Abstract
Purpose
T helper 17 cells (Th17) are one of the main pathogenic effectors in autoimmune uveitis, and IL-17A is the signature cytokine of Th17 cells. This study aims to assess serum IL-17A levels in patients with autoimmune uveitis and evaluate associations between IL-17A levels and disease characteristics.
Methods
Serum IL-17A levels from 87 autoimmune uveitis patients and 60 healthy controls were assessed using an enzyme-linked immunosorbent assay. Among this cohort, 9 patients were followed longitudinally for IL-17A levels during active and inactive stages of their disease.
Results
Median serum IL-17A levels were higher among uveitis patients compared to controls (p<0.0001). Moreover, IL-17A levels were elevated among uveitis patients with active disease compared to those with inactive disease (p = 0.0202). Among the 9 patients followed longitudinally, IL-17A levels were elevated during active disease compared to the inactive stage (p = 0.0078).
Conclusions
Serum IL-17A levels are elevated in uveitis patients, particularly in active uveitis.
Keywords: Cytokine, interleukin 17, serum, Th17, uveitis
Th17 cells are a subset of CD4+ T cells implicated in the pathogenesis of several autoimmune and autoinflammatory diseases.1 Th17 is characterized by the production of IL17A-F, IL-22, and other cytokines.2 IL-17 is the hallmark signature cytokine of Th17 cells. It activates neutrophils and is produced by both adaptive and innate immune cells.3,4 Evidence suggests that IL-17A is one of the most well-defined proinflammatory cytokines, while IL-17F and IL-22 are less defined but also play a role in the pathogenesis of autoimmune diseases.5 IL-17A, the first identified Th17 cytokine, has been shown to play a critical role in the pathogenesis of many autoimmune diseases, including rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, systemic lupus erythematosus, and psoriasis.1,6–10 All of these autoimmune diseases can be associated with uveitis, some more commonly than others.
Recent reports show that IL-17 producing Th17 cells play an important role in uveitis. Increased IL-17A transcript was detected in retinas of mice with experimental autoimmune uveoretinitis (EAU), suggesting that Th17 cells are involved in ocular pathology.11 IL-17A and IL-17F have also been shown to substantially promote ARPE-19 cells to release inflammatory mediators and disrupt the retinal pigment epithelium (RPE) monolayer’s barrier function.12 In humans, IL-17 expression was elevated in peripheral mononuclear cell (PBMC) of uveitis patients.11 IL-17 was also increased in PBMC of patients with Behçet’s disease (BD), Vogt-Koyanagi-Harada (VKH) disease, and aqueous humor of patients with birdshot chorioretinopathy (BCR), all which were associated with active intraocular inflammation.13–16 In this study, we aimed to investigate serum IL-17A cytokine levels in patients with uveitis and examine associations between cytokine levels and disease characteristics in a larger cohort compared to previous studies. We observed that levels were significantly elevated in uveitis patients compared to healthy donors. We further showed that patients with active disease also had increased IL-17A levels as compared to inactive patients. Our results indicate that increased IL-17A levels may be associated with uveitis disease activity.
MATERIAL AND METHODS
This was a case-control study that recruited 87 patients and 60 healthy donors. Sera were obtained from uveitis patients, and healthy subjects. The study complied with the tenets of the Declaration of Helsinki. All patients were seen under an institutional review board (IRB) approved clinical research protocol at the National Institutes of Health (NIH) and provided informed consent.
Sera was centrifuged at 1400 rpm for 15 min after 30 min of clotting and was stored at −80 °C. Sera were tested by ELISA for IL-17A. IL-17A ELISA kits were purchased from R&D Systems (Minneapolis, MN) and performed based on kit protocols.
Medical records of these patients were reviewed for demographic information, anatomical and etiologic classification of uveitis, inflammatory activity based on the Standardization of Uveitis Nomenclature (SUN) working group criteria, and immunomodulatory therapy.17 For the purposes of subgroup analyses, trace cells (+0.5) in the anterior chamber (AC) with no vitreous haze were categorized as “slightly active,” whereas ≥+1 cells in the AC or ≥+0.5 or more vitreous haze was considered “active” disease. Immunomodulatory therapy for uveitis treatment included various systemic agents, such as systemic corticosteroids, methotrexate, mycophenolate mofetil, cyclosporine, azathioprine, and infliximab. Clinical variables included disease activity (active, slightly active, or inactive), anatomical location (anterior uveitis, intermediate uveitis, or posterior or pan uveitis), and systemic treatment (no systemic treatment, one immunomodulatory drug, or ≥2 immunomodulatory drugs). Ocular sarcoidosis was diagnosed in patients with positive biopsy results. However, in cases where a biopsy was unavailable, the diagnosis of presumed ocular sarcoidosis was made based on the presence of granulomatous uveitis and laboratory and radiographic evidence of sarcoidosis.18,19 Behçet’s disease was diagnosed based on the criteria established by the International Study Group for Behçet’s Disease and the Behçet’s Disease Research Committee of Japan.20–22
All data were analyzed using SAS E-Guide Version 4.3 for Windows along with SAS version 9.3 (SAS Institute, Cary, NC) and GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Data are expressed as medians with confidence intervals. Nonparametric methods were used since the IL-17A levels did not follow a parametric distribution. For univariate analyses, a Kruskal-Wallis test was used to compare the levels of IL-17A among clinically relevant variables, such as demographics, anatomical location of uveitis, etiologic diagnoses, activity of uveitis, and immunosuppressive treatment. A repeat analysis was performed using the log of IL-17A levels and excluding 2 outliers (participants 4 and 36) to obtain a normal distribution, which did not change the results. A Wilcoxon matched-pairs test was used to compare the means between visits during inactive and active disease stage within the same patient. A two-tailed p value less than 0.05 was deemed statistically significant.
RESULTS
Patient characteristics are summarized in Table 1. Eighty-seven uveitis patients (52 females and 35 males) with an average age of 41.5 years (SD: 16.7, median: 42, range: 7–75 years) and 60 healthy individuals (40 females and 20 males) with an average age of 47.6 years (SD: 13.4, median: 49, range: 25–72 years) were included in our study. The most common diagnosis among uveitis patients was sarcoidosis followed by idiopathic uveitis, Behçet’s disease (BD), birdshot chorioretinopathy (BCR), and VKH. Of the 87 uveitis patients, 17 had anterior uveitis, 18 had intermediate uveitis, and 52 had posterior or pan uveitis. At the time of sample collection, 68 patients were inactive (78.2%), 19 were active, and 55 patients were on immunosuppressive treatment (63.2%).
TABLE 1.
Demographic and disease characteristics of study subjects.
| Uveitis patients (N = 87) | Healthy controls (N = 60) | |
|---|---|---|
| Mean age (Standard Deviation) | 41.5 (16.7) | 47.6 (13.4) |
| Gender (F/M) | 52:35 (1.49) | 40:20 (2.00) |
| Race/Ethnicity | 50.6% Caucasian | 53.3% Caucasian |
| 37.9% AA | 36.7% AA | |
| 11.5% Other | 10% Other | |
| Anatomical location of Uveitis | 17 Anterior Uveitis | N/A |
| 18 Intermediate Uveitis | ||
| 52 Posterior or Pan Uveitis | ||
| Eliologic Diagnoses | Sarcoidosis (n = 29) | N/A |
| Behcet’s Disease (n = 17) | ||
| Idiopathic (n = 17) | ||
| VKH (n = 5) | ||
| BCR (n = 5) | ||
| Other (n = 14) | ||
| Immunosuppressive therapy | 63.2% (n = 55) | N/A |
| Active uveitis | 21.8%, (n = 19) | N/A |
| Median serum IL-7A levels | 27.26 pg/ml (95% CI: 22.07–35.05) | 9.17 pg/ml (95% CI: 7.51–10.84) |
Serum IL-17A levels in uveitis patients (27.26 pg/mL; 95% CI: 22.07–35.05) were statistically significantly higher compared to controls (9.17 pg/mL; 95% CI: 7.51–10.84) (p<0.0001) (Figure 1A). In addition, uveitis patients with active disease (36.35 pg/mL; 95% CI: 27.26–51.93) had statistically significantly increased IL-17A levels compared to patients with inactive disease (24.67 pg/mL; 95% CI: 16.88–31.16) (p = 0.0202). Both inactive and active patients had higher levels of IL-17A compared to healthy individuals (p<0.0001) (Figure 1B). Slightly active patients (+0.5 cells in the AC only) also had lower levels of IL-17A (18.18 pg/mL; 95% CI: 6.49–45.44) compared to active patients (+1 or more cells in the AC or any vitreous haze) (36.35 pg/mL; 95% CI: 27.26–51.93) (p = 0.0420) (Figure 1C).
FIGURE 1.
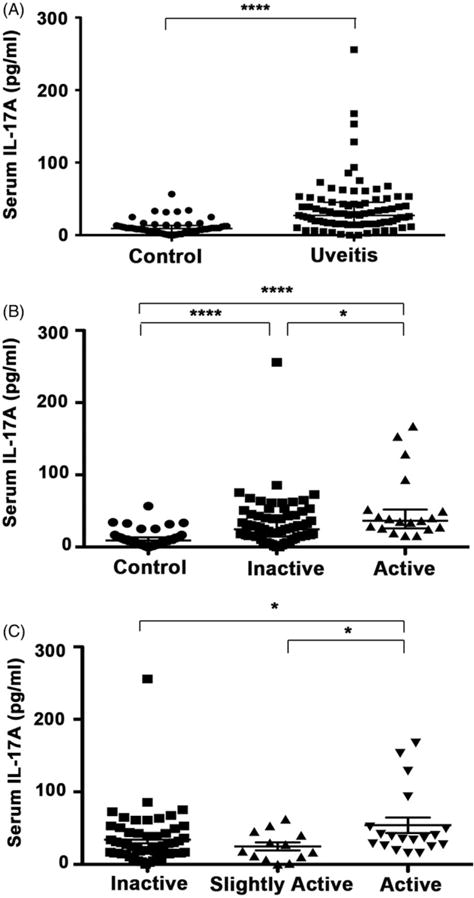
Serum IL-17A levels. (A) Median IL-17A levels were significantly increased among uveitis patients (27.26 pg/mL; 95% CI: 22.07–35.05) compared to controls (9.17 pg/mL; 95% CI: 7.51–10.84) (p<0.0001). (B) Median IL-17A was higher in patients with active disease (36.35 pg/mL; 95% CI: 27.26–51.93) compared to patients with inactive disease (24.27 pg/mL; 95% CI: 16.88–31.16) (p = 0.0202). Both inactive and active patients had higher levels of IL-17A compared to controls (p<0.0001). (C) When distinguishing between patients with minimal to no disease activity, both inactive (24.67 pg/mL; 95% CI: 18.18–33.76) and slightly active patients (18.18 pg/mL; 95% CI: 6.49–45.44) had decreased IL-17A levels compared to highly active patients (36.35 pg/mL; 95% CI: 27.26–51.93) (p = 0.0323, p = 0.0420). Kruskal-Wallis tests were used to compare groups. * and **** indicate the statistically significant p value <0.05 and 0.0001, respectively.
Uveitis patients off of immunosuppressive therapy (35.05 pg/mL; 95% CI: 25.97–53.23) had slightly higher IL-17A levels compared to those patients receiving therapy (24.67 pg/mL; 95% CI: 18.18–31.16); however, this difference was not statistically significant (p = 0.1357). While we observed no statistically significant difference between treated and untreated patients, inactive patients on treatment (18.82 pg/mL; 95% CI: 15.58–29.86) had significantly lower levels of IL-17A compared to active patients off treatment (40.89 pg/mL; 95% CI: 25.97–167.48) (p = 0.0106).
With respect to anatomical locations of uveitis, there was no difference between patients with anterior (anterior uveitis) and posterior segment involvement (intermediate, posterior, or pan uveitis) (p = 0.7603). With regards to etiology of uveitis, we did not observe any significant associations; additionally, the numbers in each group were too small to make any valid comparisons. Because univariate analysis revealed “activity” as the only significant factor, we did not attempt a multivariate analysis.
Nine patients followed longitudinally for serum IL-17 levels showed a significant difference between inactive (15.58 pg/mL; 95 CI: 9.09–36.35) and active (27.26 pg/mL; 95 CI: 16.88–41.54) stages of their disease (p = 0.0078) (Figure 2).
FIGURE 2.
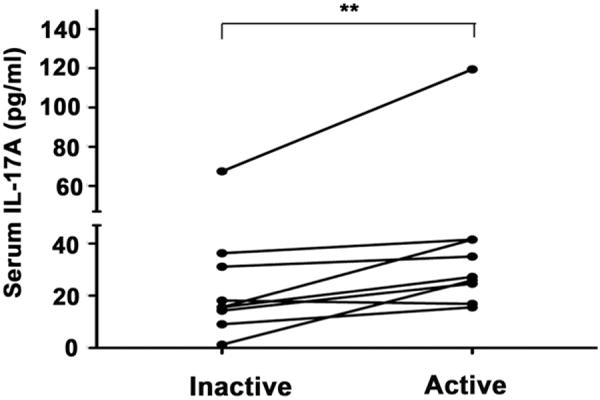
Median serum IL-17A levels were significantly higher during the active phase (27.26 pg/mL; 95 CI: 16.88–41.54) compared to the inactive phase (15.58 pg/mL; 95 CI: 9.09–36.35) of the disease among the 9 patients followed longitudinally (**p = 0.0078). A Wilcoxon matched-pairs test was used to compare the means between visits.
DISCUSSION
In this study, we examined IL-17A levels and their possible role in the pathogenesis of uveitis. The results showed that serum IL-17A levels were significantly elevated in uveitis patients compared to healthy donors as well as in patients with active uveitis compared to those with inactive disease. The role of IL-17A has been reported in several autoimmune diseases and uveitis subtypes but, despite mounting evidence in animal models, IL-17 serum levels were not assessed in a large cohort of patients with various forms of uveitis. Consistent with our results, several studies reported elevated levels of IL-17A in small cohorts of isolated uveitic syndromes as well as in uveitis associated with systemic autoimmune diseases, such as Behçet’s disease and Vogt-Koyanagi-Harada syndrome.14,15 Increased IL-17 expression has been reported both in peripheral blood and at the tissue level in granulomatous and nongranulomatous uveitis.23 Birdshot chorioretinopathy, a disease considered to be limited to the eye, is found to be associated with elevated intraocular levels of IL-17A during the active stage of the disease. Interestingly, serum levels of IL-17 were hardly detectable in their cohort.16 In our cohort of uveitis patients, with and without associated systemic autoimmune disease, serum IL-17 levels were significantly elevated compared to controls.
Our results also indicate that serum IL-17 may be an indicator of active disease in uveitis patients. The only clinical factor that was significantly associated with IL-17 levels was indeed the disease activity. IL-17 level has been associated with disease activity in systemic Behçet’s disease as well as various arthritis syndromes.24–27 While most of these studies in rheumatologic disorders have shown elevated IL-17 levels in the patients compared to controls, association with disease activity has not been as consistent.9,28 Animal studies also showed a positive correlation between Th17 cells and the severity of uveitis in experimental models of uveitis; however, no such associations with disease activity have been demonstrated in human uveitis.11
Treatment was not independently associated with IL-17A levels. Although patients receiving systemic immunomodulatory treatment tended to have lower levels of IL-17A compared to those not receiving any systemic immunomodulatory treatment (24.67 vs. 35.05 pg/mL), this difference was not significant and is likely attributable to disease quiescence induced by therapy. Since treatment and quiescence are likely to be correlated factors, it is difficult to distinguish the impact from each of these factors. However, because of limited number of patients not receiving treatment, we could not draw any conclusions regarding treatment effect. A previous study reported higher levels of IL-17 in patients with active BD uveitis not receiving treatment compared to inactive patients on infliximab, an anti-TNF alpha treatment that inhibits Th17 differentiation.29 Similarly, cyclosporine also inhibits intraocular inflammation and IL-17 production in Behçet’s disease patients.30 Therefore, it is possible that treatment itself may be directly associated with lower IL-17 levels, although it is difficult to separate this effect from the effect of quiescence induced by immunomodulatory treatment.
Interestingly, phase II and III trials using secukinumab, a fully human monoclonal antibody for targeted interleukin-17A blockade, has shown conflicting results in uveitis patients. While phase II showed favorable results, phase III results did not meet the primary outcome. Given the challenges of clinical trials in uveitis, these negative data do not refute a significant role of IL-17 in uveitis. It is important to point out the small sample size in two trials that enrolled active and inactive noninfectious uveitis without Behçet’s disease due to early termination (INSURE and ENDURE). Furthermore, the lack of significant difference between active and control groups may be due to the therapeutic effect of a high amount of concomitant immunosuppressive medication in all treatment groups in the secukinumab trial for Behçet’s disease-associated uveitis (SHIELD). Even though the primary outcome was not met, in the SHIELD trial, secukinumab was associated with a significant reduction in immunosuppressive medication and a trend for lower rate of recurrences. The authors do mention that further research is needed to determine the appropriate dosing and to identify a subset of patients who may benefit from secukinumab. Interestingly, phase II trials used intravenous administration while all phase III trials used subcutaneous administration of the monoclonal antibody.31,32
In humans, uveitic entities where IL-17 is proposed to play a role have been mostly posterior or pan uveitis syndromes, such as Behçet’s disease, VKH, BCR, and sympathetic ophthalmia.14–16,23 Therefore, we evaluated the relation between anatomical location and IL-17 levels; however, we found no significant association. This could be due to small numbers in each of these categories. However, higher levels of IL-17A have also been reported in ocular surface disease where levels correlated corneal disease severity, suggesting a broader role for IL-17 in the ocular environment.33
Our study is limited by a one-time assessment of an inflammatory cytokine as well as small numbers in different etiologic diagnosis categories and various treatments. However, this is one of the largest case-control studies focusing on serum IL-17A levels among uveitis patients that demonstrates elevated serum IL-17A levels in human uveitis and indicates a correlation with disease activity. Additionally, our results are supported by the consistent findings in the subgroup of patients followed longitudinally throughout active and inactive stages of their disease.
In summary, our results suggest that IL-17 may serve as a useful biomarker for disease activity and highlight the role of IL-17A in uveitis. Long-term longitudinal studies are needed to confirm whether IL-17 and other Th17 cytokines can reliably be used as biomarkers of disease activity or prognosis, which can have significant impact on clinical decision making. Our study may provide information that promotes understanding of the prognosis, activity of the disease, and pathogenesis.
Acknowledgments
We would like to thank the Laboratory of Immunology and National Eye Institute Intramural Research Program for their support.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Frontiers Immunol. 2012;3:129. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bending D, Newland S, Krejci A, et al. Epigenetic changes at Il12rb2 and Tbx21 in relation to plasticity behavior of Th17 cells. J Immunol. 2011;186:3373–3382. doi: 10.4049/jimmunol.1003216. [DOI] [PubMed] [Google Scholar]
- 3.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res. 2008;42:41–50. doi: 10.1007/s12026-008-8031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luger D, Caspi RR. New perspectives on effector mechanisms in uveitis. Semin Immunopathol. 2008;30:135–143. doi: 10.1007/s00281-008-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Chen X, Liu Z, et al. Expression of Th17 cytokines in skin lesions of patients with psoriasis. J Huazhong U Sci-Med. 2007;27:330–332. doi: 10.1007/s11596-007-0329-1. [DOI] [PubMed] [Google Scholar]
- 7.Cascao R, Moura RA, Perpetuo I, et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Therapy. 2010;12:R196. doi: 10.1186/ar3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CK, Lit LC, Tam LS, et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Mei Y, Pan F, Gao J, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–273. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 10.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PloS One. 2011;6:e18139. doi: 10.1371/journal.pone.0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamzaoui K, Bouali E, Ghorbel I, et al. Expression of Th-17 and RORgammat mRNA in Behçet’s disease. Med Sci Monitor. 2011;17:CR227–CR234. doi: 10.12659/MSM.881720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi W, Zhu X, Yang P, et al. Upregulated IL-23 and IL-17 in Behçet patients with active uveitis. Invest Ophthalmol Visual Sci. 2008;49:3058–3064. doi: 10.1167/iovs.07-1390. [DOI] [PubMed] [Google Scholar]
- 15.Chi W, Yang P, Li B, et al. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol. 2007;119:1218–1224. doi: 10.1016/j.jaci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper JJ, Mutis T, de Jager W, et al. Intraocular interleukin-17 and proinflammatory cytokines in HLA-A29-associated birdshot chorioretinopathy. Am J Ophthalmol. 2011;152:177–182 e171. doi: 10.1016/j.ajo.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data: results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbort CP, Rao NA, Mochizuki M, members of Scientific Committee of First International Workshop on Ocular S. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17:160–169. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 19.Thompson IA, Liu B, Sen HN, et al. Association of complement factor h tyrosine 402 histidine genotype with posterior involvement in sarcoid-related uveitis. Am J Ophthalmol. 2013;155:1068–1074 e1061. doi: 10.1016/j.ajo.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 21.The International Study Group for Behçet’s Disease. Evaluation of diagnostic (‘classification’) criteria in Behçet’s disease—towards internationally agreed criteria. Br J Rheumatol. 1992;31:299–308. [PubMed] [Google Scholar]
- 22.Behçet’s Disease Research Committee. Clinical research section recommendations. Jpn J Ophthalmol. 1974;18:291–294. [Google Scholar]
- 23.Furusato E, Shen D, Cao X, et al. Inflammatory cytokine and chemokine expression in sympathetic ophthalmia: a pilot study. Histol Histopathol. 2011;26:1145–1151. doi: 10.14670/hh-26.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wendling D, Toussirot E. Bone and matrix remodeling markers: a new tool for assessment of treatment efficacy in ankylosing spondylitis? J Rheumatol. 2007;34:1647–1649. [PubMed] [Google Scholar]
- 25.Chen WS, Chang YS, Lin KC, et al. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J Chin Med Assoc. 2012;75:303–308. doi: 10.1016/j.jcma.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Metawi SA, Abbas D, Kamal MM, Ibrahim MK. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clin Rheumatol. 2011;30:1201–1207. doi: 10.1007/s10067-011-1737-y. [DOI] [PubMed] [Google Scholar]
- 27.Hamzaoui K, Hamzaoui A, Guemira F, et al. Cytokine profile in Behçet’s disease patients: relationship with disease activity. Scand J Rheumatol. 2002;31:205–210. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 28.Londono J, Romero-Sanchez MC, Torres VG, et al. The association between serum levels of potential biomarkers with the presence of factors related to the clinical activity and poor prognosis in spondyloarthritis. Rev Bras Reumatol. 2012;52:536–544. [PubMed] [Google Scholar]
- 29.Sugita S, Horie S, Yamada Y, et al. Suppression of interleukin-17-producing T-helper 17 cells by retinal pigment epithelial cells. Jpn J Ophthalmol. 2011;55:565–575. doi: 10.1007/s10384-011-0064-9. [DOI] [PubMed] [Google Scholar]
- 30.Chi W, Yang P, Zhu X, et al. Production of interleukin-17 in Behçet’s disease is inhibited by cyclosporin A. Mol Vision. 2010;16:880–886. [PMC free article] [PubMed] [Google Scholar]
- 31.Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 32.Dick AD, Tugal-Tutkun I, Foster S, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120:777–787. doi: 10.1016/j.ophtha.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 33.Kang MH, Kim MK, Lee HJ, et al. Interleukin-17 in various ocular surface inflammatory diseases. J Korean Med Sci. 2011;26:938–944. doi: 10.3346/jkms.2011.26.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]


