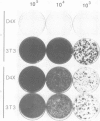
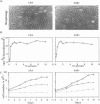
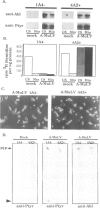
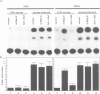
Abstract
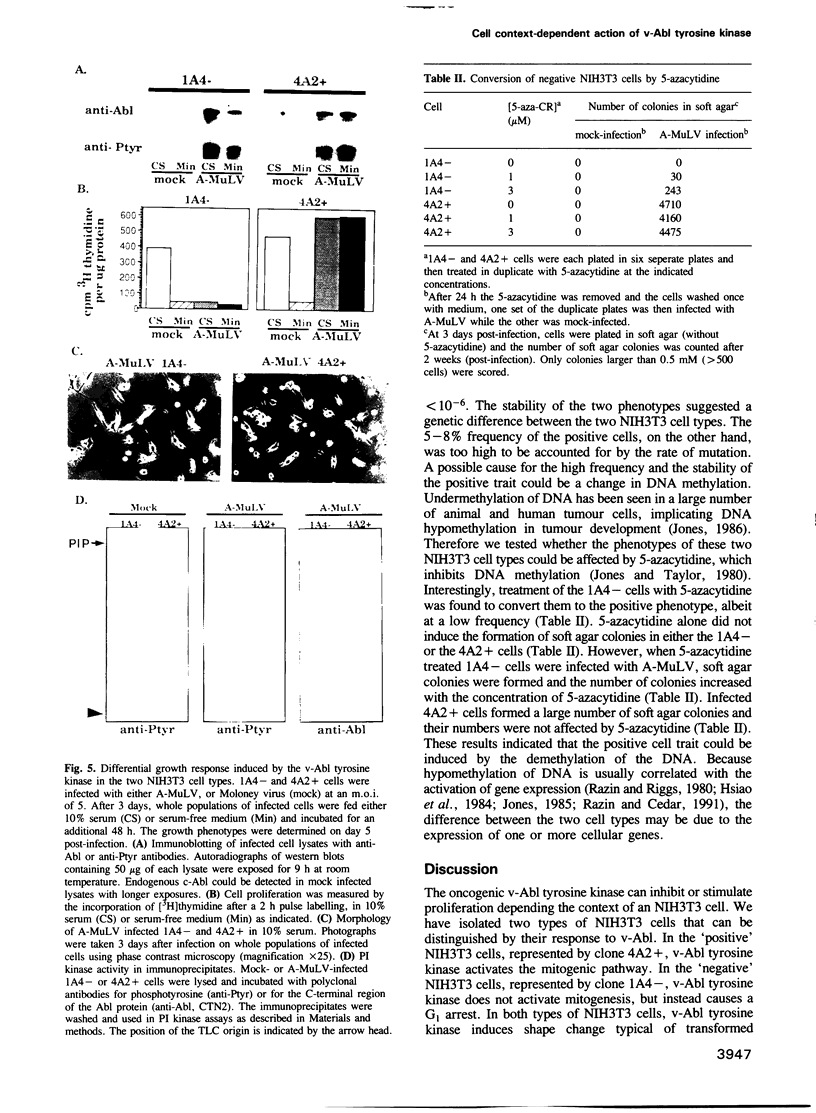
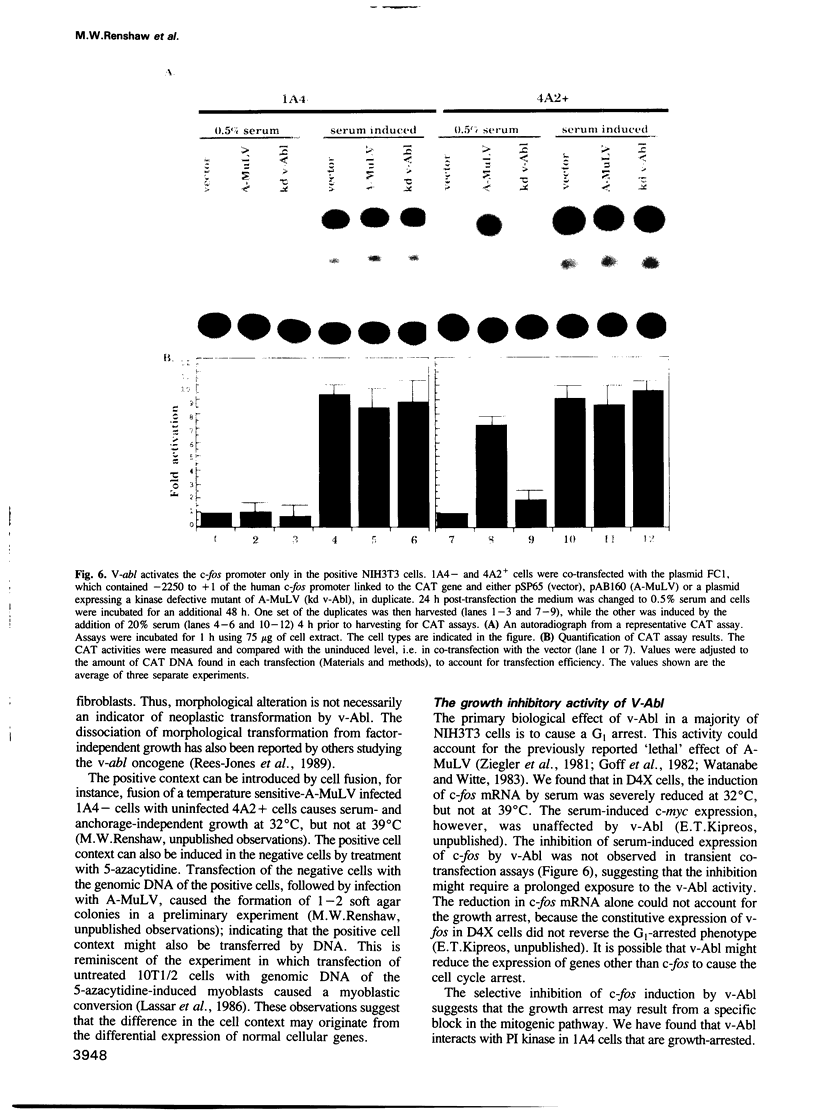
The v-abl oncogene of Abelson murine leukemia virus (A-MuLV) induces two opposite phenotypes in NIH3T3 cells. In the majority of cells, v-abl causes a growth arrest at the G1 phase of the cell cycle; while in a minority of cells, v-abl abrogates the requirement for growth factors. Using temperature sensitive mutants, it can be demonstrated that v-Abl tyrosine kinase is required for growth inhibition or stimulation. The two phenotypes are not caused by mutations or differences in the expression of v-Abl, but are dependent on the cell context. Two stable subclones of NIH3T3 cells have been isolated that exhibit similar morphology and growth characteristics. However, upon infection with A-MuLV, the 'positive' cells become serum- and anchorage-independent, whereas the 'negative' cells become arrested in G1. The positive phenotype is dominant, shown by cell fusion, and treatment with 5-azacytidine converts the negative cells to the positive phenotype. Activation of v-Abl tyrosine kinase induces the serum-responsive genes in the positive but not in the negative cells. Transactivation of the c-fos promoter by v-Abl in transient assays is also restricted to the positive cells. These results show that v-Abl tyrosine kinase is not an obligatory activator of growth, but requires a permissive cellular context to manifest its mitogenic function.
Full text
PDF
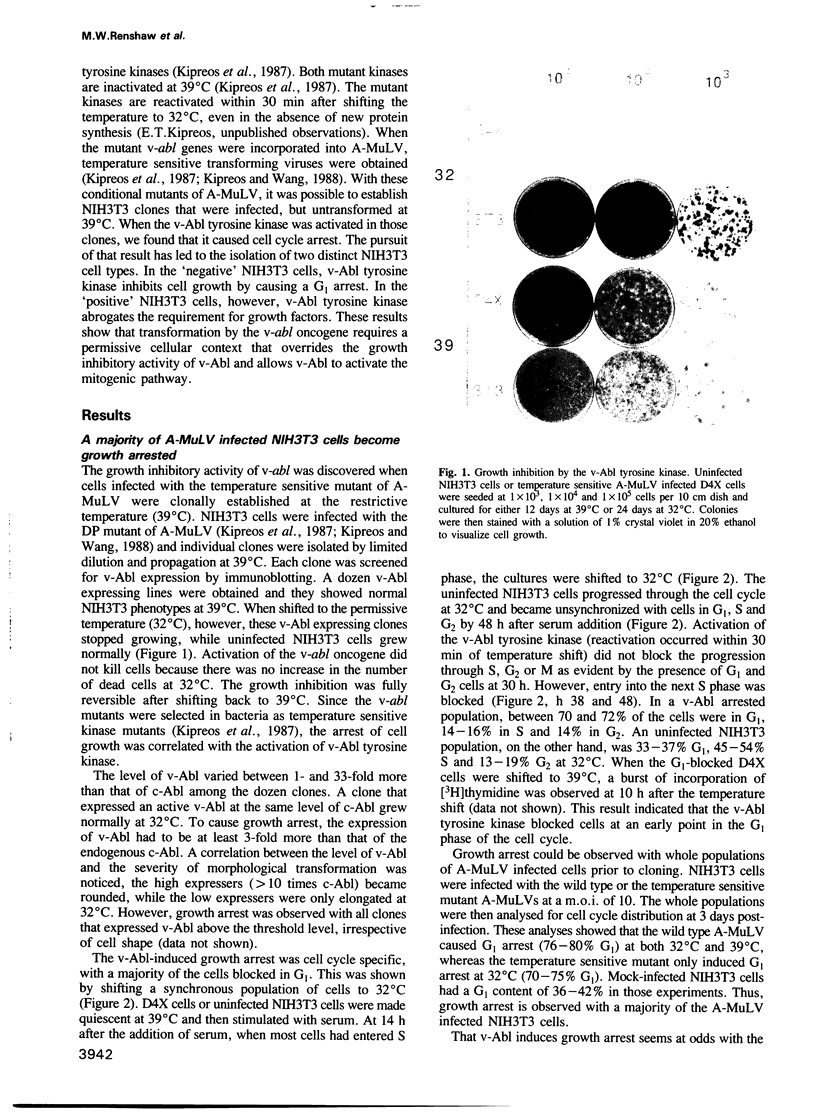
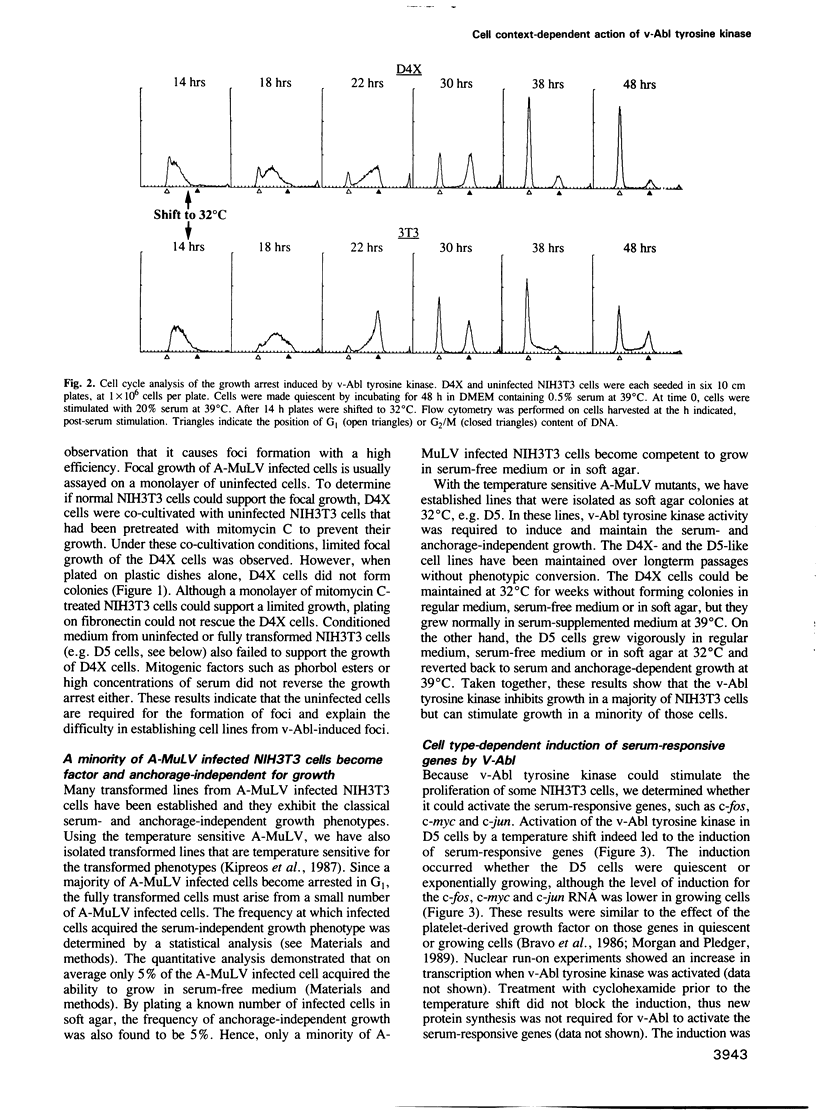
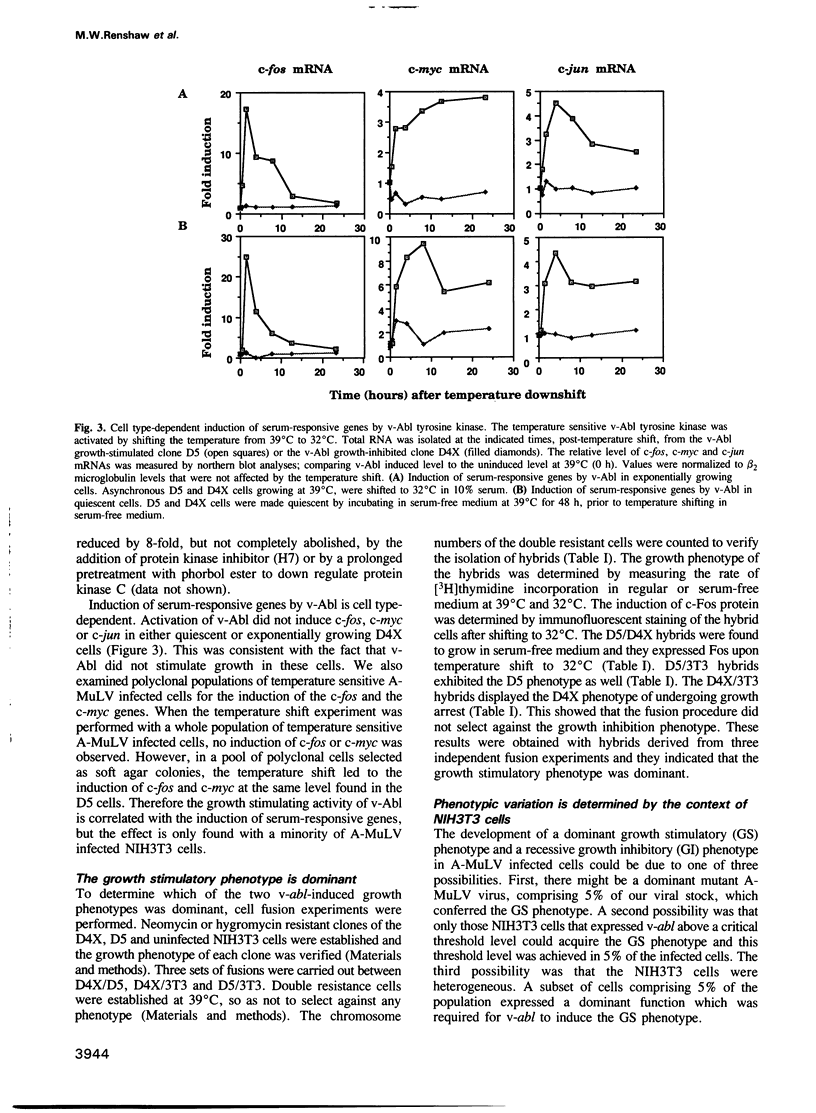

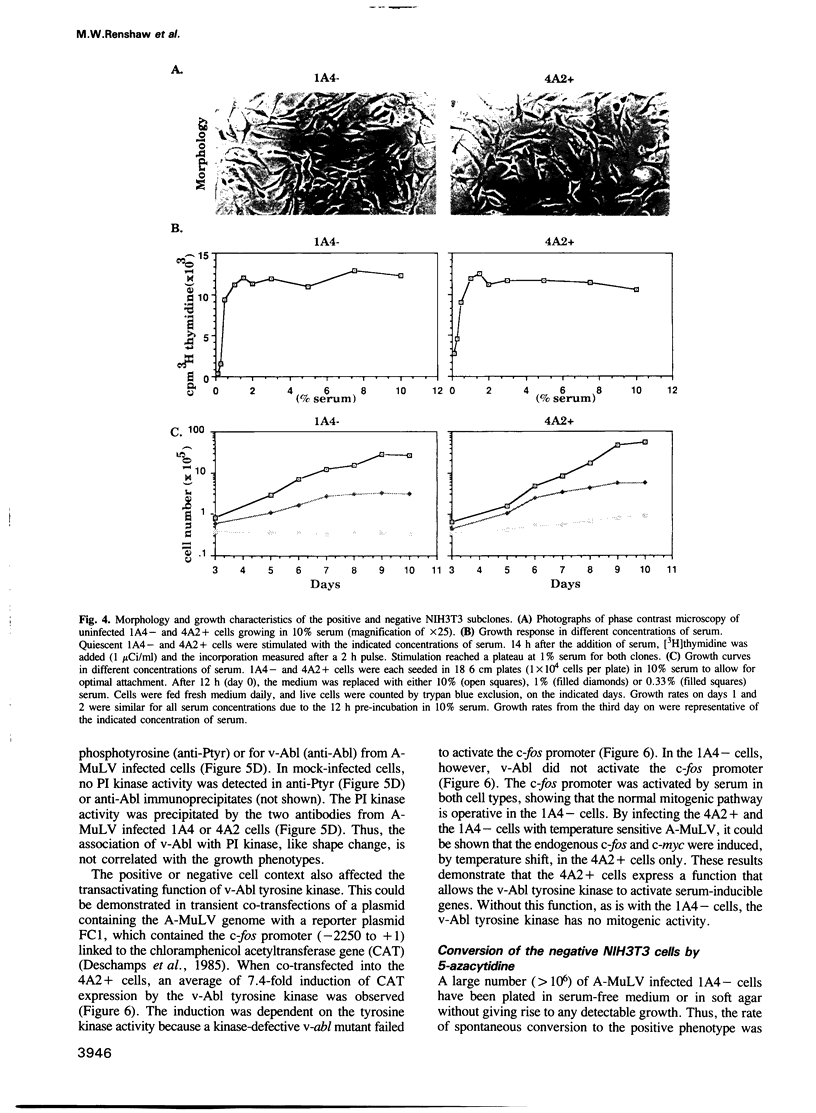



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Medrano E. E. Cell cycle perturbations in normal and transformed fibroblasts caused by detachment from the substratum. J Cell Physiol. 1983 Jan;114(1):53–60. doi: 10.1002/jcp.1041140109. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Dean M., Rosenberg N., Wang J. Y., Rapp U. R. Tyrosine kinase oncogenes abrogate interleukin-3 dependence of murine myeloid cells through signaling pathways involving c-myc: conditional regulation of c-myc transcription by temperature-sensitive v-abl. Mol Cell Biol. 1989 Dec;9(12):5685–5695. doi: 10.1128/mcb.9.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Homologous and heterologous mitogenic desensitization of Swiss 3T3 cells to phorbol esters and vasopressin: role of receptor and postreceptor steps. J Cell Physiol. 1984 Feb;118(2):133–142. doi: 10.1002/jcp.1041180205. [DOI] [PubMed] [Google Scholar]
- Cook W. D., Fazekas de St Groth B., Miller J. F., MacDonald H. R., Gabathuler R. Abelson virus transformation of an interleukin 2-dependent antigen-specific T-cell line. Mol Cell Biol. 1987 Jul;7(7):2631–2635. doi: 10.1128/mcb.7.7.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976 Mar;2(2):165–176. doi: 10.1007/BF01542629. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- De Togni P., Niman H., Raymond V., Sawchenko P., Verma I. M. Detection of fos protein during osteogenesis by monoclonal antibodies. Mol Cell Biol. 1988 May;8(5):2251–2256. doi: 10.1128/mcb.8.5.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps J., Meijlink F., Verma I. M. Identification of a transcriptional enhancer element upstream from the proto-oncogene fos. Science. 1985 Dec 6;230(4730):1174–1177. doi: 10.1126/science.3865371. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Tabin C. J., Wang J. Y., Weinberg R., Baltimore D. Transfection of fibroblasts by cloned Abelson murine leukemia virus DNA and recovery of transmissible virus by recombination with helper virus. J Virol. 1982 Jan;41(1):271–285. doi: 10.1128/jvi.41.1.271-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Kirschmeier P., Weinstein I. B. Effects of 5-azacytidine on methylation and expression of specific DNA sequences in C3H 10T1/2 cells. Mol Cell Biol. 1984 Apr;4(4):634–641. doi: 10.1128/mcb.4.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A. DNA methylation and cancer. Cancer Res. 1986 Feb;46(2):461–466. [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Mendelsohn J., Le A., Sato G. H., Lazar C. S., Gill G. N. Relation of epidermal growth factor receptor concentration to growth of human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Jun 25;259(12):7761–7766. [PubMed] [Google Scholar]
- Kipreos E. T., Lee G. J., Wang J. Y. Isolation of temperature-sensitive tyrosine kinase mutants of v-abl oncogene by screening with antibodies for phosphotyrosine. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1345–1349. doi: 10.1073/pnas.84.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipreos E. T., Wang J. Y. Reversible dependence on growth factor interleukin-3 in myeloid cells expressing temperature sensitive v-abl oncogene. Oncogene Res. 1988 Feb;2(3):277–284. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lassar A. B., Paterson B. M., Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986 Dec 5;47(5):649–656. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Goff S. P., Tabin C. J., Paskind M., Wang J. Y., Baltimore D. Cloning and analysis of reverse transcript P160 genomes of Abelson murine leukemia virus. J Virol. 1983 Mar;45(3):1195–1199. doi: 10.1128/jvi.45.3.1195-1199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshitz A., Lazar C. S., Buss J. E., Gill G. N. Analysis of morphology and receptor metabolism in clonal variant A431 cells with differing growth responses to epidermal growth factor. J Cell Physiol. 1983 Jun;115(3):235–242. doi: 10.1002/jcp.1041150304. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Morgan C. J., Pledger W. J. Cell cycle dependent growth factor regulation of gene expression. J Cell Physiol. 1989 Dec;141(3):535–542. doi: 10.1002/jcp.1041410312. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nepveu A., Fahrlander P. D., Yang J. Q., Marcu K. B. Amplification and altered expression of the c-myc oncogene in A-MuLV-transformed fibroblasts. Nature. 1985 Oct 3;317(6036):440–443. doi: 10.1038/317440a0. [DOI] [PubMed] [Google Scholar]
- Nori M., Shawver L. K., Weber M. J. A Swiss 3T3 variant cell line resistant to the effects of tumor promoters cannot be transformed by src. Mol Cell Biol. 1990 Aug;10(8):4155–4162. doi: 10.1128/mcb.10.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka H., Moskowitz M. Arrest of 3T3 cells in G1 phase in suspension culture. J Cell Physiol. 1975 Dec;87(2):213–219. doi: 10.1002/jcp.1040870209. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Razin A., Cedar H. DNA methylation and gene expression. Microbiol Rev. 1991 Sep;55(3):451–458. doi: 10.1128/mr.55.3.451-458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R. W., Goldfarb M., Goff S. P. Abelson murine leukemia virus induces platelet-derived growth factor-independent fibroblast growth: correlation with kinase activity and dissociation from full morphologic transformation. Mol Cell Biol. 1989 Jan;9(1):278–287. doi: 10.1128/mcb.9.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. M., Morla A. O., Wang J. Y. Reduction in protein tyrosine phosphorylation during differentiation of human leukemia cell line K-562. Cancer Res. 1987 Aug 1;47(15):4066–4070. [PubMed] [Google Scholar]
- Risser R., Green P. L. Abelson virus: current status of a viral oncogene. Proc Soc Exp Biol Med. 1988 Jul;188(3):235–242. doi: 10.3181/00379727-188-42732. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter V., Witte O. N., Coffman R., Baltimore D. Abelson murine leukemia virus-induced tumors elicit antibodies against a host cell protein, P50. J Virol. 1980 Nov;36(2):547–555. doi: 10.1128/jvi.36.2.547-555.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E. CD4, CD8 and the TCR-CD3 complex: a novel class of protein-tyrosine kinase receptor. Immunol Today. 1990 Nov;11(11):400–406. doi: 10.1016/0167-5699(90)90159-7. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Daley G. Q., Jackson P., Baltimore D., Cantley L. C. Activation of phosphatidylinositol 3-kinase in cells expressing abl oncogene variants. Mol Cell Biol. 1991 Feb;11(2):1107–1113. doi: 10.1128/mcb.11.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. M., Witte O. N. Site-directed deletions of Abelson murine leukemia virus define 3' sequences essential for transformation and lethality. J Virol. 1983 Mar;45(3):1028–1036. doi: 10.1128/jvi.45.3.1028-1036.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Ziegler S. F., Whitlock C. A., Goff S. P., Gifford A., Witte O. N. Lethal effect of the Abelson murine leukemia virus transforming gene product. Cell. 1981 Dec;27(3 Pt 2):477–486. doi: 10.1016/0092-8674(81)90389-5. [DOI] [PubMed] [Google Scholar]