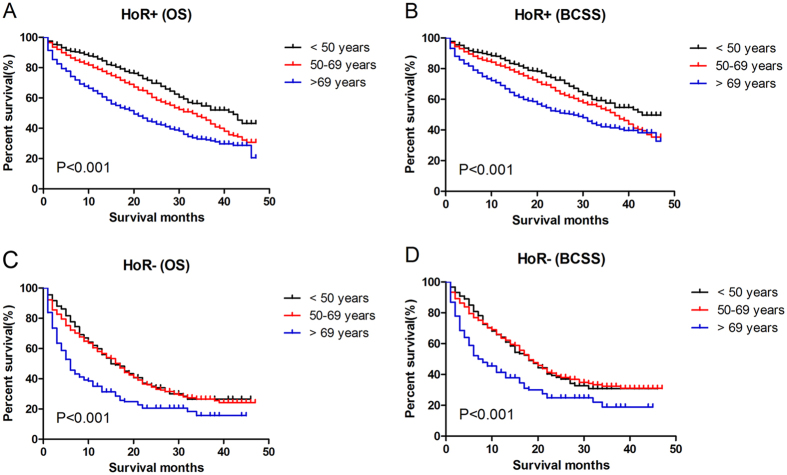
We also conducted survival analysis in subgroups according to hormone receptor status (Fig. 2). In hormone receptor positive patients, the prognosis became worse with the increase of age. However, in hormone receptor negative patients, younger group did not show better prognosis compared to middle-aged patients (P > 0.05). The middle-aged group even had slightly longer median survival time (MST) than younger group.
Figure 2.
Comparison of survival in breast cancer patients with different hormone receptor status. Kaplan Meier analysis for OS and BCSS in different subgroups were shown in the graph. In hormone receptor positive (A,B) patients, the prognosis became worse with the increase of age (P < 0.001). In hormone receptor negative patients (C,D), middle-aged patients had similar survival to younger patients (P > 0.05), though older patients still had the worst prognosis. HoR+: hormone receptor positive; HoR-: hormone receptor negative.
Comparison of survival among single site and multiple sites metastatic breast cancer patients
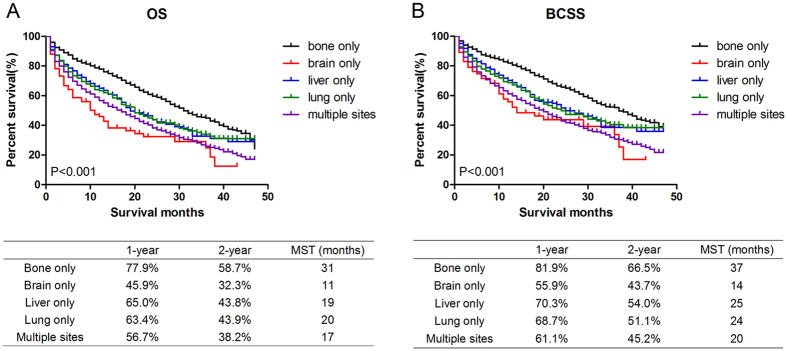
We used Kaplan-Meier analysis to compare the effects of single and multiple distant metastatic organs on survival time among the study population. The 1-year and 2-year survival rate and MST were also calculated for each group. The results suggested that there were significant differences among patients with different specific metastatic sites in OS (χ2 = 147.7, P < 0.001, Fig. 3A) and BCSS (χ2 = 145.7, P < 0.001, Fig. 3B). Patients with bone metastasis only had superior survival compared to other metastatic patients (MST: 31 months in OS and 37 months in BCSS, P < 0.001). Patients with lung or liver invasion only had similar intermediate MST (P > 0.05). However, brain metastasis only group (MST: 11 months in OS and 14 months in BCSS) and multiple sites metastasis group (MST: 17 months in OS and 20 months in BCSS) had the poorest prognosis compared to other groups (P < 0.05) and there was no significant difference between these two groups both in OS and BCSS (P > 0.05) as well. The prognosis of three age groups with single metastasis was also analysed (Supplementary Figure S1). The results suggested that the prognosis become worse with the increase of age in bone metastasis only and liver only patients (P < 0.001), but not in lung metastatic patients (P > 0.05). The statistics of brain metastasis was not analysed because of the limited number of samples.
Figure 3.
Comparison of survival in breast cancer patients with single or multiple metastatic sites. Kaplan Meier analysis for OS (χ2 = 147.7, P < 0.001, Fig. 2A) and BCSS (χ2 = 145.7, P < 0.001, Fig. 2B) were shown. The 1-year, 2-year survival rate and median survival time (MST) were listed respectively in the table below the graph. MST: median survival time.
Discussion
With the increase of cancer incidence worldwide, there will be more patients suffer from cancer invasion and metastasis. Tumor metastasis is a complicated and multi-stage process which involves cell proliferation, angiogenesis, migration and many other cell functions11. There are certain specificities in tumor dissemination and invasion to distant organs. We hope to provide deep insights into better understanding of the heterogeneity of breast cancer.
In our retrospective study, we analysed the influence of age at diagnosis on breast cancer metastasis and mortality. The incidence of breast cancer among younger women has been increased in recent years2. This phenomenon may be due to the deteriorating pollution, increased exposure to estrogen, alcohol or many other risk factors. There were also significant differences between the three age groups including race, T stage, N stage, molecular subtypes, surgery and radiation therapy, histological types, etc. Interestingly, we found that younger age at diagnosis was associated with bigger tumor size, more lymph node involvement and higher rate of TNBC. Also, younger stage IV patients were more likely to have multiple and distant lymph nodes metastasis, but less likely to have lung metastasis. Our results are generally consistent with some of the previous studies12, 13. However, another study among 3553 breast cancer patients, with 6.32 years median follow-up, revealed a reduction in risk of bone and viscera metastasis with the increase of age at diagnosis14. The MST was 1.52 (IQR: 0.7–2.9) years for patients with bone metastasis and 0.7 (IQR: 1.2–1.5) years for visceral metastasis. Hung MH, et al. suggested that younger patients (age < 35 years) were particularly at risk of brain metastasis regardless of biological subtype15. The “seed and soil hypothesis” may partially account for the phenomenon of different metastatic patterns16. It seems that different subpopulations of tumor cells favored different microenvironment of distant organs, which provides ideal condition for their invasion and proliferation. It is necessary to treat different subpopulations of tumor cells with different strategies. The underlying molecular mechanisms still need further investigations. We also hypothesize that the immune system may have important influence on the various patterns of metastasis. The younger patients usually have stronger immune response, leading to differences in tumor microenvironment. Recent research found that neutrophils can help the colonization of breast cancer initiating cells in the lung17. The roles of immune cells and inflammatory mediators, like a two-edged sword, in tumor metastasis are still controversial.
We also evaluated the effects of age at diagnosis and metastatic sites on breast cancer mortality in a large sample of population. In Kaplan Meier analysis and Cox hazard regression model, the results indicated that older age contributed significantly to the poorer prognosis both in OS and BCSS. Age at diagnosis was one of independent prognostic factors in the study population. There may be multi-factorial explanations. A lot of age-related factors may play important roles in metastasis, including accumulation of DNA damage, immune response18, chronic inflammation19, hormone level changes, etc. Furthermore, younger patients usually have higher chance to receive chemotherapy, radiation therapy and surgery. Even with distant organ metastasis, patients will still benefit from local treatment, including mastectomy20, 21. Interestingly, in hormone receptor positive patients, younger group showed better prognosis than middle-aged patients, while the two age groups had similar survival time in hormone receptor negative patients. Recent studies revealed the important roles of hormone level on distant metastasis22. The disparity in hormone level may influence the incidence and survival between prepremenopausal and postmenopausal patients. Many other researches suggested that younger breast cancer patients were characterised as more advanced stages and more aggressive in clinical behaviour23, 24. There may be other underlying factors. All of these factors contribute to the complex relationship between age and metastasis.
More importantly, we demonstrated that patients with different metastasis patterns had different survival outcomes. To be specific, bone metastasis only group had the longest MST compared to other metastatic patients, while brain metastasis only group and multiple sites metastasis group had the poorest outcome. Patients with only lung and only liver invasion had similar MST. Despite large number of bone metastatic patients, there are several effective chemotherapies, endocrine therapy and other medications such as zoledronic acid, which have distinct benefits on stage IV patients survival. Unfortunately, effective therapies are still limited for patients with brain (mainly because of the blood-brain barrier) and multiple sites metastasis currently25. The results may remind clinical physicians of treating breast cancer patients in a more individual manner.
Tremendous efforts had been made to explore the prevention and treatment of breast cancer metastasis. The concept of precise medicine had been put forward recently in order to prevent and treat cancer more individually. It was recently revealed that there were circulating tumor cells (CTCs) in the peripheral blood of breast cancer patients26. CTCs were also estimated to be an independent prognostic indicator of poor survival outcomes for triple-negative breast cancer (TNBC) patients27. Breast cancer was considered to be a systematic disease at the very early stage. If the CTCs can be detected more sensitively in the blood or by other non-invasive means, distant metastasis may be predicted more precisely before lesions in distant organs appeared. Therefore, it was necessary to prevent distant metastasis at an earlier period of the disease than we thought before.
There are also some limitations in our study. We could not collect the patients’ information of chemotherapy, endocrine therapy and many other related factors from SEER database. This may cause a certain bias in our results. In addition, the information of specific metastatic organ and Her2 status was only available after 2010 in SEER database, which may not ensure enough number of samples and enough time of follow-up in the study, especially brain metastatic samples. Besides age at diagnosis, several other factors may also influence the survival time. For example, younger metastatic patients may have higher chances to achieve chemotherapy, targeted therapy or other intensive systemic treatment. Many elder patients had co-morbidities which may lead to the exacerbation of the disease. Therefore, the results need further validation in future studies.
In conclusion, our research summarised the clinical characteristics and survival outcomes of metastatic breast cancer patients in three age groups in a large sample of population. The results may provide more evidence for precise medicine and individualized therapy. Further efforts still need to be done in order to investigate more comprehensive factors associated with breast cancer metastasis in the future.
Methods
Ethics statement
The SEER research data files were downloaded using the reference number 11443-Nov2015. The data released by the SEER database do not require informed patient consent. Our study had already been approved by the Ethical Committee and Institutional Review Board of Fudan University Shanghai Cancer Centre (FDUSCC). The methods were performed in accordance with the approved guidelines.
Data collection
SEER*Stat version 8.3.2 was utilized to filtrate and collect the information of representative patients in the research (http://seer.cancer.gov/). We chose patients from SEER 18 Regs Research Data which covered approximately 28% of the U.S. population when follow-up ended before 31/12/2013. We finally focused on 4932 eligible patients based on the following criteria: microscopically confirmed primary breast cancer patients, diagnosis between 2010 and 2013, known age at diagnosis, and de novo stage IV (AJCC 7th edition) patients. Patients with metastasis to distant lymph nodes were also included. We chose patients who were diagnosed since 2010 because the information of distant metastatic to specific organs and molecular subtypes were only available after 2010. The patients registered after 2013 were not included because we would like to ensure enough time of follow-up.
Statistical analysis
All the patients were divided into three age groups: the younger group (<50 years), middle-aged group (50–69 years) and elder group (>69 years). We used SPSS 22.0 software to analyse the information we obtained from the database. The clinical characteristics of the selected patients were compared with the Pearson’s χ2 test. The survival curves were drawn with Kaplan Meier analysis and the curves were compared with log rank test with GraphPad Prism 5.0. Cox regression models were used to identify factors which were significantly associated with overall survival (OS) and breast cancer-specific survival (BCSS). OS was defined as the time from breast cancer diagnosis to death due to any cause and BCSS from breast cancer diagnosis to death due to breast cancer. The 1-year and 2-year survival rate and median survival rate was also calculated. At the meantime, hazard ratios (HRs) and 95% confidence interval (95% CI) were also analysed. We defined P-value < 0.05 as statistically significant.
Electronic supplementary material
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81472669, 81302299). The authors would like to thank Dr. Ding Ma for his suggestion and assistance with the data analysis.
Author Contributions
W.J. and M.-T.-C. conceived and designed the study. M.-T.-C., H.-F.S., S.-P.G., L.-D.L, W.-Y.F. performed the analysis, H.-F.S., H.-L.J. and Y.-L.Y. prepared the figures and tables, M.-T.-C. analysed the results and wrote the main manuscript. All of the authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Meng-Ting Chen and He-Fen Sun contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10166-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller KD, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: a cancer journal for clinicians. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 3.Eng LG, et al. Ten-year survival in women with primary stage IV breast cancer. Breast cancer research and treatment. 2016;160:145–152. doi: 10.1007/s10549-016-3974-x. [DOI] [PubMed] [Google Scholar]
- 4.Kennecke H, et al. Metastatic behavior of breast cancer subtypes. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 5.Marinelli B, et al. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. American journal of nuclear medicine and molecular imaging. 2016;6:120–127. [PMC free article] [PubMed] [Google Scholar]
- 6.Wang XX, Jiang YZ, Li JJ, Song CG, Shao ZM. Effect of nodal status on clinical outcomes of triple-negative breast cancer: a population-based study using the SEER 18 database. Oncotarget. 2016;7:46636–46645. doi: 10.18632/oncotarget.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crivellari D, et al. Breast cancer in the elderly. Journal of clinical oncology. 2007;25:1882–1890. doi: 10.1200/JCO.2006.10.2079. [DOI] [PubMed] [Google Scholar]
- 8.Sabiani L, et al. Breast cancer in young women: Pathologic features and molecular phenotype. Breast (Edinburgh, Scotland). 2016;29:109–116. doi: 10.1016/j.breast.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Azim HA, Jr., et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clinical cancer research. 2012;18:1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, Perez EA, Hong R, Li Q, Xu B. Age-Related Disparity in Immediate Prognosis of Patients with Triple-Negative Breast Cancer: A Population-Based Study from SEER Cancer Registries. PloS one. 2015;10 doi: 10.1371/journal.pone.0128345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Inal A, et al. Pathologic and clinical characteristics of elderly patients with breast cancer: a retrospective analysis of a multicenter study (Anatolian Society of Medical Oncology) International surgery. 2014;99:2–7. doi: 10.9738/INTSURG-D-13-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liedtke C, et al. The prognostic impact of age in different molecular subtypes of breast cancer. Breast cancer research and treatment. 2015;152:667–673. doi: 10.1007/s10549-015-3491-3. [DOI] [PubMed] [Google Scholar]
- 14.Purushotham A, et al. Age at diagnosis and distant metastasis in breast cancer–a surprising inverse relationship. European journal of cancer. 2014;50:1697–1705. doi: 10.1016/j.ejca.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Hung M. H. et al. Effect of age and biological subtype on the risk and timing of brain metastasis inbreast cancer patients. PLoS One. 9, doi:10.1371/journal.pone.0089389 (2014). [DOI] [PMC free article] [PubMed]
- 16.Ribelles N, Santonja A, Pajares B, Llacer C, Alba E. The seed and soil hypothesis revisited: current state of knowledge of inherited genes on prognosis in breast cancer. Cancer treatment reviews. 2014;40:293–299. doi: 10.1016/j.ctrv.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Li X, Liu X, Liu Y. The role of tumor-associated macrophages in breast carcinoma invasion and metastasis. International journal of clinical and experimental pathology. 2015;8:6656–6664. [PMC free article] [PubMed] [Google Scholar]
- 19.Hugo HJ, Saunders C, Ramsay RG, Thompson EW. New Insights on COX-2 in Chronic Inflammation Driving Breast Cancer Growth and Metastasis. Journal of mammary gland biology and neoplasia. 2015;20:109–119. doi: 10.1007/s10911-015-9333-4. [DOI] [PubMed] [Google Scholar]
- 20.Warschkow R, et al. Improved Survival After Primary Tumor Surgery in Metastatic Breast Cancer: A Propensity-adjusted, Population-based SEER Trend Analysis. Annals of surgery. 2016;263:1188–1198. doi: 10.1097/SLA.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 21.Sofi AA, Mohamed I, Koumaya M, Kamaluddin Z. Local therapy in metastatic breast cancer is associated with improved survival. American journal of therapeutics. 2013;20:487–492. doi: 10.1097/MJT.0b013e31822119c5. [DOI] [PubMed] [Google Scholar]
- 22.Hosseini H, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540:552–558. doi: 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kataoka A, et al. Clinicopathological features of young patients (<35 years of age) with breast cancer in a Japanese Breast Cancer Society supported study. Breast Cancer. 2014;21:643–50. doi: 10.1007/s12282-013-0466-2. [DOI] [PubMed] [Google Scholar]
- 24.Park YH, et al. Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: Single institutional experience in Korea. Breast. 2015;24:213–7. doi: 10.1016/j.breast.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. Journal of neuro-oncology. 2016;127:407–414. doi: 10.1007/s11060-016-2075-3. [DOI] [PubMed] [Google Scholar]
- 26.Bidard FC, Proudhon C, Pierga JY. Circulating tumor cells in breast cancer. Molecular oncology. 2016;10:418–430. doi: 10.1016/j.molonc.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu YJ, et al. The significant prognostic value of circulating tumor cells in triple-negative breast cancer: a meta-analysis. Oncotarget. 2016;7:37361–37369. doi: 10.18632/oncotarget.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.