Abstract
Although exercise is often recommended for managing osteoarthritis (OA), limited evidence-based exercise options are available for older adults with OA. This study compared the effects of Hatha yoga (HY) and aerobic/strengthening exercises (ASE) on knee OA. Randomized controlled trial with three arms design was used: HY, ASE, and education control. Both HY and ASE groups involved 8 weekly 45-min group classes with 2–4 days/week home practice sessions. Control group received OA education brochures and weekly phone calls from study staff. Standardized instruments were used to measure OA symptoms, physical function, mood, spiritual health, fear of falling, and quality of life at baseline, 4 and 8 weeks. HY/ASE adherences were assessed weekly using class attendance records and home practice video recordings. Primary analysis of the difference in the change from baseline was based on intent-to-treat and adjusted for baseline values. Eight-three adults with symptomatic knee OA completed the study (84% female; mean age 71.6 ± 8.0 years; mean BMI 29.0 ± 7.0 kg/m2). Retention rate was 82%. Compared to the ASE group at 8 weeks, participants in the HY group had a significant improvement from baseline in perception of OA symptoms (−9.6 [95% CI −15.3, −4]; p = .001), anxiety (−1.4 [95% CI −2.7, −0]; p = .04), and fear of falling (−4.6 [−7.5, −1.7]; p = .002). There were no differences in class/home practice adherence between HY and ASE. Three non-serious adverse events were reported from the ASE group. Both HY and ASE improved symptoms and function but HY may have superior benefits for older adults with knee OA.
Keywords: Knee osteoarthritis, Hatha yoga, Aerobic and strengthening exercises, Older adults
Introduction
Osteoarthritis (OA) is a chronic degenerative musculoskeletal condition that affects many older adults worldwide [1]. In the United States, 33.6% (12.4 million) of individuals age 65 years and older are affected by OA, and projections suggest that this number will increase to be 67 million by 2030 [2]. OA is the primary reason for pain, limited range of motion, and loss of joint movement in older adults. The most commonly affected joint is the knee [3]. Symptomatic knee OA is associated with anxiety [4], fear of falling and falls [5], and impaired quality of life [6].
Because OA is a chronic condition that has no effective cure, self-management is essential. Recent practice guidelines recognize that exercise is a key element of any treatment program for OA [7]. It is recommended that engaging in moderate physical activity at least three times per week can reduce the risk of arthritis-related disability by 47% [8]. Although there is increasing evidence in the literature for the beneficial effects of exercise for managing OA [9], the evidence on comparative effectiveness of different types of exercise, including exercise adherence and satisfaction in older adults with knee OA, is limited.
A multidisciplinary guideline development group recommended “prescription of both general (aerobic fitness training) and local (strengthening) exercises as a core aspect of management for every patient with hip or knee OA” [10]. Systematic reviews have evaluated the efficacy of aerobic [11] and strengthening [12] exercises in people with OA at predominately the knee joint and found that both types of exercise programs improve strength, pain, joint tenderness, and physical function. Yoga has been used clinically as a therapeutic intervention for improving strength, posture, balance, and flexibility in older adults [13, 14] and is currently one of the fitness programs recommended by the Arthritis Foundation for individuals with arthritis in general. However, relatively little robust research has been conducted on yoga in the OA population compared to other types of exercise. A recent focused review evaluating the effectiveness and safety of yoga for knee OA identified only three randomized controlled trials [15]. Results from this review revealed that yoga improves OA symptoms and that it can be practiced safely without supervision at home.
There are relatively few head-to-head comparisons of different exercise modalities in older adults with OA. It is unclear how yoga compares to the current recommended exercise interventions. Research shows that exercise non-adherence is a common problem especially among older exercisers [16] due to poor health and mobility limitations [17]. It is unclear if a yoga program that is tailored for managing knee OA will have a better adherence rate than a traditional exercise program. Hatha yoga (HA) is the physical form of yoga that can theoretically reduce pain and stiffness associated with OA by realigning the skeletal structure, strengthening muscles around the joints, and stretching tight joint structures [18]. Its mindful, meditative way of combining breathing with stretching exercises provides the relaxation benefits and reduces the activity of the stress system that may help decrease pain sensitivity and induce analgesic effects [19]. The aims of this pilot study were to: (1) evaluate the effects of an 8-week HA program, a low-impact aerobics and strengthening exercises (ASE), and an education attention control intervention on OA-related symptoms in older adults, and (2) determine the difference in adherence to both group-based and home-base sessions as well as satisfaction between the two intervention programs. We hypothesized that participants in the HA group would demonstrate greater improvement in OA-related symptoms than participants in the ASE program and the education control intervention.
Methods
Design
This study employed a parallel randomized controlled trial design with three arms: HY, ASE, and an education attention control group. As an incentive, participants in the control group received a free yoga for OA intervention session at the end of the study. Data on OA-related outcomes were collected at baseline, 4 weeks, and 8 weeks post-randomization.
Sample
Recruitment of participants was conducted in two waves. The first wave was to pilot the study design and intervention strategies in a small number of participants. The inclusion criteria were: (1) community-dwelling adults aged 60 years or over; (2) have a self-reported medical diagnosis of symptomatic OA of knee for at least 6 months; (3) have not practiced any form of yoga for 2 months because physiological changes induced by regular exercise training are generally lost after 4–8 weeks of detraining [20, 21]; and (4) not currently participating in a supervised exercise program more than two times a week.
Additional exclusion criteria included: symptoms of joint locking to a degree that affects the individual’s balance and makes participating in a group exercise program unsafe; chronic use of assistive devices; corticosteroid injections within 3 months of study entry; hyaluronic acid injection within 6 months of study start date; history of knee surgery within the last 2 years; knee joint replacement; self-reported comorbidities including uncontrolled hypertension, unstable heart conditions, or comorbidities with overlapping symptoms (i.e., rheumatoid arthritis). Because additional funding was obtained to expand the size and duration of the study, a second wave of recruitment occurred with the same recruitment protocol except only women were recruited because it was the primary focus of the funding agency. Participants were recruited through OA-related presentations at various community and senior centers, senior programs, flyers, press releases, and community newsletters. No important changes were made to methods after trial commencement.
Potential participants were initially screened by trained research assistant (RA) over the telephone. Those who met the initial eligibility requirements were invited for a second-step screening. A second-step screening was conducted face-to-face with a trained RA to assess the presence of symptomatic knee OA using the Clinical Criteria for the Classification of Idiopathic OA of the Knee developed by the American College of Rheumatology [22]. Population based studies suggest that 30–50% of individuals with moderate to severe radiographic changes of OA are asymptomatic, and approximately 10% of individuals with moderate to severe knee pain have normal radiographic reports [23, 24]. Because the focus of this study was on symptom management, therefore individuals with symptomatic knee OA with or without an official OA diagnosis were recruited. Potential participants were excluded if they had cognitive impairment (scored less than 8 in the Short Portable Mental Status Questionnaire) [25], unstable health conditions (answered any “yes” in the Exercise Assessment and Screening for You tool) [26], treatment that may alter their OA symptoms, or functional limitations that may make exercise unsafe. All data were collected at a community center or senior housing complex where the cohort’s intervention classes were held.
Randomization was conducted by the principal investigator using a simple randomization method. The process involved randomizing participants utilizing an online software program (www.random.org) that generates random numbers. Each participant’s group assignment was placed in a sealed envelope and distributed to the participant by the principal investigator after the baseline data were collected by blinded RAs.
Intervention
Intervention classes were held in a large metropolitan area in the Upper Midwest. The HY program was designed by a group of expert yoga teachers and was composed of one 45-min class per week for eight weeks and additional 30 min/day, four times/week of yoga practice at home during the intervention period. Sessions included poses in the seated, supine, prone, and standing positions; breathing exercises, and relaxation/mindfulness training. Key yoga poses included “easy” seated pose, reclining bound angle, half locust variation, head to knee pose, bridge, standing forward fold, chair pose, mountain pose, warrior I and II, tree pose variation, reclining hamstring stretch with hip opener with strap, reclining twist, and relaxation pose. A progressive series of poses with props such as yoga mats, blocks, straps, blankets, and chairs were used during class, and poses were modified when needed based on the participants’ physical abilities to increase confidence and the ability to remain in the pose and achieve benefits. Each class consisted of approximately 8–10 yoga poses with a 2–3 new, variable poses were introduced at each session. A registered yoga instructor who was experienced in working with older adults with functional limitations taught all HY classes.
The ASE program consisted of eight weekly group-based classes that involved 15 min of mild aerobic exercise that served as a full body warm up, and 30 min of strengthening exercises including both isometric (without moving the joints) and isotonic (moving the joints) exercises. Additionally, participants were asked to practice the aerobic portion of the program for 15–30 min/day, four times/week, and the strengthening exercise 30 min/day, two times/week on non-consecutive days at home. The ASE program was progressive in nature. It was based on the current Arthritis Foundation recommendations and taught by a certified arthritis exercise instructor who taught all the ASE classes. Specific types of ASE included head rotations, shoulder flexion/extension, torso 360° rotation (circles in both direction), shoulder circles, marching in place, heel and toe raises, overhead arm reaches, side bends, torso twist (gentle 30°), seated side steps alternating sides, and ankle circles. Props such as elastic bands and chairs were used during the class.
Extra measures were taken to provide a safe and acceptable environment for both HY and ASE groups: (a) a small class size (n ≤ 10 participants/class) and programs specifically designed for older adults with knee OA, (b) each participant was provided a tool (yoga mat for the HY group, elastic band for the ASE group) for use in group-based classes and home practices; (c) home practice handouts with pictures and written instruction were distributed after each class; and (d) the intervention sites were conveniently located in the metropolitan areas with adequate parking space to allow easy access.
Participants in the education group received preprinted education brochures from the Arthritis Foundation on how to manage OA pain, and physical activity and exercise for OA. Each participant in the control group received weekly telephone calls from a RA during the 8-week intervention period. They were asked about their OA symptoms and general health status.
Measures
Demographic and health information were collected from all participants at baseline. Standardized instruments that were validated in older and/or OA populations were used for collecting outcome data at baseline, 4 weeks and at 8 weeks. These instruments took approximately 20–30 min to complete.
Primary outcome measure
OA symptoms including pain, stiffness, and physical function were measured using the 24-item Western Ontario and McMaster Universities OA Index scale (LK scale 3.1) (WOMAC) [27]. Each symptom is measured using a 5-point Likert scale where 0 represents having no symptom and 4 represents having a severe symptom. OA pain was also measured using the Visual Analog Scale, and a single question that asked participants to record the average number of pain medications (prescription and over-the-counter) they used for knee OA per day.
Secondary outcome measures
Physical performance of the lower extremities was assessed using the Short Physical Performance Battery (SPPB) [28] which consists of three components: repeated chair stands, balance, and timed 8 foot walk. Categorical scores (range 0–4) for the 8 foot walk and chair stands were based on timed quartiles previously established in a large population. Walking speed was timed using the 50 foot walk test [29].
Mood including anxiety and depression was measured using the Hospital Anxiety and Depression Scale (HADS) [30]. The tool is a 14-item self-report scale that consists of two subscales: 7 items on depression and 7 items on anxiety. The scale was designed to screen for mood disorders in general (non-psychiatric) medical outpatients.
Fear of Falling was assessed by the Fall Efficacy Scale-International [31]. It measures participants’ level of concern about falling during social and physical activities inside and outside the home whether or not the person actually does the activity.
Spiritual health was assessed using the Self-Transcendence (SF) Scale [32]. SF refers to expanded self-boundaries and awareness [33]. A positive association was found between SF and positive coping factors for those suffering with chronic disease [34].
Quality of life was assessed using the Short Form Health Survey; the SF-12 which measures both physical and mental component summary scales [35]. The SF-12 survey contains questions that assess limitations in role functioning as a result of physical and emotional health.
Program Satisfaction was assessed at the end of the 8-week intervention using an investigator-developed questionnaire. Participants were asked to rate their satisfaction through perceived enjoyment of class, the ease of class, and likelihood to recommend class to others using a 4-point Likert scale with “1” representing “not at all” and “4” representing “definitely.”
Adherence to the intervention programs including both class attendance and frequency of home practice were evaluated using weekly class attendance records, home practice video recordings, and self-recorded yoga/exercise log sheets. Each participant in the HY and ASE groups were given a video camera with both written and verbal instructions at the beginning of the intervention program. No changes were made to the study outcomes after the trial commenced.
Data analysis
The primary objective of this study was to compare the change in self-reported OA symptoms among the HY, ASE and education control groups from baseline to 8 weeks. Data from a recent pilot study [36] indicated an overall SD of 16.5 for the total WOMAC and a correlation between time points of 0.6. The sample size of 30 per group was calculated to have 80% power to detect a difference in the change (slope) of 35% over time (change of 11.3 on the WOMAC total score) with Type I error set to 0.05 and using a repeated measures analysis of covariance (ANCOVA) controlling for baseline values.
Descriptive statistics were used to describe the main characteristics of the participants, frequency of home practice, and study retention. Data were collected from all participants who were enrolled in the study for intent-to-treat analysis, carrying forward the previous measurement if missing. ANOVA was used to analyze group differences at baseline in demographic information, health status, and outcome measures. To evaluate treatment differences, between HY versus ASE and HY versus control, means were adjusted by baseline values and contrasts with 95% confidence intervals were calculated. The p values were not adjusted for the multiple comparisons. Data were analyzed using SAS version 9.4 by a biostatistician who was blinded to the group assignment. If the calculated p value was less than 0.05, the result was considered to be statistically significant.
Study quality was monitored by two established researchers who served on the Data Safety and Monitoring Board (DSMB) at mid-point of the study. Progress reports including participant recruitment, retention/attrition with reasons for dropout from the study, and a summary of adverse events were sent to DSMB members for independent review.
Results
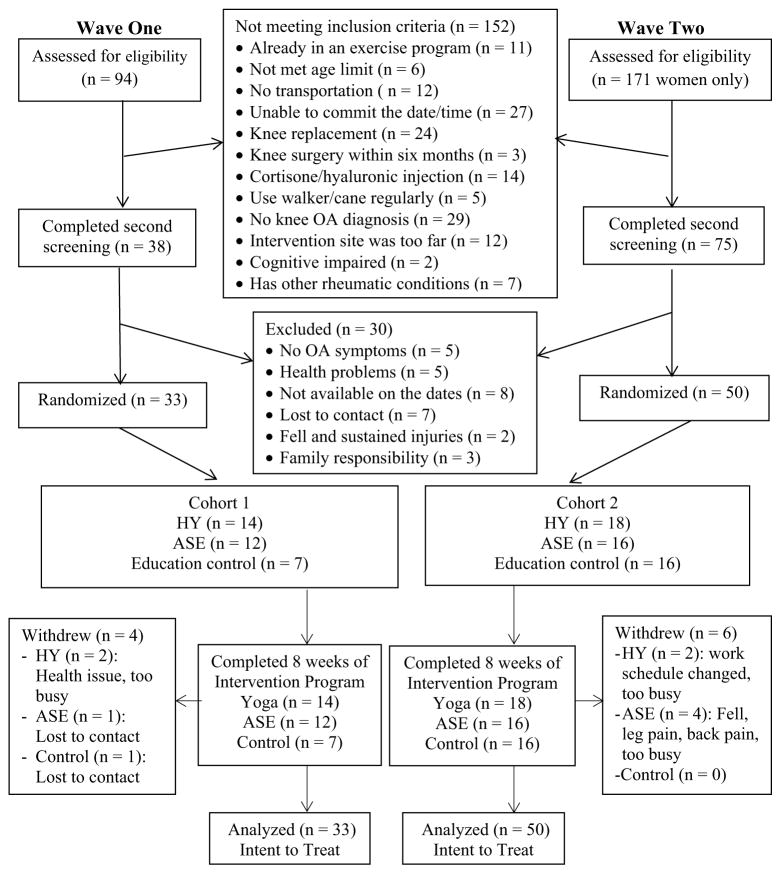
Recruitments took place from March to September 2013 for wave one and from April 2014 to November 2015 for wave two. A total of 265 potential participants were screened by telephone to determine initial eligibility. Of those, 113 (43%) were included in the second-step screening. Potential participants were excluded for a variety of reasons, the major ones being unable to commit the date/time or no medical diagnosis of OA in the knee. Among the people who completed the second-step screening, 83 (73%) participants were randomized into one of the three groups (Fig. 1).
Fig. 1.
CONSORT Flow Diagram depicts the study recruitment, screening, enrollment, and allocation of group assignments as well as the number of data analyzed
The characteristics of the randomized participants and group differences are shown in Table 1. Age, OA pain level, and fear of falling were noted to be statistically different among the three groups at baseline. The uneven group sizes are due to the short funding period in wave one, and two of the intervention classes in wave two began before the ideal sample size was reached to avoid having classes during the extreme winter months. Therefore, less participants were randomized into the control group to maximize the sample size in both HY and ASE groups.
Table 1.
Baseline demographic and clinical characteristics of the sample
| Categories | HY (n = 32) Mean (SD) |
ASE (n = 28) Mean (SD) |
Control (n = 23) Mean (SD) |
p value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 68.9 (7.7) | 74.4 (7.5) | 71.8 (8.0) | 0.03 |
| BMI | 29.8 (6.3) | 29.2 (7.1) | 27.8 (7.9) | 0.61 |
| Education (years) | 15.3 (2.1) | 16.1 (2.2) | 16.2 (3.2) | 0.30 |
| Health status and OA symptoms | ||||
| Comorbidities, no. | 1.5 (1.3) | 1.6 (1.8) | 1.5 (1.2) | 0.93 |
| WOMAC-total (0–96) | 39.4 (16.8) | 42.1 (21.0) | 34.1 (14.8) | 0.28 |
| WOMAC-pain (0–20) | 7.9 (2.8) | 7.7 (4.4) | 6.3 (3.1) | 0.20 |
| WOMAC-stiffness (0–8) | 4.4 (1.9) | 4.5 (1.8) | 3.5 (1.6) | 0.11 |
| WOMAC-function (0–68) | 27.1 (13.2) | 29.9 (15.9) | 24.3 (11.3) | 0.36 |
| Pain scale (0–10) | 6.2 (2.5) | 4.9 (2.0) | 5.0 (2.1) | 0.04 |
| Physical functions | ||||
| SPPB Global (0–12) | 9.9 (1.9) | 9.8 (2.2) | 9.4 (2.9) | 0.73 |
| SPPB-chair (0–4) | 2.5 (1.1) | 2.9 (1.4) | 2.3 (1.5) | 0.27 |
| SPPB-chair (s) | 14.3 (6.6) | 12.1 (4.0) | 13.6 (8.9) | 0.47 |
| SPPB-balance (0–4) | 3.6 (0.8) | 3.3 (1.1) | 3.6 (1.1) | 0.39 |
| SPPB-8′ walk (0–4) | 3.7 (0.6) | 3.6 (0.7) | 3.5 (0.9) | 0.70 |
| SPPB-8′ walk (s) | 2.9 (0.8) | 2.8 (0.9) | 3.2 (1.3) | 0.46 |
| 50′ Walk (s) | 12.5 (3.0) | 12.4 (3.4) | 15.1 (9.4) | 0.16 |
| Psychosocial well-being | ||||
| HADS-A (0–21) | 5.5 (3.3) | 4.9 (3.7) | 4.4 (3.8) | 0.52 |
| HADS-D (0–21) | 4.0 (3.0) | 4.4 (2.4) | 3.3 (1.8) | 0.33 |
| STS (15–60) | 50.6 (7.2) | 52.3 (5.4) | 52.1 (8.2) | 0.60 |
| FESI (16–64) | 25.8 (8.5) | 28.7 (9.3) | 23.0 (5.4) | 0.05 |
| SF-12 PCS (0–100) | 38.9 (9.7) | 37.6 (11.1) | 39.1 (10.5) | 0.86 |
| SF-12 MCS (0–100) | 53.5 (9.8) | 52.4 (10.2) | 58.1 (7.4) | 0.09 |
Bold values denote a statistically significant difference (p values ≤ .05)
WOMAC Western Ontario and McMaster Universities Osteoarthritis Index (lower scores = better state), SPPB Short Physical Performance Battery (higher scores = better state), HADS Hospital Anxiety and Depression Scale (lower score = better state), STS Self-Transcendence Scale, FESI Fall Efficacy Scale-International (lower score = better state), QOL quality of life, SF-12 Health related Short Form 12 (higher scores = better state), PCS physical component summary, MCS mental component summary
Dropout rates of participants among the three groups were: HY group (n = 4, 13%), ASE group (n = 5, 18%), and education control group (n = 1, 4%). One non-study related and two non-serious adverse events related to study participation were noted. All occurred among participants in the ASE group: one participant fell on ice and experienced back pain; one participant with a history of herniated disk had recurring low back pain; and a third participant developed leg pain half way through the program which may have been related to a preexisting condition of left patella femoral pain syndrome.
Effects of interventions on OA symptoms at 4 and 8 weeks
The primary outcome in this study was self-reported OA symptoms. Compared to participants in the ASE group at 8 weeks, participants in the HY group had greater symptom improvement (WOMAC total: −9.6 [95% CI −15.3, −4.0]; p = .001). Specifically, less knee pain (WOMAC pain index scores: −1.4 [95% CI −2.7, −0.1]; p = .04 and Visual Pain Analog scores: −1.1 [95% CI −2.2, −0.1]; p = .03), and higher perceived function (WOMAC function index scores: −7.6 [95% CI 11.9, −3.3]; p =.001) were reported (Table 2).
Table 2.
Effects of interventions on outcome variables at 8 weeks
| Variable | HY (n = 32) Mean (95% CI) |
ASE (n = 28) Mean (95% CI) |
Control (n = 23) Mean (95% CI) |
HY versus ASE Mean (95% CI) |
p value | HY versus control Mean (95% CI) |
p value |
|---|---|---|---|---|---|---|---|
| Health status and OA symptoms | |||||||
| Pain medications | 1.1 (0.5, 1.7) | 1.0 (0.3, 1.6) | 1.3 (0.7, 2.0) | 0.1 (−0.8, 0.9) | 0.858 | −0.3 (−1.2, 0.6) | 0.556 |
| WOMAC-total (0–96) | 26.4 (22.5, 30.2) | 36.0 (31.9, 40.2) | 35.9 (31.3, 40.4) | −9.6 (−15.3, −4.0) | 0.001 | −9.5 (−15.5, −3.5) | 0.002 |
| WOMAC-pain (0–20) | 5.1 (4.1, 6.0) | 6.5 (5.5, 7.4) | 6.5 (5.4, 7.6) | −1.4 (−2.7, −0.1) | 0.038 | −1.5 (−2.9, −0.0) | 0.045 |
| WOMAC-stiffness (0–8) | 3.2 (2.7, 3.7) | 3.9 (3.3, 4.4) | 4.0 (3.3, 4.6) | −0.7 (−1.4, 0.1) | 0.093 | −0.8 (−1.6, 0.1) | 0.070 |
| WOMAC-function (0–68) | 18.2 (15.3, 21.1) | 25.8 (22.7, 28.9) | 25.2 (21.8, 28.7) | −7.6 (−11.9, −3.33) | 0.001 | −7.1 (−11.6, −2.5) | 0.003 |
| Pain scale (0–10) | 4.0 (3.3, 4.7) | 5.2 (4.4, 5.9) | 5.2 (4.4, 6.0) | −1.1 (−2.2, −0.1) | 0.030 | −1.2 (−2.2, −0.1) | 0.031 |
| Physical functions | |||||||
| SPPB Global (0–12) | 10.6 (10.1, 11.1) | 10.7 (10.2, 11.2) | 9.9 (9.3, 10.4) | −0.1 (−0.8, 0.6) | 0.732 | 0.7 (−0.0, 1.4) | 0.058 |
| SPPB-chair (0–4) | 3.3 (3.0, 3.6) | 3.1 (2.8, 3.4) | 2.8 (2.4, 3.1) | 0.2 (−0.2, 0.6) | 0.401 | 0.5 (0.1, 1.0) | 0.025 |
| SPPB-chair (s) | 10.6 (9.5, 11.7) | 11.0 (9.8, 12.2) | 11.1 (9.7, 12.4) | −0.4 (−2.0, 1.3) | 0.654 | −0.5 (−2.2, 1.2) | 0.571 |
| SPPB-balance (0–4) | 3.5 (3.3, 3.8) | 3.7 (3.5, 4.0) | 3.5 (3.2, 3.8) | −0.2 (−0.6, 0.1) | 0.233 | 0.0 (−0.4, 0.4) | 0.983 |
| SPPB-8′ walk (0–4) | 3.8 (3.7, 4.0) | 3.9 (3.7, 4.1) | 3.4 (3.2, 3.7) | 0.0 (−0.3, 0.2) | 0.770 | 0.4 (0.1, 0.7) | 0.006 |
| SPPB-8′ walk (s) | 2.6 (2.3, 2.9) | 2.5 (2.2, 2.9) | 3.1 (2.7, 3.5) | 0.1 (−0.4, 0.5) | 0.743 | −0.5 (−1.0, −0.0) | 0.040 |
| 50′ Walk (s) | 13.0 (12.3, 13.7) | 12.9 (12.2, 13.6) | 13.0 (12.2, 13.8) | 0.1 (−1.0, 1.1) | 0.877 | 0.0 (−1.1, 1.1) | 0.977 |
| Psychosocial well-being | |||||||
| HADS-A (0–21) | 3.8 (2.9, 4.7) | 5.2 (4.2, 6.2) | 4.4 (3.3, 5.6) | −1.4 (−2.7, −0.0) | 0.044 | −0.6 (−2.1, 0.8) | 0.379 |
| HADS-D (0–21) | 3.8 (3.1, 4.5) | 4.2 (3.5, 5.0) | 3.7 (2.8, 4.5) | −0.4 (−1.5, 0.6) | 0.396 | 0.1 (−1.0, 1.2) | 0.833 |
| STS (15–60) | 51.7 (50.0, 53.4) | 51.3 (49.5, 53.1) | 52.5 (50.5, 54.6) | 0.4 (−2.1, 2.9) | 0.752 | −0.9 (−3.5, 1.8) | 0.521 |
| FESI (16–64) | 23.9 (21.9, 25.9) | 28.5 (26.4, 30.7) | 27.7 (25.3, 30.1) | −4.6 (−7.5, −1.7) | 0.002 | −3.8 (−6.9, −0.7) | 0.016 |
| SF-12 PCS (0–100) | 41.5 (38.6, 44.5) | 38.8 (35.4, 42.1) | 39.0 (35.5, 42.4) | 2.7 (−1.7, 7.2) | 0.227 | 2.6 (−2.0, 7.1) | 0.269 |
| SF-12 MCS (0–100) | 55.2 (52.2, 58.2) | 53.8 (50.4, 57.2) | 52.8 (49.2, 56.4) | 1.4 (−3.1, 6.0) | 0.528 | 2.5 (−2.3, 7.2) | 0.302 |
ANCOVA comparing 8 week results with 8 weeks means adjusted for baseline, and 95% confidence interval were reported Bold values denote a statistically significant difference (p values ≤ .05)
WOMAC Western Ontario and McMaster Universities Osteoarthritis Index, SPPB Short Physical Performance Battery, HADS Hospital Anxiety and Depression Scale, STS Self-Transcendence Scale, FESI Fall Efficacy Scale-International, SF-12 Health related Short Form 12, PCS physical component summary, MCS mental component summary
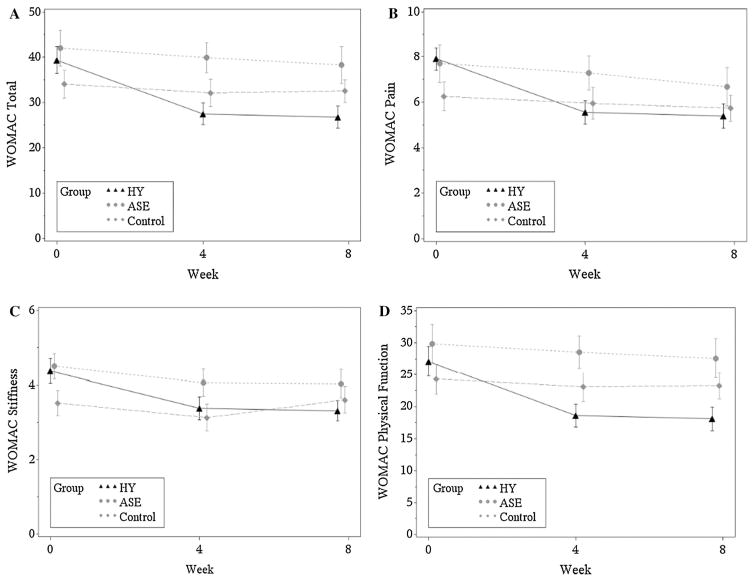
Similar effects were seen when compared to participants in the education control (WOMAC total: −9.5 [95% CI −15.5, −3.5]; p = .002; WOMAC pain index scores: −1.5 [95% CI −2.9, −0.0]; p = .05; WOMAC function index scores: −7.1 [95% CI −11.6, −2.5]; p = .003) and Visual Pain Analog scores: −1.2 [95% CI −2.2, −0.1]; p = .03) (Table 2). The positive effects of HY on symptom perception based on the WOMAC total and subscales were demonstrated at 4 weeks and sustained at 8 weeks (Fig. 2).
Fig. 2.
Effects of HY, ASE, and Education Control on knee OA symptoms at baseline, 4 and 8 weeks. a WOMAC total mean scores, b WOMAC pain mean scores, c WOMAC stiffness mean scores, and d WOMAC function mean scores
Effects of interventions on secondary outcomes at 8 weeks
The HY group took less time to complete the SPPB repeated chair stands (categorical score: .5 [95% CI .1, 1.0]; p = .03) and shorter time to complete the 8′ walk (categorical score: .4 [95% CI .1, .7]; p = .006, and in seconds: −.5 [95% CI −1.0, 0.0]; p = .04) than the education control group. Participants in the HY group had significantly lower anxiety (−1.4 [95% CI −2.7, 0]; p = .04) than the ASE group and had less fear of falling than the ASE group (−4.6 [95% CI −7.5, −1.7]; p =.002) as well as the education control group (−3.8 [95% CI −6.9, −.7]; p = .02) at the end of the intervention program at 8 weeks (Table 2).
Exercise adherence
Class adherence
More than half of the participants in both group (HY: n = 20, 63% vs. ASE: n = 16, 57%) participated ≥50% of classes. There was no difference in class attendance between the two exercise groups (p = .67).
Home practice adherence
The average number of minutes/week (HY: 79 ± 54 (0–278) vs. ASE: 56 ± 33 (0–126), p = .11) and the number of days/week (HY: 3 ± 1 (0–6) vs. ASE: 3 ± 1 (0–5), p = .28) were not significantly different.
Exercise satisfaction
No statistically significant differences in program satisfaction between the HY and ASE groups on enjoyment (p = .18), ease of use (p = .36), and recommendations of the program to others (p = .52).
Discussion
This is one of the first studies comparing the effectiveness of different types of exercise and education in the management of knee OA in older adults. While ASE have previously been shown to reduce pain and disability in subjects with symptomatic knee OA [10], the results of this study indicate that participants in the 8-week HY intervention program had greater improvements in pain and self-reported function, lower extremity strength, anxiety, and fear of falling compared with those participating in the ASE and control groups.
The positive effect of HY on measures of pain and self-reported function in this study confirmed previous findings in other yoga for OA studies [15]. The greater beneficial effect of yoga on OA symptoms than traditionally recommended ASE may be related its use of meditative practices and how they affect the perception of pain. Well-chosen yogic postures can strengthen muscles and correct posture; coupled with breathing and relaxation, yoga may help individuals deal with the reactive aspects of chronic pain. [37] During self-awareness-based meditation techniques, the person attempts to nurture the role of an observer and a detached witness of all subjective phenomena including pain [38]. Instead of being a victim of chronic pain, this practice may enable the person to take charge of their pain experience and change the perception of it [39]. Similar results with different kinds of meditative practices have also demonstrated improvement on pain states [40]. It is noteworthy that the physical function scores were not significantly different between the HY and ASE groups. This finding concurs with previous studies that found both interventions improve muscular strength, balance, coordination, joint range of motion, and reaction time [9, 41].
“Fear of falling” is highly prevalent among older adults. Research demonstrated that fear may contribute to the risk of falling in older adults [42]. The HY program included poses that required participants to get up and down from the floor. Yoga might have provided the participants not only the physical practice and skills but the confidence they need to reduce their level of concern about falling. People who think they have a low risk of falling may be more likely to engage in physical activity, and therefore, retain strength and prevent functional disability. In addition to poses, HY incorporates breathing and relaxation exercises in the practice, which may have contributed to the positive finding on anxiety. A meta-analysis investigating the effectiveness of yoga as a complementary treatment for psychiatric disorders found that yoga is a safe and effective option for lowering anxiety [43].
It is noteworthy that at baseline, most participants scored positively on spiritual health and quality of life before the intervention began; therefore, ceiling effects might have contributed to the findings. In general, independent living older adults who volunteered to be in a research study may have a positive outlook in life [44].
The differences in baseline characteristics could be contributed by the small sample size. Despite simple randomization’s usefulness in mitigating selection bias and forming the basis for statistical analysis, it cannot avoid any imbalances in the distribution of key baseline covariates due to chance. In future clinical trials, using a larger sample size or a randomization method such as a covariate-adaptive randomization to help achieve covariate balance at baseline will decrease excess noise in clinical trial data to allow for maximal power of detecting the treatment effect in primary and secondary outcome analyses [45].
Safety of exercise for individuals with preexisting health conditions including knee OA can be a concern, especially for those who are older. In this study, there were no exercise-related injuries with HY, whereas three participants in the ASE group experienced possible exercise-related injuries. Yoga is gentle and adaptable to accommodate the needs and limitations of older adults. It may be a safer exercise option for those who have functional limitations because of OA or other preexisting musculoskeletal conditions.
The dropout rates between the two intervention groups are not significantly different (13 vs. 18%). Both are lowered than the average dropout rate of 21% reported in a meta-analysis of 36 exercise studies that focused on the general adult population [46]. Although overall class participation met expectations, many participants in both groups did not adhere to the recommended exercise prescription during their home practice. Future research studies are needed to determine effective strategies to improve home-based exercise adherence in older adults with OA, and the optimal dose of home practice for sustaining therapeutic effects.
Limitations
There are several study limitations. First, the sample size is slightly below the expected level due to short funding period and weather. Nonetheless, significant findings in group comparisons were noted in symptom perception, physical performance, anxiety, and fear of falling. Second, because the majority of the sample was comprised of Caucasian and well-educated women, the homogenous sample limit the generalizability of findings. Third, participants are self-selected volunteers; they may be more active and motivated than the typical older adults with knee OA. Lastly, although using a video camera was an effective way to capture home HY/ASE practices and served as an objective measurement tool, it created subject burden and a learning curve that many participants experienced during the first 2 weeks of the program. Alternatively, the video camera might have impacted some of the participants positively and promoted their home practice adherence.
In conclusion, the results of this study suggest that an 8-week HY program is safe and effective in improving symptom perception in older adults with knee OA, and that it may have superior benefits than ASE and education alone on physical function of the lower extremities, anxiety, and fear of falling. These findings provide support for the Arthritis Foundation’s recommendation for the use of yoga as an exercise intervention that could be incorporated as a part of a regular OA treatment program.
Acknowledgments
The authors want to thank Catherine Justice, Registered Yoga Teacher, and Mary Clapp, Certified Personal Trainer for implementing the intervention classes, and the Directors of the Jewish Community Centers for providing the classrooms and assisting in recruitment.
Funding This study was funded by the University of Iowa Hartford Center Geriatric Nursing Excellent Pilot Grant, and Deborah E. Powell Center of Mature Women’s Health and Research Grants. It is also supported in part by the National Center for Advancing Translational Sciences Award UL1TR000114. The study sponsors played no role in study design, methods, participant recruitment, data collection, data analysis or development of this manuscript.
Footnotes
Trial registration The full trial protocol is available at clinicaltrials.gov (NCT02525341).
Authors’ contributions The authors CC and JW conceived the study and carried out the design. UB provided exercise consultation and assisted with the development of the ASE intervention program. TM participated in the design of the study and provided geriatric consultations. KR provided statistical consultations. MM performed the statistical analyses. CC, KR, and MM interpreted the results. CC drafted the manuscript. All authors critically reviewed, revised, and approved the final manuscript.
Compliance with ethical standards
Conflict of interest The authors, Corjena Cheung, Jean Wyman, Ulf Bronas, Teresa McCarthy, Kyle Rudser, and Michelle Mathison declare that they have no financial, personal, or potential conflict of interests that could potentially or inappropriately influence (bias) their work and conclusions have no conflict of interest. The University of Minnesota’s Institutional Review Board granted approval of the study.
Ethical standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study. Measures were taken throughout the study to ensure safety to prevent yoga or exercise-related injury. Participants’ data were kept private and confidential.
References
- 1.Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26(3):371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hootman JL, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheumatol. 2006;54(1):226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention. [Accessed 12 May 2016];Osteoarthritis. http://www.cdc.gov/arthritis/basics/osteoarthritis.htm.
- 4.Axford J, Butt A, Heron C, et al. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clin Rheumatol. 2010;29(11):1277–1283. doi: 10.1007/s10067-010-1547-7. [DOI] [PubMed] [Google Scholar]
- 5.Ng CT, Tan MP. Osteoarthritis and falls in the older person. Age Ageing. 2013;42(5):561–566. doi: 10.1093/ageing/aft070. [DOI] [PubMed] [Google Scholar]
- 6.Alkan BM, Fidan F, Tosun A, et al. Quality of life and self-reported disability in patients with knee osteoarthritis. Mod Rheumatol. 2014;24(1):166–171. doi: 10.3109/14397595.2013.854046. [DOI] [PubMed] [Google Scholar]
- 7.Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the US Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43(6):701–712. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW, Messier SP, Rejeski WJ, et al. Physical exercise and the prevention of disability in activities of daily living in older persons with osteoarthritis. Arch Intern Med. 2001;161(19):2309–2316. doi: 10.1001/archinte.161.19.2309. [DOI] [PubMed] [Google Scholar]
- 9.Hunter DJ, Eckstein F. Exercise and osteoarthritis. J Anat. 2009;214(2):197–207. doi: 10.1111/j.1469-7580.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66(3):622–636. doi: 10.1002/art.38290. [DOI] [PubMed] [Google Scholar]
- 11.Roddy E, Zhang W, Doherty M, et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee—the MOVE consensus. Rheumatology. 2005;44(1):67–73. doi: 10.1093/rheumatology/keh399. [DOI] [PubMed] [Google Scholar]
- 12.Bennell KL, Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J Sci Med Sport. 2001;14(1):4–9. doi: 10.1016/j.jsams.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Patel NK, Newstead AH, Ferrer RL. The effects of yoga on physical functioning and health related quality of life in older adults: a systematic review and meta-analysis. J Altern Complement Med. 2012;18(10):902–917. doi: 10.1089/acm.2011.0473. [DOI] [PubMed] [Google Scholar]
- 14.Roland KP, Jakobi JM, Jones GR. Does yoga engender fitness in older adults? A critical review. J Aging Phys Act. 2011;19(1):62–79. doi: 10.1123/japa.19.1.62. [DOI] [PubMed] [Google Scholar]
- 15.Cheung C, Park J, Wyman JF. Effects of yoga on symptoms, physical function, and psychosocial outcomes in adults with osteoarthritis: a focused review. Am J Phys Med Rehabil. 2016;95(2):139–151. doi: 10.1097/PHM.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 16.Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39(5):1056–1061. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Belza B, Walwick J, Shiu-Thornton S, et al. Older adult perspectives on physical activity and exercise: voices from multiple cultures. Prev Chronic Dis. 2004;1(4):A09. [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor MJ. Yoga therapeutics: an ancient, dynamic systems theory. Tech Orthop. 2003;18:115–125. [Google Scholar]
- 19.Ferrari ML, Thuraisingam S, Känel RV. Expectations and effects of a single yoga session on pain perception. Int J Yoga. 2015;8(2):154–157. doi: 10.4103/0973-6131.158486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson B. ACSM’s guidelines for exercise testing and prescription 9th Ed. J Can Chiropr Assoc. 2014;58(3):328. [Google Scholar]
- 21.O’Connor FG, editor. ACSM’s sports medicine: a comprehensive review. Lippincott Williams & Wilkins; Philadelphia: 2013. [Google Scholar]
- 22.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee American Rheumatology Classification. Arthritis Rheum. 1986;29(8):1039–1048. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 23.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27(6):1513–1517. [PubMed] [Google Scholar]
- 24.Lethbridge-Çejku M, Scott WW, Reichle R, et al. Association of radiographic features of osteoarthritis of the knee with knee pain: data from the Baltimore Longitudinal Study of Aging. Arthritis Rheum. 1995;8(3):182–188. doi: 10.1002/art.1790080311. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffe E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 26.Resnick B, Ory MG, Hora K, et al. A proposal for a new screening paradigm and tool called Exercise Assessment and Screening for You (EASY) J Aging Phys Act. 2008;16(2):215–233. doi: 10.1123/japa.16.2.215. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 28.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 29.Grace EM, Gerecz EM, Kassam YB, et al. 50-foot walking time: a critical assessment of an outcome measure in clinical therapeutic trials of antirheumatic drugs. Br J Rheumatol. 1988;27(5):372–374. doi: 10.1093/rheumatology/27.5.372. [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Yardley L, Beyer N, Hauer K, et al. Development and initial validation of the Falls Efficacy Scale International (FES-I) Age Ageing. 2005;34(6):614–619. doi: 10.1093/ageing/afi196. [DOI] [PubMed] [Google Scholar]
- 32.Reed PG. Self-transcendence and mental health in oldest-old adults. Nurs Res. 1991;40(1):5–11. [PubMed] [Google Scholar]
- 33.Reed PG. Demystifying self-transcendence for mental health nursing practice and research. Arch Psychiatr Nurs. 2009;23(5):397–400. doi: 10.1016/j.apnu.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Molzahn AE. Spirituality in later life: effect on quality of life. J Gerontol Nurs. 2007;33(1):32–39. doi: 10.3928/00989134-20070101-07. [DOI] [PubMed] [Google Scholar]
- 35.Ware JE, Kosinski M, Keller SD. A 12-item Short Form health survey. Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Cheung C, Wyman JF, Resnick B, Savik K. Yoga for managing knee osteoarthritis in older women: a pilot randomized controlled trial. BMC Complement Altern Med. 2014;14:160. doi: 10.1186/1472-6882-14-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Med Clin N Am. 2009;93(1):83–100. doi: 10.1016/j.mcna.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Vallath N. Perspectives on Yoga inputs in the management of chronic pain. Indian J Palliat Care. 2010;16(1):1–7. doi: 10.4103/0973-1075.63127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nespor K. Pain management and yoga. Int J Psychosom. 1991;38(1–4):76–81. [PubMed] [Google Scholar]
- 40.Morone NE, Greco CM. Mind-body interventions for chronic pain in older adults: a structured review. Pain Med. 2007;8:359–375. doi: 10.1111/j.1526-4637.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 41.Chin A, Paw MJ, van Uffelen JG, et al. The functional effects of physical exercise training in frail older people. Sports Med. 2008;38(9):781–793. doi: 10.2165/00007256-200838090-00006. [DOI] [PubMed] [Google Scholar]
- 42.Hadjistavropoulos T, Delbaere K, Fitzgerald TD. Reconceptualizing the role of fear of falling and balance confidence in fall risk. J Aging Health. 2011;23(1):3–23. doi: 10.1177/0898264310378039. [DOI] [PubMed] [Google Scholar]
- 43.Smith C, Hancock H, Blake-Mortimer J, et al. A randomised comparative trial of yoga and relaxation to reduce stress and anxiety. Complement Ther Med. 2007;15(2):77–83. doi: 10.1016/j.ctim.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Thoits P, Hewitt LN. Volunteer work and well-being. J Health Soc Behav. 2001;1:115–131. [PubMed] [Google Scholar]
- 45.Lin Y, Zhu M, Su Z. The pursuit of balance: an overview of covariate-adaptive randomization techniques in clinical trials. Contemp Clin Trials. 2015;45:21–25. doi: 10.1016/j.cct.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Kelley GA, Kelley KS. Dropouts and compliance in exercise interventions targeting bone mineral density in adults: a meta-analysis of randomized controlled trials. J Osteoporosis. 2013;2013:1–19. doi: 10.1155/2013/250423. [DOI] [PMC free article] [PubMed] [Google Scholar]