Abstract
The progressive development of civilization and intensive industrialization has contributed to the global pollution of the natural environment by heavy metals, especially the soil. Degraded soils generally contain less organic matter, and thus, their homeostasis is more often disturbed, which in turn manifests in changes in biological and physicochemical properties of the soil. Therefore, new possibilities and solutions for possible neutralization of these contaminations are sought, inter alia, through reclamation of degraded land. At present, the use of additives supporting the reclamation process that exhibit heavy metal-sorbing properties is becoming increasingly important in soil recovery. Research was conducted to determine the role of compost in stabilizing the microbial and biochemical balance of the soil due to the significant problem of heavy metal-contaminated areas. The study was conducted on loamy sand, to which zinc was applied at the following doses: 0, 250, 500, 750, 1000, and 1250 mg Zn2+ kg−1 DM of soil. Compost was introduced to the appropriate objects calculated on the basis of organic carbon content in the amount of 0, 10, and 20 g Corg kg−1 DM of soil. The study was conducted over a period of 20 weeks, maintaining soil moisture at 50% capillary water capacity. Zinc significantly modified soil microbiome status. The abundance of microorganisms and their biological diversity and the enzymatic activity of the soil were affected. The negative effects of contaminating zinc doses were alleviated by the introduction of compost into the soil. Organic fertilization led to microbial growth intensification and increased biochemical activity of the soil already 2 weeks after compost application. These effects persisted throughout the experiment. Therefore, it can be stated that the use of compost is an appropriate method for restoring normal functions of soil ecosystems contaminated with zinc.
Keywords: Zinc, Soil, Contamination, Compost, Biostimulation, Microorganisms and enzymes
Introduction
The intense growth of the human population entails the need to increase agricultural production and crop area. Therefore, modern agriculture is facing the great challenge of providing sufficient quantity and quality of food for humanity. In turn, the increase of urbanization and the diversity and growth dynamics of contaminations present in the soil environment endanger both crop yields and their wholesomeness. Particular attention is paid to the heavy metal contents of the soil due to their persistence in the environment, as evidenced by works on the role of trace elements in the soil environment and their penetration into the trophic chain (Verma and Dwivedi 2013; Vrščaj et al. 2008; Wyszkowska et al. 2013). Some of these elements are essential for the correct growth of plants and the functioning of complex systems of biological processes that could not exist without their presence (Chibuike and Obiora 2014). Others do not affect the functioning of living organisms and the course of biological processes occurring in the soil environment, thus their occurrence in the soil may be undesirable. Zinc belongs to the elements involved in various biological processes. However, from an ecological point of view, excessive amounts of this element result in a number of adverse effects (Wyszkowska et al. 2013; Wyszkowska et al. 2017; Strachel et al. 2017a; Strachel et al. 2017b).
Crop yield is dependent on soil fertility, which in turn is determined by the microbiological and biochemical properties of the soil, which are closely interrelated. The state of the soil microbiome, both in terms of quantity and diversity, influences the activity of soil enzymes. The decomposition of organic matter, and hence the release of nutrients is the result of enzyme involvement in these processes (Chaperon and Sauvé 2007; Kızılkaya et al. 2004; Utobo and Tewari 2015). Therefore, it is very important for modern agriculture to maintain a high biochemical activity of the soil. Zinc in moderate amounts contributes to the stimulation of soil microbiome and its activity, while in excessive doses it disturbs soil homeostasis. The abundance and diversity of soil microorganisms is disrupted, which can also contribute to the deterioration of soil biochemical properties (Boros et al. 2011; Wyszkowska et al. 2016). Bioavailability of these elements is the most important aspect of heavy metal pressure in the soil. The total quantity of heavy metal may remain unchanged but its toxicity is dependent on its unbound form (Luo et al. 2012). This balance is determined by specific soil environment conditions, i.e., pH, organic matter content, and sorption complex volume (Aydinalp and Marinova 2003). Soils heavily degraded by heavy metals need a sufficient amount of stabilized organic matter, which not only delivers nutrients and reduces their losses, but also shows the ability to bind these elements. Replenishment of organic matter in contaminated soil can be achieved through the application of compost, which effectively increases soil fertility. Compost supplies vast quantities of nutrients to the soil, which become more available and assimilable to soil organisms. In addition, it improves soil physicochemical properties such as soil density, soil porosity, water and heat capacity, soil sorption and aeration (Adugna 2016; Babalola et al. 2012; Civeira 2010; Pane et al. 2015). The presence of organic matter in the compost also contributes to the promotion of microorganisms in the soil environment, increasing their abundance and biodiversity as well as biological activity (Adugna 2016; Kim et al. 2015; Pane et al. 2015). Compost properties make it suitable for reclamation of heavy metal contaminated areas, however, it must be sanitary safe and free from additional pollutant loads.
Effective methods of restoring soil homeostasis are still sought due to the progressive contamination of the soil environment by heavy metals. This was a prerequisite for this research, which primary purpose was to assess the suitability of stabilized compost in restoring microbial and biochemical balance of the soil.
Material and Methods
Soil Material
Loamy sand from the Didactic and Experimental Station in Tomaszkowo (north-eastern Poland), located in the Olsztyn Lakeland, was used for the experiment. This area is dominated by typical brown soils formed mainly on sands and clays. The material was sampled from the topsoil layer (0–20 cm), which was classified as Eutric Cambisols (World Reference Base of Soil Resources 2014). Complete characterization of soil material is shown in Table 1
Table 1.
Selected physicochemical properties of the soil
| Parameter | Unit | Value |
|---|---|---|
| Soil texture | ||
| < 0.002 | % | 1.49 |
| 0.002–0.05 | 17.98 | |
| 0.05–2.00 | 80.56 | |
| pH | – | 5.60 |
| Total organic carbon | g kg−1 DM of soil | 10.00 |
| Total nitrogen | 0.58 | |
| Available cations | ||
| P | mg kg−1 DM of soil | 96.32 |
| K | 179.08 | |
| Mg | 50.17 | |
| Exchangeable cations | ||
| K+ | 217.73 | |
| Ca2+ | mg kg−1 DM of soil | 568.60 |
| Na+ | 100.34 | |
| Mg2+ | 64.52 | |
| Hydrolytic acidity (HAC) | 18.66 | |
| Total exchangeable bases (TEB) | mM(+) kg−1 DM of soil | 40.00 |
| Cation exchange capacity (CEC) | 58.66 | |
| Base saturation (BS) | % | 68.19 |
| Total zinc | 22.68 | |
| Available zinc | mg kg−1 DM of soil | 9.13 |
Compost Characteristics
The compost used in the experiment was produced by Nolet (Poland). It was produced by aerobic composting of coniferous sawdust and turkey litter. It was characterized by the following parameters: pH (in 1 M KCl)—7.08; organic carbon content—402.0 g kg−1; total nitrogen content—23.1 g kg−1; cations in the assimilable form (g kg−1): P—4.7, K—10.2, Mg—2.2; exchangeable cations (g kg−1): K+—0.8, Ca2+—2.7; Na+—0.3; and Mg2+—0.5.
Experimental Conditions
The introduction of heavy metals into the soil can lead to its degradation by reducing the nutrients necessary for the growth and development of living organisms, mainly microorganisms. Replenishment of nutrient deficiencies, especially in degraded areas, is essential for the proper functioning of soil ecosystems. Therefore, the study was conducted to determine the effect of differentiated doses of zinc and compost on soil biological properties. Model studies were carried out under strictly controlled conditions in order to avoid the influence of other factors on the microbiological and biochemical properties of the soil. In 150 cm3 glass beakers, 100 g of air dry mass of soil material was added, into which zinc and compost were put in appropriate objects. Zinc was applied as an aqueous solution of ZnCl2 at a dose of: 0, 250, 500, 750, 1000, and 1250 mg Zn2+ kg−1 DM of soil. Compost calculated based on organic carbon content was used in the amount corresponding to the level of 0, 10, and 20 g Corg kg−1 DM of soil. In the experiment, 108 glass beakers were prepared, i.e., 6 beakers with different doses of zinc × 3 doses of compost, and it means 18 combinations in 3 replications (54 in total). Samples were prepared separately for two study terms (54 samples × 2 terms, it means 108 experimental beakers). After thorough homogenizing of soil samples, they were incubated at 25 °C for 2 and 20 weeks. Throughout the experiment, the moisture content of soil samples was maintained at 50% of the capillary water capacity.
Microbiological, Biochemical and Physicochemical Analyses of the Soil
In week 2 and 20 of the incubation period, the number of the following groups of microorganisms was determined: organotrophic bacteria on Bunt and Rovira (1955) soil extracts, oligotrophic and copiotrophic bacteria—medium with peptone and meat extract, according to Oht and Hattori (1983), actinomycetes—Küster and Williams medium with nystatin and actidione antibiotics, according to Parkinson et al. (1971), and fungi—on glucose-peptone agar with Bengal rose and aureomycin according to Martin (1950). The ecophysiological diversification index (EP) was determined based on the abundance of microorganisms (organotrophic bacteria, actinomycetes, fungi), on the basis of which the diversity of the soil environment was inferred (De Leij et al. 1993). Colony growth observations were performed at 1 day intervals for a period of 8 days. The enzymatic activities of dehydrogenases (Öhlinger 1996), catalase and urease (Alef and Nannipieri 1998) and acid phosphatase, alkaline phosphatase, and β-glucosidase (Alef et al. 1998) were also determined in week 2 and 20 of soil incubation. A detailed procedure for determining the activity of the enzymes tested was described in the work of Borowik et al. (2014). The biostimulation index (IFb) was also calculated, on the basis of which the effect of compost on microbial multiplication and soil enzyme activity was evaluated, according to the formula provided by Kaczyńska et al. (2015):
where A b—enzyme activity or number of microorganisms in soil with compost, A—enzyme activity or number of soil microorganisms without added compost.
Soil physicochemical analyses were carried out in week 20 of soil incubation, which included the determination of pH in 1 mol dm−3 KCl, hydrolytic acidity, sum of exchangeable alkaline cations, organic carbon content, and total nitrogen content and exchangeable cations (K+, Na+, Ca2+, Mg2+), according to methods described in Harris’s work (2006). Soil microbiological, biochemical, and physicochemical analyses were performed in three replicates.
Statistical Analysis
Based on the results obtained, statistical analysis was performed using the Statistica 12 (StatSoft, Inc. 2014) software package. Statistical analysis included percentage involvement of factors of the observed diversity—η 2; principal component analysis (PCA) of microbial counts and enzyme activities; homogeneous groups determination by the Tukey’s honestly significant difference (HSD) test using ANOVA (at P < 0.01); correlation matrix between microbiological, biochemical, and physicochemical properties of the soil.
Results and Discussion
Soil Microbiome
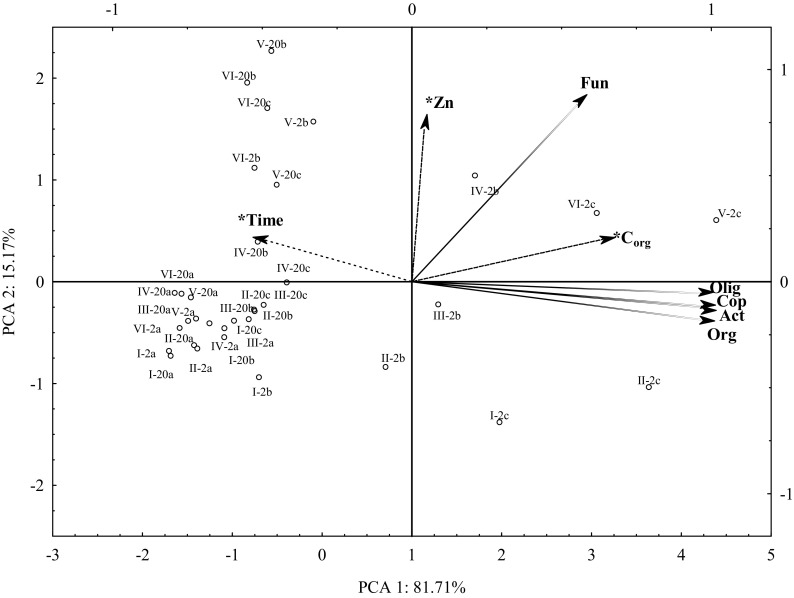
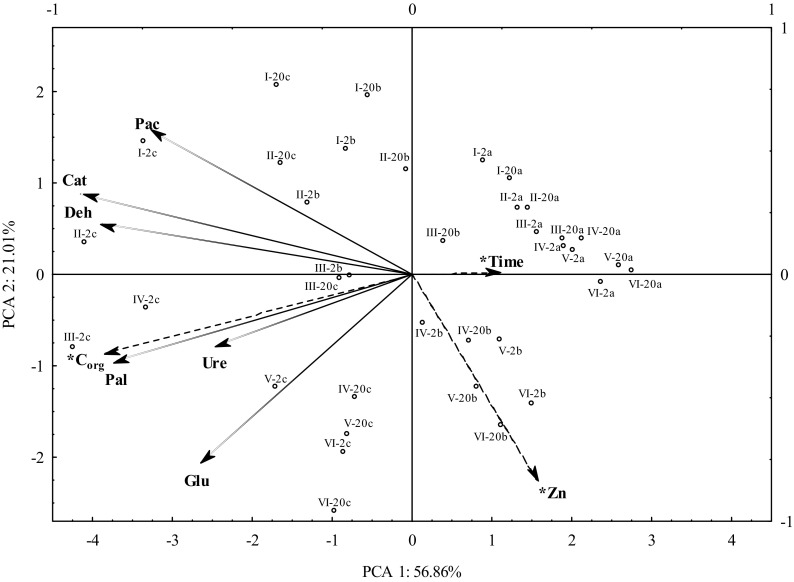
The soil environment is a very complex structure, but at the same time, it is a natural storage of diverse substances, including heavy metals, which can lead to unsustainable soil ecosystems. Zinc is one of these elements, which on the one hand is present in the soil environment as a microelement and plays a vital role in the proper functioning of living organisms. On the other hand, when it occurs in the soil in excessive amounts, it can become toxic, leading to the disruption of its biological and chemical balance (Pardo et al. 2014; Soares et al. 2015). On the basis of the results of the present study, it can be concluded that all analyzed factors (zinc dose, compost fertilization, and soil incubation time) significantly influenced microbiological properties of the soil (Table 2). Compost soil fertilization most highly affected the count of organotrophic bacteria, oligotrophic bacteria, and actinomycetes, zinc dosage—fungi count, while soil incubation time—the number of copiotrophic bacteria. Principal component analysis also confirmed these dependencies (Fig. 1). It was found that two basic groups were formed. The first group consisted of vectors representing the count of organotrophic bacteria, copiotrophic bacteria, oligotrophic bacteria, and actinomycetes. This group was positively correlated with the dose of zinc and compost, and negatively with soil incubation time. The second group was formed by a vector representing fungi that positively correlated with the dose of zinc and compost and soil incubation time. The positioning of the vectors representing the variables suggested that microorganisms forming the first group (organotrophic bacteria, copiotrophic bacteria, oligotrophic bacteria, and actinomycetes) reacted similarly to the factors used in the experiment. Considering the distribution of the cases, it was observed that zinc effect on soil microorganisms varied over time. At week 2 of the treatment, zinc at doses from 250 to 1250 mg Zn2+ kg−1 contributed to growth stimulation of copiotrophic bacteria, oligotrophic bacteria, and fungi. In the case of actinomycetes, a decrease in their number was recorded following the introduction of zinc at 1000 and 1250 mg Zn2+ kg−1. Organotrophic bacteria also responded to zinc reduction with a lower count, but after its administration at a dose of 1250 mg Zn2+ kg−1. In the 20th week of soil incubation, the count of oligotrophic bacteria and fungi increased after zinc introduction into the soil at all tested doses, identically to week 2. At the same time point, zinc doses of 1000 and 1250 mg Zn2+ kg−1 inhibited the growth of copiotrophic bacteria and actinomycetes, and doses of 500 to 1250 mg Zn2+ kg−1 inhibited the multiplication of organotrophic bacteria. It was also observed that the count of organotrophic bacteria, copiotrophic bacteria, oligotrophic bacteria, and actinomycetes was higher at week 2, and that of fungi at week 20. This is indicated by the opposite arrangement of the vectors describing these groups relative to the variable vector representing soil incubation time. The excessive amounts of heavy metals may result in the reduction of the soil microbiome pool. In many works (Pérez-de-Mora et al. 2006; Wang et al. 2007; Zhou et al. 2017), microbial biomass was reduced due to heavy metal action. In turn, Sang-Hwan et al. (2009) noted the negative impact of these elements on soil respiration.
Table 2.
Percentage proportion of the observed variability factors η 2
| Factors | Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Org | Cop | Olig | Act | Fun | Deh | Cat | Pac | Pal | Ure | Glu | |
| Dose of Zn | 5.47 | 3.00 | 11.03 | 4.38 | 40.34 | 17.10 | 33.47 | 65.14 | 7.72 | 0.21 | 16.32 |
| Dose of Corg | 37.91 | 27.96 | 42.88 | 39.26 | 32.52 | 29.07 | 50.93 | 25.13 | 56.23 | 58.54 | 56.38 |
| Incubation time | 23.59 | 35.45 | 17.02 | 23.76 | 1.21 | 20.80 | 3.82 | 0.11 | 10.84 | 16.96 | 6.00 |
| Dose of Zn × dose of Corg | 2.38 | 1.25 | 6.50 | 1.41 | 13.30 | 5.23 | 6.09 | 2.09 | 7.95 | 0.45 | 14.13 |
| Dose of Zn × incubation time | 2.19 | 2.53 | 2.58 | 2.73 | 4.76 | 5.50 | 1.64 | 3.01 | 2.62 | 5.58 | 2.58 |
| Dose of Corg × incubation time | 25.73 | 26.56 | 15.91 | 24.27 | 3.48 | 18.82 | 2.88 | 1.83 | 12.32 | 12.98 | 0.98 |
| Dose of Zn × dose of Corg × incubation time | 1.54 | 1.32 | 3.26 | 1.97 | 3.13 | 3.48 | 1.01 | 2.66 | 2.32 | 5.17 | 3.60 |
| Error | 1.18 | 1.93 | 0.82 | 2.22 | 1.24 | 0.01 | 0.16 | 0.04 | 0.00 | 0.11 | 0.01 |
Zn zinc, Org organotrophic bacteria, Cop copiotrophic bacteria, Olig oligotrophic bacteria, Act actinomycetes, Fun fungi, Deh dehydrogenases, Cat catalase, Pac acid phosphatase, Pal alkaline phosphatase, Ure urease, Glu β-glucosidase
Fig. 1.
The count of selected groups of microorganism in zinc-contaminated soil supplemented with compost (PCA analysis). Org organotrophic bacteria, Cop copiotrophic bacteria, Olig oligotrophic bacteria, Act actinomycetes, Fun fungi; dose of zinc (mg Zn2+ kg−1 DM soil): I—0, II—250, III—500, IV—750, V—1000, VI—1250; dose of Corg (g kg−1 DM soil): a—0, b—10, c—20; incubation time: 2—two weeks, 20—twenty weeks
Increasing emphasis is being placed on the reclamation of soils under heavy metal pressure due to the need of sustaining proper condition of the soil environment. Soil remediation techniques are based primarily on two processes: extraction and stabilization of soil contaminants. Extraction is a costly process, thus it is better to use “in situ” stabilization techniques that require less financial effort and can be applied in larger areas contaminated with heavy metals (Pérez de Mora et al. 2005; Lee et al. 2009). According to Baker et al. (2011), organic matter content can be of great importance in maintaining good microbiological condition of the soil subjected to heavy metal pressure. In own research, the introduction of compost into the soil was highly stimulating on soil microbiome, as evidenced by the arrangement of vectors in particular cases. Both in the 2nd and 20th week of incubation, the highest counts of copiotrophic bacteria, oligotrophic bacteria, actinomycetes, and fungi were recorded in objects with the highest compost dosage (20 g Corg kg−1 DM of soil). A slightly different relationship to soil compost was observed for organotrophic bacteria. Their growth in the 2nd week was the highest in soil with the compost amount of 20 g Corg kg−1 DM of soil, while at week 20, the number of these bacteria remained at a similar level after the introduction of compost at 10 and 20 g Corg kg−1 DM of soil. Soil microbiome changes largely depend on the state of the environment and the dose of compost used (Baker et al. 2011). Nevertheless, the dynamics of changes in soil is the greatest in the initial period after compost application, as evidenced by increased CO2 emission from the soil, which gradually decreases over time (Debosz et al. 2002). Compost added to the soil environment primarily increases the pool of microorganisms, which is closely associated with soil processes triggered by the microbiome (Chang et al. 2007). Stimulation of autochthonous microorganisms, as a result of introducing nutrients contained in compost, improves the biochemical properties of the soil environment (Marinari et al. 2000). In addition, the efficiency of microorganisms in the metabolism of organic carbon sources is higher and the colonization of plant roots by fungi increases (Civeira 2010). The strongly degraded soils are deprived of organic matter; hence, the addition of compost leads to an improvement of general soil condition, through supplementing the carbon sources and other nutrients (Pane et al. 2015). In addition, organic matter plays a fundamental role in the proper functioning of soil ecosystems and determines soil fertility and its resistance to environmental degradation factors (Civeira 2010). The study of Baker et al. (2011) showed that the soil after compost application was dominated by bacteria. In turn, based on the analysis of fatty acids, Bastida et al. (2008) observed an increase in the ratio of G+ to G− bacteria after compost application to the soil. In addition, similar changes were observed with respect to mycorrhizal fungi in relation to total fungi number. The biostimulation index (IFb) shown in Table 3 confirmed the beneficial effect of compost on the development of soil microorganisms. Mean IFb index values calculated in all zinc doses and experimental time points indicated that soil supplementation with compost had the most favorable impact on the development of actinomycetes (IFb = 10.78). It was also observed that the positive effect of compost on the microbiological properties of the soil was more effective when introduced at a dose 20 g Corg kg−1 DM of soil. Vinhal-Freitas et al. (2010) also observed the stimulating effect of compost applied at doses of 10 and 20 g kg−1 of soil on the propagation of microorganisms. Taking into account soil incubation time, it was noted that biostimulation index values were higher at week 2 than at week 20. This higher stimulating effect of compost in the second week of soil incubation resulted from the supply of the proper amount of fresh organic matter to the soil, which provided nutrients for microorganisms. In time, organic matter was mineralized, resulting in fewer nutrients needed for the proliferation of microorganisms at week 20 (Adugna 2016). In the 20th incubation week, the introduction of compost at a dose of 10 mg Corg kg−1 DM of soil. Negatively affected the proliferation of the copiotrophic bacteria, because IFb index values in objects with zinc dosages from 500 to 1250 mg Zn2+ kg−1 DM of soil were lower than 1.
Table 3.
Effect of compost on the abundance of soil microorganisms expressed by the biostimulation index (IFb)
| Dose of zinc (mg Zn2+ kg−1) | Org | Cop | Olig | Act | Fun | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incubation time (weeks) | ||||||||||
| 2 | 20 | 2 | 20 | 2 | 20 | 2 | 20 | 2 | 20 | |
| 10 g Corg kg−1 DM of soil | ||||||||||
| 0 | 3.61c,d | 1.79a | 4.88a,b,c | 1.21a | 5.85c,d,e | 5.53a,b | 4.56c | 3.42a,b,c,c | 1.20b | 10.22a,b |
| 250 | 3.71c,d | 2.45a | 4.06b,c | 1.01a | 6.37c,d | 1.80b | 4.39c | 2.04b,c | 2.97a,b | 3.16c,d,e |
| 500 | 2.26c,d | 3.48a | 3.79b,c | 0.79a | 6.06c,d | 2.66a,b | 4.60c | 1.35c | 2.76a,b | 1.56c |
| 750 | 5.33b,c,d | 3.21a | 6.89a,b,c | 0.73a | 2.25d,e | 3.84a,b | 15.81a,b,c | 1.00c | 6.33a,b | 2.16c |
| 1000 | 2.91c,d | 2.30a | 2.88c | 0.96a | 2.38d,e | 2.39a,b | 7.65b,c | 2.42a,b,c | 7.06a,b | 4.99b,c |
| 1250 | 1.60d | 3.14a | 4.02b,c | 0.85a | 1.72e | 1.81b | 2.33c | 2.69a,b,c | 6.84a,b | 4.50b,c |
| Average | 3.24 | 2.73 | 4.42 | 0.93 | 4.11 | 3.01 | 6.56 | 2.15 | 4.53 | 4.43 |
| r | − 0.38 | 0.49 | − 0.18 | − 0.62 | − 0.89 | − 0.58 | 0.11 | − 0.17 | 0.94 | − 0.38 |
| 20 g Corg kg−1 DM of soil | ||||||||||
| 0 | 9.99a,b,c | 1.90a | 14.87a,b | 1.47a | 11.60a,b | 4.95a,b | 26.85a,b,c | 4.72a,b,c,c | 5.79a,b | 16.22a |
| 250 | 8.42a,b,c,d | 1.99a | 9.45a,b,c | 1.21a | 7.60a,b,c | 1.89b | 13.25a,b,c | 2.75a,b,c | 6.81a,b | 3.59c,d,e |
| 500 | 7.36a,b,c,d | 3.43a | 8.85a,b,c | 1.12a | 13.17a | 1.99b | 9.24b,c | 3.10a,b,c | 4.26a,b | 1.78c |
| 750 | 14.06a | 3.04a | 14.52a,b,c | 1.25a | 5.32c,d,e | 6.34a | 32.65a,b,c | 2.04b,c | 6.64a,b | 1.58c |
| 1000 | 11.85a,b | 2.74a | 16.04a | 1.51a | 9.05a,b,c | 4.59a,b | 51.38a | 6.41a,b | 7.92a | 3.00d,e |
| 1250 | 8.27a,b,c,d | 3.36a | 16.45a | 1.13a | 9.00a,bc, | 3.96a,b | 46.83a,b | 7.22a | 8.69a | 4.18b,c |
| Average | 9.99 | 2.74 | 13.36 | 1.28 | 9.29 | 3.95 | 30.03 | 4.37 | 6.69 | 5.06 |
| r | 0.18 | 0.73 | 0.53 | − 0.22 | − 0.31 | 0.23 | 0.74 | 0.57 | 0.69 | − 0.60 |
Homogeneous groups were marked with the same letters separately for each group of microorganisms and soil incubation time (two-way ANOVA performed using Tukey’s T test at P < 0.01)
Org organotrophic bacteria, Cop copiotrophic bacteria, Olig oligotrophic bacteria, Act actinomycetes, Fun fungi, r Pearson’s linear correlation coefficient
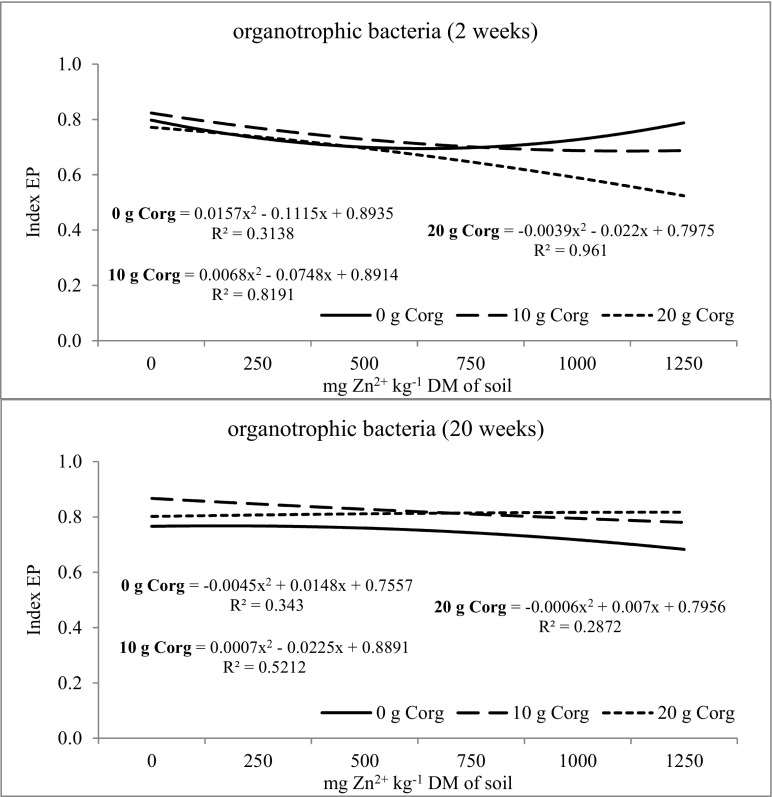
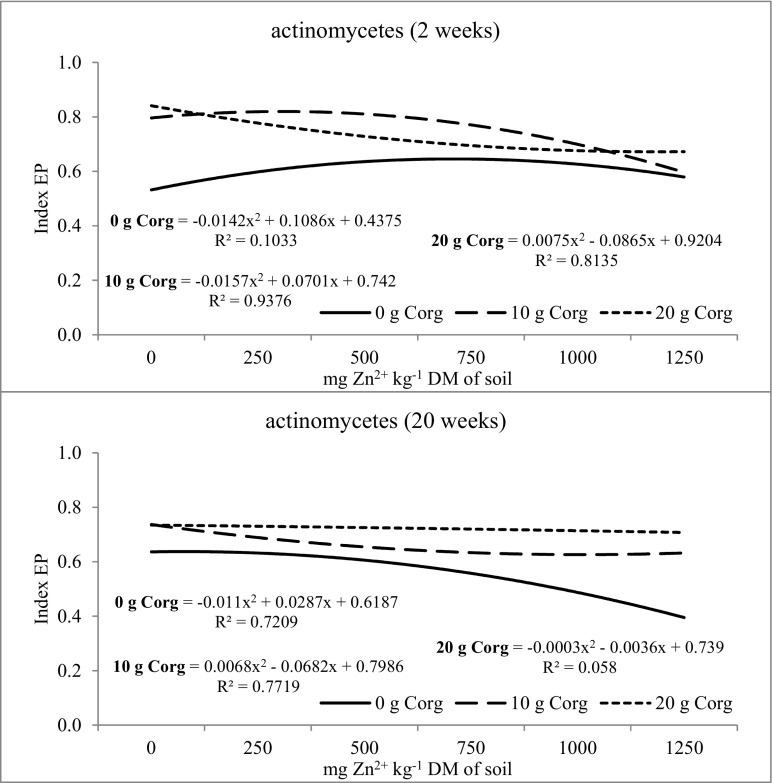
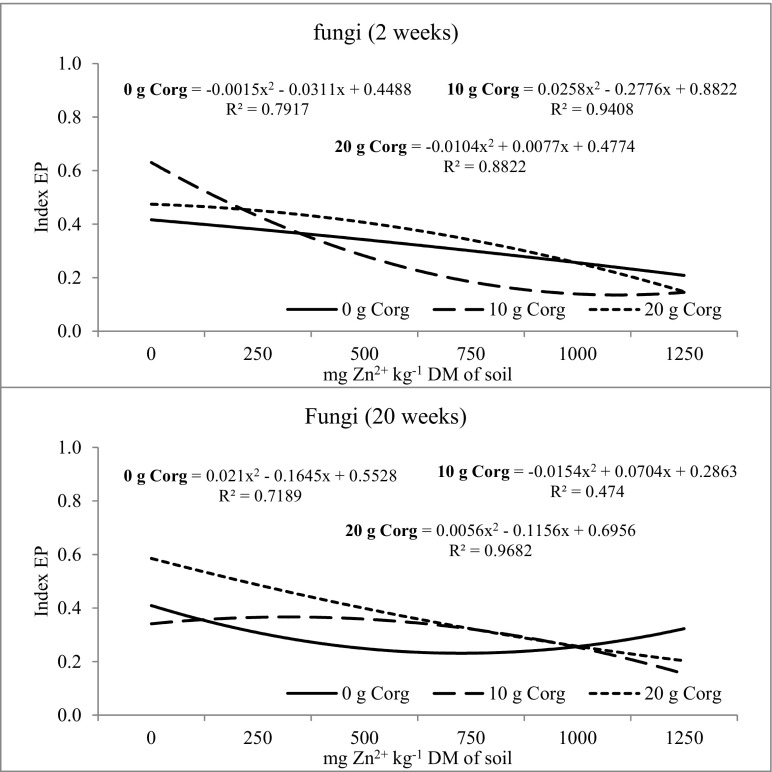
Biodiversity of microorganisms determined by the soil ecophysiological diversification index (EP) was also modified by the investigated factors (Figs. 2, 3, and 4). The value of the EP index of organotrophic bacteria at week 2 and 20 of the experiment was similar (Fig. 2). It was recorded that the mean EP index value at week 2 and 20 of soil incubation was 0.740. Zinc dose did not significantly influence the ecophysiological diversification index of organotrophic bacteria, while at week 2, the largest changes were caused by the dose of 250 mg of Zn2+ kg−1 and at week 20—by the dose of 1250 mg of Zn2+ kg−1. In the 2nd week of soil incubation, compost introduction at a dose of 10 and 20 g Corg kg−1 DM of soil reduced the diversity of organotrophic bacteria. Although the biodiversity of organotrophic bacteria was reduced in the 2nd week after compost application, its stimulating effects on the multiplication of these bacteria was noted. The introduction of compost has increased the amount of available carbon, thereby increasing the number of organotrophic bacteria. However, at week 20, the increase of the EP index value in the objects with composts was already observed, indicating an increase in the biodiversity of these bacteria. The diversity of actinobacteria was altered by the tested factors (Fig. 3). The EP value was observed to increase in the second week of soil incubation upon zinc application to the soil at doses from 250 to 750 mg Zn2+ kg−1, while at week 20—upon zinc doses of 250 and 500 mg Zn2+ kg−1. The prolonged retention of high zinc doses in non-fertilized objects resulted in decreased biodiversity of actinomycetes. The introduction of compost into the soil contributed to the increase of actinomycetes biodiversity. This was particularly evident at week 20 of the study in soil samples, to which compost was added at 10 and 20 g Corg kg−1 DM of soil. The ecophysiological diversification index was altered by the zinc and compost dosage and soil incubation time (Fig. 4). Zinc caused a decrease in the EP index when present in excessive amounts in the soil. In week 2, the dose of 1000 mg of Zn2+ kg−1 caused the largest changes, while 750 mg of Zn2+ kg−1 in week 20. Compost introduction to the soil exerted a varied effect on the EP index value in fungi. At week 2, the EP index was at a similar level in objects both with and without compost. At week 20, a positive effect of compost on the increase of fungal biodiversity was observed. Increased biodiversity of the soil microbiome under compost influence could indicate its protective role against the action of heavy metals, including zinc. Compost supplementation can also affect microbiome by promoting microorganisms that are more resistant to heavy metals in the soil. It can be stated that compost fulfills its role in stabilizing the microbial diversity of soil contaminated with zinc.
Fig. 2.
The ecophysiological diversification index (EP) of organotrophic bacteria in zinc-contaminated soil supplemented with compost. 0 g Corg—soil without compost addition; 10 g Corg—soil with 10 g compost calculated based on the organic carbon content; 20 g Corg—soil with 20 g compost calculated based on the organic carbon content
Fig. 3.
The ecophysiological diversification index (EP) of actinomycetes in zinc-contaminated soil supplemented with compost. 0 g Corg—soil without compost addition; 10 g Corg—soil with 10 g compost calculated based on the organic carbon content; 20 g Corg—soil with 20 g compost calculated based on the organic carbon content
Fig. 4.
The ecophysiological diversification index (EP) of fungi in zinc-contaminated soil supplemented with compost. 0 g Corg—soil without compost addition; 10 g Corg—soil with 10 g compost calculated based on the organic carbon content; 20 g Corg—soil with 20 g compost calculated based on the organic carbon content
Soil Enzymes
The investigated factors (zinc dose, compost dose, and soil incubation time) also had a significant effect on soil biochemical properties (Table 2). Acid phosphatase activity was also primarily affected by zinc dose (in 65.14%). In turn, compost dose affected dehydrogenase activities in 29.07%, catalase—in 50.93%; urease—in 58.54%; alkaline phosphatase—in 56.23; and β-glucosidase—in 56.38%. Enzymatic activity time was the least affected by soil incubation, from 0.11% (acid phosphatase activity) to 20.80% (dehydrogenase activities). Results of principal component analysis, shown in Fig. 5, revealed that zinc caused significant changes in the enzymatic activity of the soil, but its activity changed over time. Zinc applied to the soil at doses of 250 to 1250 mg Zn2+ kg−1 was a potent inhibitor of the activity of dehydrogenases, catalase, and acid phosphatase. Based on the literature data (Borowik et al. 2014; Chaperon and Sauvé 2007; Kucharski et al. 2011; Strachel et al. 2017a, dehydrogenases are the most sensitive enzymes to excessive amounts of zinc in the environment. According to Yang et al. (2006), catalase also shows high sensitivity to zinc. In the current study, urease activity increased with the introduction of 250 and 500 mg of Zn2+ kg−1 to the soil in week 2 and from 250 to 750 mg Zn2+ kg−1 in week 20. The inverse relationship was recorded in the case of β-glucosidase. Alkaline phosphatase activity decreased after zinc addition in doses from 500 to 1250 mg Zn2+ kg−1. Kucharski et al. (2011) and Borowik et al. (2014) also observed negative effects of zinc on soil enzymatic activity, especially when applied at contaminating doses. They also noted that this inhibitory effect was long lasting. Moreno et al. (2009) reported that the activity of soil enzymes, i.e., urease, acid phosphatase, and β-glucosidase, decreased with the dose of this element in zinc-contaminated soil. The decrease in β-glucosidase activity in zinc-stressed soil was noted by, among others, Castaldi et al. (2004), Hinojosa et al. (2004), Kunito et al. (2001), and Moreno et al. (2009). Studies of other authors also confirmed that the activity of soil enzymes, i.e., dehydrogenases (Pérez-de-Mora et al. 2006; Sang-Hwan et al. 2009), phosphatases (Pérez-de-Mora et al. 2006; Wang et al. 2007), urease (Sang-Hwan et al. 2009), β-glucosidase, and arylsulfatase (Pérez-de-Mora et al. 2006), could be affected by heavy metals. In this study, the introduction of compost into the soil contributed positively to the activity of the analyzed soil enzymes. However, higher neutralizing effects of compost on the biochemical properties of zinc-contaminated soil were visible in objects with doses of 20 g Corg kg−1 DM of soil. Compost addition to the soil contaminated with zinc doses from 250 to 1250 mg Zn2+ kg−1 contributed to the increase in β-glucosidase activity. Moreno et al. (2009) believed that organic matter introduction into the soil reduced the negative effects of zinc on soil enzymes and even stimulated their activity. This stimulating effect of compost on enzymes indicates the strong influence of organic matter on the increase of microbiome abundance, and thus the improvement of soil biochemical properties. Enzyme secretion by microorganisms is increased as a result of microbial proliferation and development (Babalola et al. 2012; Civeira 2010). The increase in enzyme activity may also result from zinc binding by organic matter, which occurs through the adsorption and formation of chelated bonds with complex properties (Jiao et al. 2011; Wyszkowska et al. 2015). In addition, the highest activity was observed in the objects in the 2nd week of soil incubation, both without compost and with its addition. Soil biostimulation indices (IFb) showed that compost not only alleviated the negative effects of zinc, but also activated soil enzymes (Table 4). According to mean IFb values, calculated based on all zinc doses and experimental time points, compost had the greatest stimulating effect on soil enzyme activities in the following order (from the highest to the lowest): urease (22.79), dehydrogenases (19.51), alkaline phosphatase (13.96), catalase (2.79), β-glucosidase (2.50), and acid phosphatase (1.52). Compost exerted a more stimulating effect on dehydrogenases, catalase, and alkaline phosphatase activities at week 2 of soil incubation, while on urease, acid phosphatase, and β-glucosidase activities at week 20. As in the case of soil microorganisms, the activity of soil enzymes was considerably higher in objects with compost dose of 20 g Corg kg−1 DM of soil. Study conducted by Vinhal-Freitas et al. (2010) confirmed the beneficial effect of compost on soil enzymatic activity. It was recorded that compost introduced into the soil, particularly at a dose of 20 g kg−1 DM of soil, significantly increased the activity of β-glucosidase, acid phosphatase, and alkaline phosphatase when compared to the variant without organic matter addition. After 20 weeks of incubation, zinc contamination significantly affected microbiological, biochemical, and physicochemical properties of the soil. Table 5 shows the correlation coefficients between the variables tested and soil parameters. There was a highly significant negative correlation observed between zinc dose and the abundance of organotrophic bacteria, copiotrophic bacteria and actinomycetes, and dehydrogenase and catalase activities. A significantly positive correlation was noted between zinc dose and fungal count and β-glucosidase activity. The zinc dose also significantly negatively correlated with the physicochemical properties of the soil, except for soil hydrolytic acidity. These changes in environmental conditions also significantly correlated with the results obtained. The number of microorganisms and soil enzymes considerably decreased with the increase in soil environment acidity, with the exception of fungi and β-glucosidase. The introduction of compost into the soil significantly modified the physicochemical properties of the soil environment. Compost is a rich source of biogenes, i.e., C, N, and P, thus, it increases the pool of available nutrients (Baker et al. 2011; Farrell and Jones 2010). The type of substance from which compost is produced largely determines its properties. Many studies (Baker et al. 2011; Farrell and Jones 2010; Pérez de Mora et al. 2005; Pérez-de-Mora et al. 2006; Zhou et al. 2017) reported an increase in the pH value after compost application to the soil, which reduced the bioavailability of trace elements. The magnitude of the effect can be determined by the amount of compost introduced. The higher the compost dose, the more limited zinc bioavailability (Taiwo et al. 2016). The study of Paradelo et al. (2011) analyzed in detailed metal chelates and found that organic matter could play a key role in the immobilization of trace elements by forming compounds with them. Moreover, the modification of soil physicochemical properties caused changes in soil microbiome. In the study of Pardo et al. (2014), the activity of soil enzymes (cellulase, β-glucosidase, urease) was positively correlated with the pH and availability of biogenic elements (C, N, P, K), whereas negatively correlated with heavy metal content. Compost addition, by increasing organic matter content, stabilizes the microbial processes in the soil by changing the C/N ratio (Taiwo et al. 2016). Many authors pointed to the increase of microbial C biomass after compost application (Baker et al. 2011; Bastida et al. 2008; Pérez de Mora et al. 2005; Pérez-de-Mora et al. 2006; Zhou et al. 2017). According to Chang et al. (2007), soil respiration, nitrification rate and stimulation of enzymatic activity (dehydrogenases, cellulases, β-glucosidases, urease, arylsulfase, acid phosphatase, and alkaline phosphatase)are also elevated with increasing microbial C biomass. These effects were increased with the applied dose of compost. There was a positive effect of compost on enzymes, i.e., dehydrogenases (Bastida et al. 2008; Pérez de Mora et al. 2005), acid phosphatase (Baker et al. 2011), alkaline phosphatase (Baker et al. 2011; Bastida et al. 2008), arylsulfase (Baker et al. 2011; Pérez de Mora et al. 2005), urease (Bastida et al. 2008), β-glucosidase (Baker et al. 2011; Bastida et al. 2008; Debosz et al. 2002; Pérez de Mora et al. 2005); and FDA (Debosz et al. 2002) hydrolytic activity. Moreover, it was observed that the positive effects of soil composting can persist for a long time—19 months (Bastida et al. 2008). In turn, Baker et al. (2011) noted the positive effect of compost on enzyme activity even after 711 days. This allows concluding that compost is a material that has a long-term positive effect on the microbiological, biochemical, and physicochemical properties of the soil, thereby reducing the negative effects of soil contamination with zinc. In the present study, compost showed a positive effect on microbial and biochemical properties of the soil already in the second week of the experiment, and these effects persisted throughout the experiment.
Fig. 5.
The activity of selected enzymes in zinc-contaminated soil supplemented with compost (PCA analysis). Deh dehydrogenases, Cat catalase, Ure urease, Pac acid phosphatase, Pal alkaline phosphatase, Glu β-glucosidase; dose of zinc (mg Zn2+ kg−1 DM soil): I—0, II—250, III—500, IV—750, V—1000, VI—1250; dose of Corg (g kg−1 DM soil): a—0, b—10, c—20; incubation time: 2—two weeks, 20—twenty weeks
Table 4.
Effect of compost on the abundance of soil enzymes expressed by the biostimulation index (IFb)
| Dose of zinc (mg Zn2+ kg−1) | Deh | Cat | Ure | Pac | Pal | Glu | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation time of soil (weeks) | ||||||||||||
| 2 | 20 | 2 | 20 | 2 | 20 | 2 | 20 | 2 | 20 | 2 | 20 | |
| 10 g Corg kg−1 DM of soil | ||||||||||||
| 0 | 3.41d | 3.02c,d | 2.08e,f | 2.10e | 8.89c,d | 9.26b | 1.05e | 1.48f,g,h | 3.77d | 2.28g | 1.42h | 1.12i |
| 250 | 6.02c,d | 2.36c,d | 2.61c,d,e | 1.97e | 7.86d | 10.83b | 1.35c | 1.42g,h | 6.81d | 4.68e,f,g | 1.75g | 1.03i |
| 500 | 12.52c,d | 1.90d | 2.38e | 2.14d,e | 5.35d | 9.95b | 1.15d | 1.52f,g,h | 13.74c | 6.49d,e | 1.72g | 1.55g,h |
| 750 | 24.57c,d | 3.38c,d | 2.48d,e | 1.87e | 5.33d | 10.30b | 1.07e | 1.19i | 15.53c | 7.23d,e | 1.94f | 2.59f |
| 1000 | 7.09c,d | 4.42b,c | 1.71e,f | 1.93e | 4.11d | 23.56b | 1.05e | 1.81c,d | 5.03d | 7.79d | 2.20e | 3.75d |
| 1250 | 7.84c,d | 4.32b,c | 1.17f | 2.05e | 4.50d | 39.00b | 1.11d,e | 1.63d,e,f | 4.12d | 6.61d,e | 2.61c | 4.79b |
| Average | 10.23 | 3.23 | 2.07 | 2.01 | 6.01 | 17.150 | 1.13 | 1.51 | 8.17 | 5.85 | 1.94 | 2.47 |
| r | 0.26 | 0.74 | − 0.70 | − 0.34 | − 0.92 | 0.834 | − 0.32 | 0.40 | − 0.02 | 0.83 | 0.96 | 0.96 |
| 20 g Corg kg−1 DM of soil | ||||||||||||
| 0 | 6.24 | 3.30c,d | 3.76a,b,c | 3.07b,c | 34.56a | 28.13b | 1.12d,e | 1.60e,f,g | 7.74d | 3.63f,g | 1.91f | 1.09i |
| 250 | 13.15c,d | 2.74c,d | 3.89a,b | 2.72c | 30.89a | 31.25b | 1.43b | 1.91c | 14.75c | 5.59d,e,f | 2.24e | 1.45h |
| 500 | 30.18c | 4.30b,c | 3.78a,b | 2.63c,d | 17.40b | 31.85b | 1.46b | 1.75c,d,e | 35.43a,b | 13.75c | 2.62b,c | 1.63g |
| 750 | 111.72a | 5.78b | 4.77a | 3.01b,c | 14.89b,c | 26.12b | 1.66a | 1.35h,i | 39.18a | 17.40b | 2.51d | 2.99e |
| 1000 | 82.19b | 6.21a,b | 3.92a,b | 3.48a,b | 10.28c,d | 55.33b | 1.29c | 2.37b | 38.76a | 21.54a | 2.70b | 4.31c |
| 1250 | 113.44a | 8.05a | 3.58b,c,d | 3.86a | 7.67d | 119.33a | 1.73a | 2.86a | 33.20b | 19.99a,b | 3.40a | 6.62a |
| Average | 59.49 | 5.06 | 3.95 | 3.13 | 19.28 | 48.67 | 1.45 | 1.97 | 28.18 | 13.65 | 2.56 | 3.01 |
| r | 0.90 | 0.96 | 0.02 | 0.76 | − 0.96 | 0.77 | 0.67 | 0.70 | 0.80 | 0.95 | 0.93 | 0.94 |
Homogeneous groups were marked with the same letters separately for each group of enzymes and soil incubation time (two-way ANOVA performed using Tukey’s T test at P < 0.01)
Deh dehydrogenases, Cat catalase, Ure urease, Pac acid phosphatase, Pal alkaline phosphatase, Glu β-glucosidase, r Pearson’s linear correlation coefficient
Table 5.
Correlation coefficients between studied variables and enzyme activities, the count of microorganisms and physicochemical properties of the soil
| Variables | Zn | Org | Cop | Olig | Act | Fun | Deh | Cat | Pac | Pal | Ure | Glu | pH | HAC | TEB | CEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Org | − 0.32* | |||||||||||||||
| Cop | − 0.64** | 0.38** | ||||||||||||||
| Olig | − 0.08 | 0.74** | 0.35** | |||||||||||||
| Act | − 0.49** | 0.70** | 0.75** | 0.55** | ||||||||||||
| Fun | 0.70** | 0.05 | − 0.53** | 0.09 | − 0.22 | |||||||||||
| Deh | − 0.71** | 0.27 | 0.39** | 0.10 | 0.46** | − 0.34* | ||||||||||
| Cat | − 0.62** | 0.65** | 0.59** | 0.49** | 0.82** | − 0.19 | 0.80** | |||||||||
| Ure | − 0.80** | 0.62** | 0.66** | 0.36** | 0.81** | − 0.36** | 0.78** | 0.92** | ||||||||
| Pac | 0.06 | 0.71** | 0.34* | 0.78** | 0.70** | 0.29* | 0.02 | 0.53** | 0.40** | |||||||
| Pal | 0.19 | 0.53** | 0.22 | 0.67** | 0.59** | 0.46** | 0.08 | 0.53** | 0.32* | 0.92** | ||||||
| Glu | 0.52** | 0.29* | − 0.26 | 0.46** | 0.06 | 0.83** | − 0.25 | 0.06 | − 0.11 | 0.63** | 0.76** | |||||
| pH | − 0.83** | 0.50** | 0.55** | 0.27* | 0.63** | − 0.36** | 0.91** | 0.87** | 0.90** | 0.22 | 0.21 | − 0.16 | ||||
| HAC | 0.84** | − 0.61** | − 0.68** | − 0.43** | − 0.75** | 0.41** | − 0.72** | − 0.85** | − 0.92** | − 0.41** | − 0.31* | 0.09 | − 0.90** | |||
| TEB | − 0.40** | 0.81** | 0.49** | 0.70** | 0.84** | 0.08 | 0.59** | 0.92** | 0.81** | 0.77** | 0.74** | 0.37** | 0.73** | − 0.77** | ||
| CEC | − 0.32* | 0.81** | 0.44** | 0.71** | 0.82** | 0.16 | 0.54** | 0.89** | 0.76** | 0.79** | 0.78** | 0.43** | 0.67** | − 0.70** | 0.99** | |
| BS | − 0.49** | 0.82** | 0.49** | 0.69** | 0.79** | 0.07 | 0.58** | 0.88** | 0.83** | 0.73** | 0.68** | 0.38** | 0.76** | − 0.83** | 0.97** | 0.96** |
Zn zinc, Org organotrophic bacteria, Cop copiotrophic bacteria, Olig oligotrophic bacteria, Act actinomycetes, Fun fungi, Deh dehydrogenases, Cat catalase, Pac acid phosphatase, Pal alkaline phosphatase, Ure urease, Glu β-glucosidase, HAC hydrolytic acidity, TEB exchangeable bases, CEC cation exchange capacity, BS base saturation (*p < 0.05, **p < 0.01; n = 53)
Conclusions
Soil contamination with zinc significantly modified the microbiological, biochemical, and physicochemical soil properties. The application of excessive amounts of zinc to the soil reduced the number of organotrophic bacteria, copiotrophic bacteria, oligotrophic bacteria, and actinomycetes. The stimulating effect of this metal on fungi propagation was observed. The activity of soil enzymes, important due to their involvement in the circulation of key biogenic elements, was suppressed. Compost—as a rich source of biogenes—was shown to stimulate the growth of microorganisms and to increase the microbial diversity of the soil environment. An increase in the enzymatic activity of the soil was another positive effect of compost application. Organic matter suppressed the negative effects associated with excessive doses of zinc. Intensive influence of compost was observed already after 2 weeks of its application. Nevertheless, these effects persisted throughout the experiment. The reaction of the soil environment was determined by both the zinc dose and the amount of organic matter introduced. This stimulating effect of compost on soil biological properties can be conducive to reducing toxic effects of zinc. Therefore, compost can be successfully used in the bioremediation of soils contaminated with this element.
Acknowledgements
Scientific research was financed by the Ministry of Science and Higher Education funds for statutory activity.
References
- Adugna G. A review on impact of compost on soil properties, water use and crop productivity. Academic Research Journal Agricultural Science and Research. 2016;4(3):93–104. [Google Scholar]
- Alef, K., & Nannipieri, P. (1998). Methods in applied soil microbiology and biochemistry. In K. Alef & P. Nannipieri (Eds.), Academic Press, Harcourt Brace & Company (pp. 316–365). Publishers: London.
- Alef, K., Nannipieri, P., & Trazar-Capeda, C. (1998). Phosphatase activity in: methods in applied soil microbiology and biochemistry. In K. Alef & P. Nannipieri (Eds.), Academic Press Harcourt Brace & Company (pp. 335–344). Publishers: London.
- Aydinalp C, Marinova S. Distribution and forms of heavy metals in some agricultural soils. Polish Journal of Environmental Studies. 2003;12:629–633. [Google Scholar]
- Babalola OA, Adesodun JK, Olasantan FO, Adekunle AF. Responses of some soil biological, chemical and physical properties to short-term compost amendment. International Journal of Soil Science. 2012;7:28–38. doi: 10.3923/ijss.2012.28.38. [DOI] [Google Scholar]
- Baker LR, White PM, Pierzynski GM. Changes in microbial properties after manure, lime, and bentonite application to a heavy metal-contaminated mine waste. Applied Soil Ecology. 2011;48(1):1–10. doi: 10.1016/j.apsoil.2011.02.007. [DOI] [Google Scholar]
- Bastida F, Kandeler E, Moreno JL, Ros M, García C, Hernández T. Application of fresh and composted organic wastes modifies structure, size and activity of soil microbial community under semiarid climate. Applied Soil Ecology. 2008;40(2):318–329. doi: 10.1016/j.apsoil.2008.05.007. [DOI] [Google Scholar]
- Boros E, Baćmaga M, Kucharski J, Wyszkowska J. The usefulness of organic substances and plant growth in neutralizing the effects of zinc on the biochemical properties of soil. Fresenius Environmental Bulletin. 2011;20(12):3101–3109. [Google Scholar]
- Borowik A, Wyszkowska J, Kucharski J, Baćmaga M, Boros-Lajszner E, Tomkiel M. Sensitivity of soil enzymes to excessive zinc concentrations. Journal of Elementology. 2014;19(3):637–648. [Google Scholar]
- Bunt JS, Rovira AD. Microbiological studies of some subantarctic soils. Journal of Soil Science. 1955;6:119–128. doi: 10.1111/j.1365-2389.1955.tb00836.x. [DOI] [Google Scholar]
- Castaldi S, Rutigliano FA, Virzo de Santo A. Suitability of soil microbial parameters as indicators of heavy metal pollution. Water, Air, and Soil Pollution. 2004;158(1):21–35. doi: 10.1023/B:WATE.0000044824.88079.d9. [DOI] [Google Scholar]
- Chang EH, Chung RS, Tsai YH. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Science & Plant Nutrition. 2007;53:132–140. doi: 10.1111/j.1747-0765.2007.00122.x. [DOI] [Google Scholar]
- Chaperon S, Sauvé S. Toxicity interaction of metals (Ag, Cu, Hg, Zn) to urease and dehydrogenase activities in soils. Soil Biology and Biochemistry. 2007;39(9):2329–2338. doi: 10.1016/j.soilbio.2007.04.004. [DOI] [Google Scholar]
- Chibuike GU, Obiora SC. Heavy metal polluted soils: effect on plants and bioremediation methods. Applied and Environmental Soil Science. 2014;2014:1–12. doi: 10.1155/2014/752708. [DOI] [Google Scholar]
- Civeira G. Influence of municipal solid waste compost on soil properties and plant are establishment in peri-urban environments. Chilean Journal of Agricultural Research. 2010;70(3):446–453. doi: 10.4067/S0718-58392010000300012. [DOI] [Google Scholar]
- De Leij FAAM, Whipps JM, Lynch JM. The use of colony development for the characterization of bacterial communities in soil and on roots. Microbial Ecology. 1993;27:81–97. doi: 10.1007/BF00170116. [DOI] [PubMed] [Google Scholar]
- Debosz K, Petersen SO, Kure LK, Ambus P. Evaluating effects of sewage sludge and household compost on soil physical, chemical and microbiological properties. Applied Soil Ecology. 2002;19(3):237–248. doi: 10.1016/S0929-1393(01)00191-3. [DOI] [Google Scholar]
- Farrell M, Jones DL. Use of composts in the remediation of heavy metal contaminated soil. Journal of Hazardous Materials. 2010;175(1–3):575–582. doi: 10.1016/j.jhazmat.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Harris, D. C. (2006). Quantitative chemical analysis. Michelson Laboratory Chine Lake USA. WH Freeman and Company 7th edition, 1008.
- Hinojosa MB, García-Ruíz R, Viñegla B, Carreira JA. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biology and Biochemistry. 2004;36(10):1637–1644. doi: 10.1016/j.soilbio.2004.07.006. [DOI] [Google Scholar]
- Jiao XG, Gao CS, Lü GH, Sui YY. Effect of long-term fertilization on soil enzyme activities under different hydrothermal conditions in northeast China. Agricultural Sciences in China. 2011;10(3):412–422. doi: 10.1016/S1671-2927(11)60020-5. [DOI] [Google Scholar]
- Kaczyńska G, Borowik A, Wyszkowska J. Soil dehydrogenases as an indicator of contamination of the environment with petroleum products. Water, Air & Soil Pollution. 2015;226(11):372. doi: 10.1007/s11270-015-2642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Shim CK, Kim YK, Hong SJ, Park JH, Han EJ, Kim JH, Kim SC. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. The Plant Pathology Journal. 2015;31(3):259–268. doi: 10.5423/PPJ.OA.02.2015.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kızılkaya R, Aşkın T, Bayraklı B, Sağlam M. Microbiological characteristics of soils contaminated with heavy metals. European Journal of Soil Biology. 2004;40(2):95–102. doi: 10.1016/j.ejsobi.2004.10.002. [DOI] [Google Scholar]
- Kucharski J, Wieczorek K, Wyszkowska J. Changes in the enzymatic activity in sandy loam soil exposed to zinc pressure. Journal of Elementology. 2011;16(4):577–589. [Google Scholar]
- Kunito T, Saeki K, Goto S, Hayashi H, Oyaizu H, Matsumoto S. Copper and zinc fractions affecting microorganisms in long-term sludge-amended soils. Bioresource Technology. 2001;79(2):135–146. doi: 10.1016/S0960-8524(01)00047-5. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Lee J-S, Choi YJ, Kim J-G. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere. 2009;77(8):1069–1075. doi: 10.1016/j.chemosphere.2009.08.056. [DOI] [PubMed] [Google Scholar]
- Luo XS, Yu S, Zhu YG, Li XD. Trace metal contamination in urban soils of China. Science of the Total Environment. 2012;421–422:17–30. doi: 10.1016/j.scitotenv.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Marinari S, Masciandaro G, Ceccanti B, Grego S. Influence of organic and mineral fertilisers on soil biological and physical properties. Bioresource Technology. 2000;72(1):9–17. doi: 10.1016/S0960-8524(99)00094-2. [DOI] [Google Scholar]
- Martin J. Use of acid rose bengal and streptomycin in the plate method for estimating soil fungi. Soil Science. 1950;69:215–233. doi: 10.1097/00010694-195003000-00006. [DOI] [Google Scholar]
- Moreno JL, Bastida F, Ros M, Hernández T, García C. Soil organic carbon buffers heavy metal contamination on semiarid soils: Effects of different metal threshold levels on soil microbial activity. European Journal of Soil Biology. 2009;45(3):220–228. doi: 10.1016/j.ejsobi.2009.02.004. [DOI] [Google Scholar]
- Öhlinger R. Dehydrogenase activity with the substrate TTC. In: Schinner F, Öhlinger R, Kandler E, Margesin R, editors. Methods in soil biology. Berlin: Springer Verlag; 1996. pp. 241–243. [Google Scholar]
- Oht H, Hattori T. Oligotrophic bacteria on organic debris and plant roots in paddy field. Soil Biology & Biochemistry. 1983;1:1–8. [Google Scholar]
- Pane C, Celano G, Piccolo A, Villecco D, Spaccini R, Palese AM, Zaccardelli M. Effects of on-farm composted tomato residues on soil biological activity and yields in a tomato cropping system. Chemical and Biological Technologies in Agriculture. 2015;2:4. doi: 10.1186/s40538-014-0026-9. [DOI] [Google Scholar]
- Paradelo R, Villada A, Barral MT. Reduction of the short-term availability of copper, lead and zinc in a contaminated soil amended with municipal solid waste compost. Journal of Hazardous Materials. 2011;188(1–3):98–104. doi: 10.1016/j.jhazmat.2011.01.074. [DOI] [PubMed] [Google Scholar]
- Pardo T, Clemente R, Alvarenga P, Bernal MP. Efficiency of soil organic and inorganic amendments on the remediation of a contaminated mine soil: II. Biological and ecotoxicological evaluation. Chemosphere. 2014;107:101–108. doi: 10.1016/j.chemosphere.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Parkinson, D., Gray, F. R. G., & Williams, S. T. (1971). Methods for studying the ecology of soil microorganisms. Blackweel Scientific Publications Oxford and Einburg, IBP Handbook, 19: 116 pp.
- Pérez de Mora A, Ortega-Calvo JJ, Cabrera F, Madejón E. Changes in enzyme activities and microbial biomass after “in situ” remediation of a heavy metal-contaminated soil. Applied Soil Ecology. 2005;28(2):125–137. doi: 10.1016/j.apsoil.2004.07.006. [DOI] [Google Scholar]
- Pérez-de-Mora A, Burgos P, Madejón E, Cabrera F, Jaeckel P, Schloter M. Microbial community structure and function in a soil contaminated by heavy metals: effects of plant growth and different amendments. Soil Biology and Biochemistry. 2006;38(2):327–341. doi: 10.1016/j.soilbio.2005.05.010. [DOI] [Google Scholar]
- Sang-Hwan L, Jin-Soo L, Youn Jeong C, Jeong-Gyu K. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere. 2009;77(8):1069–1075. doi: 10.1016/j.chemosphere.2009.08.056. [DOI] [PubMed] [Google Scholar]
- Soares MAR, Quina MJ, Quinta-Ferreira RM. Immobilisation of lead and zinc in contaminated soil using compost derived from industrial eggshell. Journal of Environmental Management. 2015;164:137–145. doi: 10.1016/j.jenvman.2015.08.042. [DOI] [PubMed] [Google Scholar]
- StatSoft Inc. 2014. Statistica (data analysis software system), version 12.0. Available at www.statsoft.com. Accessed 21 June 2017.
- Strachel R, Wyszkowska J, Baćmaga M. The effect of nitrogen on the microbiological and biochemical properties of zinc-contaminated soil. Journal of Environmental Engineering and Landscape Management. 2017;25(1):13–22. doi: 10.3846/16486897.2016.1184154. [DOI] [Google Scholar]
- Strachel R, Wyszkowska J, Baćmaga M. The influence of nitrogen on the biological properties of soil. Bulletin of Environmental Contamination and Toxicology. 2017;98:426–432. doi: 10.1007/s00128-016-1977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo AM, Gbadebo AM, Oyedepo JA, Ojekunle ZO, Alo OM, Oyeniran AA, Onalaja OJ, Ogunjimi D, Taiwo OT. Bioremediation of industrially contaminated soil using compost and plant technology. Journal of Hazardous Materials. 2016;304:166–172. doi: 10.1016/j.jhazmat.2015.10.061. [DOI] [PubMed] [Google Scholar]
- Utobo EB, Tewari L. Soil enzymes as bioindicators of soil ecosystem status. Applied Ecology and Environmental Research. 2015;13(1):147–169. [Google Scholar]
- Verma R, Dwivedi P. Heavy metal water pollution—a case study. Recent Research in Science and Technology. 2013;5(5):98–99. [Google Scholar]
- Vinhal-Freitas IC, Wangen DRB, de Siqueira Ferreira A, Corrêa GF, Wendling B. Microbial and enzymatic activity soil after organic compost. Sociedade Brasilleria de Ciência do Solo. 2010;34:757–764. [Google Scholar]
- Vrščaj B, Poggio L, Marsan FA. A method for soil environmental quality evaluation for management and planning in urban areas. Landscape and Urban Planning. 2008;88(2–4):81–94. [Google Scholar]
- Wang YP, Shi JY, Lin Q, Chen XC, Chen YX. Heavy metal availability and impact on activity of soil microorganisms along a Cu/Zn contamination gradient. Journal of Environmental Sciences. 2007;19:848–853. doi: 10.1016/S1001-0742(07)60141-7. [DOI] [PubMed] [Google Scholar]
- World Reference Base of Soil Resources . International soil classification system for naming soils and creating legends for soil maps (World Soils Resources Raports 106) Rome, Italy: FAO; 2014. [Google Scholar]
- Wyszkowska J, Borowik A, Kucharski J, Baćmaga M, Tomkiel M, Boros-Lajszner E. The effect of organic fertilizers on the biochemical properties of soil contaminated with zinc. Plant, Soil and Environnent. 2013;59(11):500–504. [Google Scholar]
- Wyszkowska J, Boros-Lajszner E, Kucharski J. Microbiological and biochemical properties of soil contaminated with zinc and fertilized with fermented bark, keratin and cellulose. Fresenius Environmental Bulletin. 2015;24(12a):4519–4526. [Google Scholar]
- Wyszkowska J, Boros-Lajszner E, Borowik A, Baćmaga M, Kucharski J, Tomkiel M. Implication of zinc excess on soil health. Journal of Environmental Science and Health - Part B. 2016;51(5):261–270. doi: 10.1080/10934529.2015.1128726. [DOI] [PubMed] [Google Scholar]
- Wyszkowska J, Boros-Lajszner E, Borowik A, Kucharski J, Baćmaga M, Tomkiel M. Changes in the microbiological and biochemical properties of soil contaminated with zinc. Journal of Elementology. 2017;22(2):437–451. [Google Scholar]
- Yang ZX, Liu SQ, Zheng DW, Feng SD. Effects of cadium, zinc and lead on soil enzyme activities. Journal of Environmental Sciences. 2006;18(6):1135–1141. doi: 10.1016/S1001-0742(06)60051-X. [DOI] [PubMed] [Google Scholar]
- Zhou R, Liu X, Luo L, Zhou Y, Wei J, Chen A, Tang L, Wu H, Deng Y, Zhang F, Wang Y. Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. International Biodeterioration & Biodegradation. 2017;118:73–81. doi: 10.1016/j.ibiod.2017.01.023. [DOI] [Google Scholar]