Abstract
Purpose of review
Pediatric uveitis is relatively uncommon, accounting for only 5–10% of all patients with uveitis. However, owing to high prevalence of complications and devastating outcomes, its lifetime burden can be significant.
Recent findings
Immunomodulatory therapy has been associated with better outcomes in noninfectious pediatric uveitis. However, effective treatments are limited by medication-related complications, including multiorgan toxicities and systemic side effects.
Summary
We review the current therapies available to treat pediatric uveitis, discuss novel and future therapies, and provide clinical recommendations utilizing these new agents. The consideration for treatment regimens in noninfectious pediatric uveitis is multifactorial. Understanding past, present, and future technology will aid in treatment of a complex and refractory disease.
Keywords: biologic agents, immunosuppressive therapy, juvenile idiopathic arthritis, pediatric uveitis, uveitis
INTRODUCTION
Pediatric uveitis is relatively uncommon, accounting for only 5–10% of all patients with uveitis. Owing to the extremely high prevalence of complications and disease burden over a lifetime, it presents a specific challenge. Historically, effective treatments were limited by complications of their own, including multiorgan toxicities and systemic side-effects. Better treatment options may dramatically improve visual prognosis, which worsens with delayed diagnosis and disease duration. Among pediatric uveitis cases, 25–33% results in severe vision loss [1]. Most patients are White females with median diagnosis at 8.6–9.4 years of age. The most common etiologic diagnosis is juvenile idiopathic arthritis (JIA) followed by pars planitis. Most individuals have an insidious onset and persistent duration, with anterior uveitis as the most common anatomical location. Structural complications such as cystoid macular edema (CME) and hypotony are common and 10–30% can be classified as legally blind at presentation [2,3]. Evidence suggests that early and more aggressive therapy improves ocular outcomes [4,5]. Immunomodulatory therapy has been associated with reduced risk of complication, and control of inflammation within 3 years has been associated with better visual outcomes. Several studies have indicated that strict control of inflammation and the use of immunosuppressive drugs significantly reduce the risk of vision loss [6,7▪].
There are relatively few medications with specific Food and Drug Administration (FDA) indications for pediatric uveitis. Methotrexate (MTX; Rheumatrex; Dava Pharmaceuticals, Fort Lee, New Jersey, USA, Trexall; Teva Pharmaceuticals, Philadelphia, USA) has been approved for use in children with JIA. Adalimumab (Humira; Abbott Laboratories, Abbott Park, Illinois, USA) is approved for JIA in patients over age 4. Safety and efficacy of many newer therapies are not well established in children. Oral and topical corticosteroids are approved for use in children, however the safety of fluocinolone acetonide intravitreal implant (Retisert; Bausch and Lomb, New York, USA) has not been established in patients less than 12 years [8]. The dexamethasone (Ozurdex; Allerga, Irvine, California, USA) implant also lacks safety information for children [9]. The current approach includes corticosteroids to treat acute inflammation with rapid instatement of corticosteroid-sparing agents (antimetabolites, T-cell inhibitors, alkylating agents, biologics). This review provides an understanding of current and future treatment strategies, and discusses newer generation biologics and corticosteroid implant options in children (Table 1) [10].
Table 1.
Mechanisms and side-effects of selected treatments for pediatric uveitis
| Category | Drug (trade name) | Mechanism of action | Side-effects |
|---|---|---|---|
| Glucocorticoid | Deltasone (Prednisone) | Multiple mechanisms | Cataracts, weight gain, stunted growth (children), acne, osteoporosis |
| Antimetabolites | Methotrexate (Trexall) | Inhibits dihydrofolate reductase | Bone marrow suppression, stomatitis, hair loss, hepatotoxicity |
| Azathioprine (Imuran) | A purine nucleoside analogue that interferes with DNA and RNA synthesis | Bone marrow suppression, gastrointestinal symptoms | |
| Mycophenolate mofetil (Cellcept) | Inhibits inosine monophosphate dehydrogenase | Diarrhea, nausea, neutropenia, infection | |
| T-cell inhibitors | Cyclosporine (Neoral, Sandimmune, Gengraf) | Calcineurin inhibitor | Nephrotoxicity, tremor, hirsutism, gum hyperplasia, hypertension |
| a Voclosporin | Hypertension, decreased renal function, pyrexia, arthralgia [10] | ||
| Tacrolimus (Prograf) | Nephrotoxicity, hypertension, neurotoxicity, hepatitis, diabetes | ||
| a Sirolimus (Rapamune) | Inhibits mTOR pathway | Nephrotoxicity, hypersensitivity reaction, peripheral edema, hypertriglyceridemia, hypertension, hypercholesterolemia, thrombocytopenia | |
| Alkylating agents | Chlorambucil (Leukeran) | DNA crosslinking | Bone marrow suppression, malignancy, sterility, infection |
| Cyclophosphamide (Cytoxan) | Bone marrow suppression, hemorrhagic cystitis, malignancy, sterility, alopecia, infection | ||
| Biologics | Infliximab (Remicade) | TNF inhibitor | Infection, tuberculosis, lymphoma, autoantibodies, demyelinating disease, worsening heart failure; local skin reaction at injection site (golimumab, adalimumab) |
| a Adalimumab (Humira) | |||
| Golimumab (Simponi) | |||
| Certolizumab pegol (CIMZIA) | |||
| Etanercept (Enbrel) | |||
| Anakinra (Kineret) | Anti-IL1R | Injection site reaction, infection | |
| Canakinumab (Ilaris) | Anti-IL1β Ab | Injection site reaction, infection | |
| Daclizumab (Zenapax) | Anti-IL2R Ab | Infection, lymphoproliferative disorder, severe hypersensitivity reactions; Note: No longer commercially available | |
| a Tocilizumab (Actemra) | Anti-IL6R Ab | Infection, hypercholesterolemia, infusion-related reaction | |
| Rituximab (Rituxan) | CD20R Ab | Infection, infusion-related reaction, neutropenia | |
| Abatacept (Orencia) | T-cell costimulation inhibitor | Infection, malignancy | |
| Intravitreal corticosteroid implants | Dexamethasone (Ozurdex) | Multiple mechanisms | Significant IOP increase, cataract formation (less than fluocinolone acetate) |
| Fluocinolone acetonide (Retisert) | Multiple mechanisms | Significant IOP increase and cataract formation |
Ab, antibody; mTOR, mammalian target of rapamycin; R, receptor.
Potential compounds for the treatment of uveitis (clinicaltrials.gov).
CAUSE
Noninfectious anterior uveitis is most prevalent among North American populations. Most pediatric uveitis cases are idiopathic. JIA is the most common systemic association of pediatric uveitis. JIA patients typically have chronic, bilateral, nongranulomatous, anterior uveitis with insidious onset. In North America, there is an estimated incidence of 4.9–6.9 per 100 000 person-years and prevalence of 13–30 per 100 000 population [7▪]. Oligoarticular onset JIA is the type most likely associated with uveitis. JIA patients have a high risk of developing band keratopathy, cataract, and posterior synechiae [1]. Risk factors for vision loss include presence of uveitis before arthritis, complications at initial examination, short duration between arthritis onset and uveitis, young age, multiple episodes, and male sex [11].
Treatment varies with severity and response to therapy, and should be tailored to cause and anatomic location. Infectious agents such as Toxoplasma, Toxocara, Bartonella, or those causing Lyme disease syphilis, or tuberculosis, and viruses can be common causes of pediatric uveitis [12], especially in developing countries, and should be ruled out. In developed countries, infectious uveitis constitutes 11–13% of all pediatric uveitides [13]. A growing body of evidence supports infection as the causative or triggering event in presumed idiopathic uveitis [14]. PCR analysis of aqueous and vitreous specimens can be helpful in such cases [15]. In noninfectious uveitis, the ultimate goal is to start immunosuppressives early and taper off therapy after a sufficient period of quiescence. The ‘sufficient’ period is currently controversial, but there is consensus that at least 2–3 years of complete quiescence is needed before discontinuing immunomodulatory therapy.
CURRENT TREATMENTS
Current standard medical therapy for pediatric uveitis combines an older generation of medications that have been in use for decades, such as corticosteroids, with both old and new generation immunomodulatory agents. Corticosteroids are the mainstay therapy for noninfectious uveitis, but prolonged use can have significant side effects. Topical corticosteroids are effective for early control of uveitis, but a long-term corticosteroid-sparing immunomodulatory therapy plan should be discussed at the time of diagnosis, particularly for patients with ocular complications or who are at risk for new complications [16]. The most commonly used topical corticosteroid is prednisolone acetate 1%, however rimexolone 1% may be less likely to cause glaucoma [17]. Difluprednate Ophthalmic Emulsion 0.05%, a new and more potent topical corticosteroid, allows less frequent dosing but is more likely to cause corticosteroid-induced ocular hypertension. In a cohort of 14 pediatric uveitis cases (26 eyes), 50% of eyes developed a significant intraocular pressure increase [18].
Since the 1970s, peribulbar and intravitreal corticosteroids, most commonly triamcinolone acetonide, have been used to treat uveitis [19,20]. This modality is more effective in treating intermediate and posterior uveitis and has less systemic effects, but greater risk of cataract and glaucoma. Prolonged use of topical corticosteroids and repeated periocular injections further increases the risk of glaucoma and cataract in children [16]. Chronic topical corticosteroid use more frequently than three times a day is associated with increased risk of cataracts as well [21]. If uveitis requires extended or frequent corticosteroid drops, it is favorable to initiate systemic immunomodulatory therapy.
Long-term systemic corticosteroids are associated with adrenal suppression, causing growth retardation due to premature epiphyseal closure [22]. Other side-effects include weight gain, infection, osteoporosis, and hyperglycemia. Most pediatric uveitis patients requiring frequent corticosteroid drops will ultimately need immunosuppressive treatment. Systemic corticosteroids can be used as a short-term bridge to immunosuppressive therapy in patients not controlled with topical therapy.
The efficacy of NSAIDs has not been studied in depth for their specific role in treating uveitis. They are not considered a significant part of treatment regimen for pediatric uveitis.
IMMUNOMODULATORY AGENTS
Growing evidence supports earlier and more aggressive immunomodulatory therapy in pediatric uveitis. Studies indicate that systemic treatment with both conventional immunosuppressives and newer biological agents results in better outcomes.
Antimetabolites
MTX is commonly used as a first-line immunomodulatory agent in pediatric uveitis because of its long track record of both safety and efficacy. MTX is a folic acid analogue that inhibits dihydrofolate reductase and de-novo synthesis of purines. Folic acid supplementation prevents side effects [20,23]. Early aggressive treatment of JIA with MTX has significantly improved outcomes in pediatric uveitis, with about 60–80% of children showing a favorable response [24]. Long-term MTX use has been associated with a lower risk of relapse after its discontinuation [16].
Second-line immunosuppressives include azathioprine (AZA; Imuran; GlaxoSmithKline, Research Triangle Park, North Carolina, USA), mycophenolate mofetil (MMF; Cellcept; Genentech, South San Francisco, California, USA), and cyclosporine. AZA is a purine synthesis inhibitor interfering with DNA replication and RNA transcription. There are few studies regarding utility of AZA in pediatric uveitis. In JIA-associated active uveitis, AZA monotherapy was successful in controlling inflammation in 76% of cases, and in 56% when used in combination therapy. Its corticosteroid-sparing effect was moderate-to-poor in most cases, limiting its use in pediatric uveitis treatment [25].
MMF inhibits inosine monophosphate dehydrogenase, a pathway of guanosine nucleoside synthesis, used by B and T cells [20]. MMF is generally used for non-JIA uveitis, either as a first-line corticosteroid-sparing agent or as a second-line agent in MTX failures [26].
T-cell inhibitors
Cyclosporine A (CsA; Sandimmune; Novartis, East Hanover, New Jersey, USA, Gengraf; Abbott Laboratories, Neoral; Novartis), a calcineurin inhibitor, inhibits T-cell activation by blocking transcription of genes for cytokines such as interleukin-2 (IL-2). A powerful corticosteroid-sparing agent has been shown to be both effective and relatively well tolerated in low doses in treating refractory posterior uveitis in adults and children [27]. Cyclosporine is particularly effective in Behcet’s disease-associated uveitis. There are few data regarding the efficacy of CsA therapy in childhood uveitis. A recent study of 82 children with JIA uveitis showed that CsA was minimally efficacious as monotherapy, but had a 50% success rate when used in combination with MTX [28].
Nephropathy is of concern in adult patients, but less so in children, possibly because of greater pediatric CsA clearance [29]. A retrospective case series in children with uveitis showed the efficacy of CsA in combination therapy but, more importantly, showed minimal adverse effects when used in low doses (<5.0 mg/kg per day). To minimize dose-dependent CsA-induced nephropathy we, and many others, maintain a CsA dose of 5 mg/kg per day or less [29].
Tacrolimus (FK506; Prograf Astellas Pharma, Northbrook, Illinois, USA) is a newer calcineurin inhibitor with a similar mechanism to CsA. It is not as widely used as CsA for treatment of ocular inflammatory disease despite a possibly better side-effect profile.
Alkylating agents
Chlorambucil (Leukeran; GlaxoSmithKline) and cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, New Jersey, USA) cross-link DNA strands and decrease DNA synthesis, preventing cell division. Alkylating agents are typically avoided in children because of serious potential long-term side effects, except in cases of life-threatening systemic autoimmune diseases such as lupus, Behcet’s disease, or other serious vasculitides [16]. The serious long-term side effects include leukopenia, gonadal atrophy, and malignancy [20].
NEWER TREATMENTS
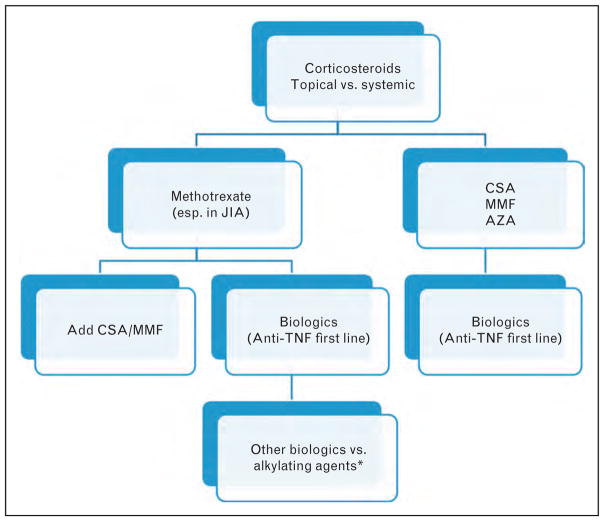
Biologic drugs refer to drugs made from human or animal proteins derived from genes. Many are designed to inhibit specific components of the immune system that play key roles in inducing inflammation. Biologics have made an enormous impact in the treatment of pediatric uveitis in the last decade (Fig. 1) [30▪].
FIGURE 1.
Treatment algorithm for noninfectious pediatric uveitis. Corticosteroid (CS) implants (i.e., Ozurdex and Retisert) are not regularly used in children because of risks of cataract and glaucoma and their implications in children [30▪]. *Alkylating agents are not commonly used due to serious long-term adverse effects, especially in children. Source: Original.
TUMOR NECROSIS FACTOR INHIBITORS
Tumor necrosis factor (TNF) is a cytokine that plays a role in inducing inflammation in autoimmune processes, and has been found at increased levels in aqueous humor and serum of patients with uveitis. Anti-TNF-α agents have successfully been used to treat various forms of uveitis both in adults and children in the past decade [31]. Often these medications are selected when patients fail to respond to conventional immunosuppressive therapy and are at a high risk for visual loss. Anti-TNF drugs used in uveitis patients include infliximab (Remicade; Janssen-Horsham Biotech Inc., Malvern, Pennsylvania, USA), adalimumab, golimumab (Simponi; Janssen Biotech Inc.), and certolizumab (CIMZIA; UCB Inc., Atlanta, Georgia, USA). A fifth anti-TNF drug, etanercept (Enbrel Immunex Corporation, Thousand Oaks, California, USA), is no longer used in uveitis patients because of concerns of exacerbation or new-onset ocular inflammation when used to treat rheumatologic disorders [16]. It is important to rule out tuberculosis prior to treatment with TNF inhibitors.
Infliximab and adalimumab
Infliximab was the first commercially available drug targeting TNF-α for treatment of adult rheumatoid arthritis (RA); however, it is not FDA-approved for the treatment of JIA [32▪▪,33]. Infliximab is 70–75% humanized chimeric mouse-human antibody. It is approved for use in various inflammatory diseases in adults, but the only pediatric indication is Crohn’s disease in children over age 6. The recommended dose is three initial infliximab infusions at 0, 2, and 6 weeks in a dosage of 5 mg/kg; followed by maintenance therapy at a dose of 5 mg/kg every 8 weeks [33] (Fig. 2).
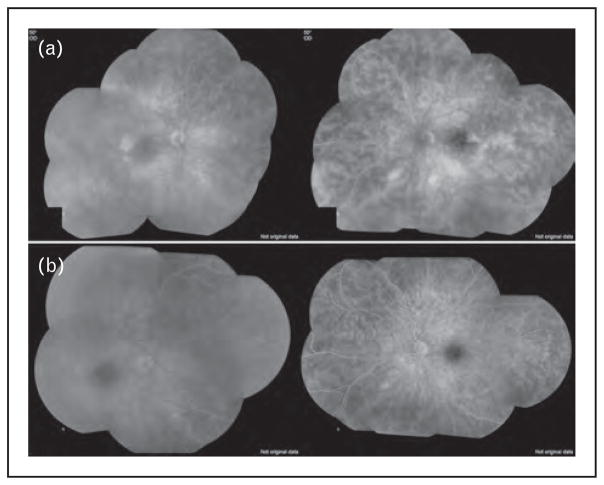
FIGURE 2.
A 16-year-old White girl with idiopathic noninfectious intermediate uveitis. She failed treatment with mycophenolate mofetil (a) but responded favorably when infliximab treatment was added (b) at 5 mg/kg dose. Note the improvement in retinal vascular leakage within 3 months of therapy. Source: Original.
Adalimumab is a fully human monoclonal antibody against TNF-alpha and the third TNF-blocker approved in the USA [33–36]. It is FDA-approved for the treatment of RA, psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, inflammatory bowel disease in adults, and polyarticular JIA in patients over age 4 and over 15 kg [34]. It is used either as monotherapy or in combination with MTX [32▪▪]. The recommended dose is 20 mg every other week for children weighing 15–30 kg; 40 mg every other week for children weighing more than 30 kg [34].
Neither infliximab nor adalimumab is approved specifically for the treatment of uveitis, but studies indicate that both can be effective. A recent prospective study reported improvement in 88.2% of pediatric patients with uveitis treated with infliximab or adalimumab, wherein half achieved remission. After 1-year follow-up of 108 patients with JIA-associated uveitis, the remission rate for adalimumab was 67.4 versus 42.8% with infliximab in a prospective registry [37▪▪]. Adalimumab has been valuable for chronic recurrent anterior uveitis [32▪▪]. Another recent prospective study of 38 pediatric uveitic eyes showed adalimumab to be good adjuvant therapy. Improvement was seen in about 76% of cases, stabilization in 16%, and relapse in 8% [38▪▪]. A large prospective study of 131 adult patients with refractory uveitis showed similar results with 85% of patients able to reduce at least 50% of their baseline immunosuppressives [39▪▪]. Data are equivocal regarding whether adalimumab or infliximab therapy is superior in uveitis treatment. Some studies suggest a slightly better remission rate with adalimumab [23]; others suggest that there is no difference between the two [40]. Adalimumab has the convenience of subcutaneous administration, more stable serum concentrations, and better safety profile. Infliximab has a faster onset for immediate ocular inflammatory control; however, tolerability is limited due to infusion reactions and higher risk of infection [16]. Infusion reactions are probably related to the development of human antichimeric antibodies and can be minimized with concomitant use of MTX [32▪▪].
Other tumor necrosis factor inhibitors
Certolizumab pegol (CIMZIA) and golimumab are the two newest TNF antagonists. Certolizumab pegol is a PEGylated Fab fragment of a humanized anti-TNF antibody, without an Fc-region, potentially decreasing the risk for infectious diseases [32▪▪]. Certolizumab is FDA-approved for the treatment of RA and Crohn’s disease in adults at a dose of 400 mg given subcutaneously every 2 weeks for three loading doses. For maintenance, 400 mg monthly or 200 mg every 2 weeks is used. Golimumab is approved for treatment of RA, psoriatic arthritis, and ankylosing spondylitis in adults [32▪▪,41] at a dose of 30 mg/m2 every 4 weeks, with maximum dose of 50 mg subcutaneously once monthly. There is a clinical trial in progress looking at higher dosages (100 mg per dose) of golimumab in adults with RA [Clinicaltrials.gov, NCT00299546]. Current use of certolizumab and golimumab in children is off-label, but studies suggest efficacy in select refractory cases with JIA or Behcet’s disease [41–43]. A multicenter randomized-withdrawal trial to evaluate the efficacy and safety of golimumab in pediatric patients with active polyarticular JIA and poor response to MTX is expected to be completed in 2016 [Clinicaltrials.gov, NCT01230827].
OTHER BIOLOGIC THERAPIES
Alternate biologic agents, such as anakinra (Kineret; Amgen, Thousand Oaks, California, USA), canakinumab (Ilaris; Novartis), daclizumab (Zenapax, Hoffman La Roche, USA), abatacept (Orencia; Bristol-Myers Squibb), rituximab (Rituxan; Genentech), and tocilizumab (Actemra; Genentech), are used in patients refractory to anti-TNF agents. There are limited data on the efficacy and safety of these agents, particularly in children. Risk of opportunistic infections and malignancies and other serious potential side effects should be carefully considered before use. Concomitant use of multiple biologics can dramatically increase risk and is generally contraindicated.
Daclizumab
Daclizumab is a humanized monoclonal antibody against IL-2Rα receptor (CD25) and inhibits IL-2-mediated pro-inflammatory responses [44]. Daclizumab has been safely and effectively used in intermediate and posterior uveitis in adults and children as well as active JIA-associated anterior uveitis [45]. Its side-effect profile has been mild, but it was pulled from the market because of diminishing demand.
Interleukin-1 inhibitors
Anakinra is a recombinant IL-1 receptor antagonist (IL-1Ra) that blocks the biologic activity of IL-1 by competitively inhibiting IL-1 binding to the receptor. It is approved for the treatment of RA in adults. It has also been used in IL-1-mediated autoinflammatory diseases [46]. Despite evidence from animal models of uveitis, there is no clear evidence of its benefit in human uveitis. Canakinumab is a human anti-IL-1β monoclonal antibody, which neutralizes the activity of IL-1β. IL-1β plays a dominant role in the pathophysiology of several autoinflammatory conditions, including JIA [47]. The recommended dose is 150 mg for children weighing more than 40 kg, and 2 mg/kg for those weighing 15–40 kg, administered every 8 weeks as a single dose via subcutaneous injection [48]. A recent study in patients with Blau syndrome showed clinical improvement with canakinumab [49] (Fig. 3 [50]). Both, canakinumab and anakinra, are being extensively studied in clinical trials for several inflammatory and chronic conditions.
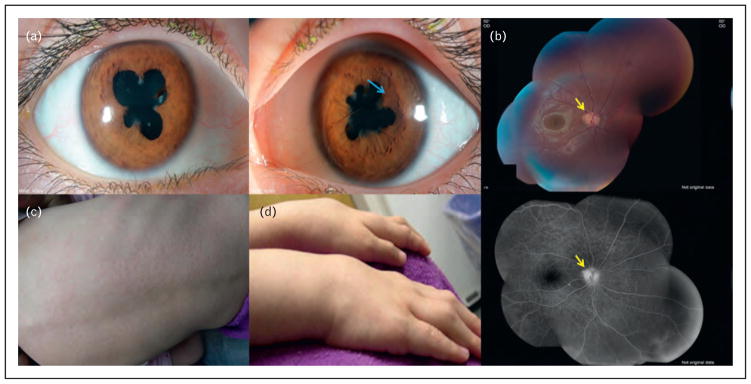
FIGURE 3.
A 7-year-old girl with Blau syndrome (NOD2 mutation) associated granulomatous panuveitis complicated with (a) posterior synechiae in both eyes secondary to anterior uveitis as well as iris nodules (blue arrow) in the left eye. (b) She had a small peripapillary granuloma (yellow arrows) in the right eye. She also had a history of (c) skin lesions and (d) arthritis, which are features of Blau syndrome [50]. Source: Original except the two pictures of hands and back (obtained permission from Elsevier by Copyright Clearance Center) [50].
Interleukin-6 inhibitors
Tocilizumab is a humanized monoclonal IL-6 receptor antagonist. IL-6 is a pleiotropic pro-inflammatory cytokine involved in the inflammatory immune response. Several case reports highlight interest in tocilizumab for the treatment of uveitis refractory to anti-TNF, including idiopathic uveitis, Birdshot chorioretinopathy [51], and Behcet’s disease [52]. More recently, tocilizumab was successfully used in adult patients with JIA uveitis refractory to immunosuppressants and anti-TNF [53▪]. There are a few ongoing clinical trials studying the safety, tolerability, and bioactivity of tocilizumab in patients with noninfectious uveitis, JIA-associated uveitis or Behcet’s disease Additionally, sirukumab by Janssen Research and Development, Spring House, Pennsylvania, USA [54] and sarilumab by Regeneron, Tarrytown, New York, USA and Sanofi, Bridgewater, New Jersey, USA are under investigation [www.clinicaltrials.gov (http://www.clinicaltrials.gov/ct2/results?term=sarilumab].
Rituximab
Rituximab is a murine-human antibody against CD20, a B lymphocyte antigen, inducing apoptosis and temporarily depleting B cells for 6–9 months. Rituximab is FDA-approved for the treatment of lymphoma and leukemia, RA, granulomatosis with polyangiitis (formerly Wegener’s granulomatosis), and microscopic polyangiitis. Rituximab can be helpful in peripheral ulcerative keratitis, scleritis, and refractory JIA uveitis that fail to respond to anti-TNF agents [55]. Rituximab is administered by intravenous infusion. The most frequent side-effects are infusion reactions, which can be suppressed with concomitant intravenous corticosteroids. Neutropenia, reactivation of hepatitis B virus, heart failure, and progressive multifocal leukoencephalopathy have also been reported [56]. Safety and efficacy of rituximab in children is unknown.
Abatacept
Abatacept, a soluble CTLA4-IgFCg fusion protein, binds to CD80/CD86 on antigen-presenting cells, thereby preventing T-cell activation [32▪▪]. It has been FDA-approved for JIA and RA. Although there are limited data to show the efficacy of this drug in pediatric and adult uveitis, numerous case reports indicated improvement of anti-TNF refractory uveitis within 2 weeks to 6 months [57–59]. Abatacept has a mild side-effect profile, but infection and malignancy can occur [60].
Local treatments
Injectable or surgically implantable corticosteroid devices provide a novel approach for the treatment of refractory uveitis. Implants provide long-term, slow release of intraocular corticosteroids [60].
The first FDA-approved implantable device for noninfectious posterior uveitis was fluocinolone acetonide (Retisert), containing 0.59 mg of fluocinolone acetonide, which is released over 30 months [8]. A recent retrospective study conducted on six eyes of children with intractable posterior uveitis showed controlled inflammation in all and significant visual acuity improvement in half. However, concerns for development of cataract and secondary glaucoma remain [30▪]. Another recent development, Ozurdex, is a biodegradable dexamethasone implant, and has been reported to have an improved side-effect profile, at least in adults [61]. There are very limited data showing the efficacy and safety in children. One recent retrospective case series in 14 eyes of 11 children with posterior segment uveitis showed control of inflammation and improvement in CME and visual acuity in all but one eye within a month, but 31% had a relapse within 6 months with a median time to relapse at 4 months [62▪▪]. A retrospective series showed Retisert and Ozurdex to be comparable in efficacy; however, cataract progression and need for glaucoma treatment and surgery were higher with the Retisert implant [63▪].
FUTURE DIRECTIONS
As our understanding of the mechanisms involved in ocular inflammatory diseases expands, new therapies will emerge. Currently, there are over 40 clinical trials for the treatment of uveitis registered at clinical trials database (clinicaltrials.gov) that span five continents. Therapeutic agents used range from intravitreal or suprachoroidal injections of novel agents and orally administered tolerance inducing peptides to novel small molecules and biologic agents. A small minority of these trials focuses on childhood uveitis. There are several potential therapeutic agents that are currently under investigation in other rheumatologic or retinal disorders. For example, biologics that target IL-17, IL12/23, IL15, IL-22, and B-cell activating factor (BAFF) can also prove useful in pediatric uveitis in the future. Small molecules, different intravitreal drug delivery techniques for sustained or combined drug delivery, small interfering RNA (siRNA), microRNA technology, and gene transfer techniques hold promise for the treatment of uveitis. More clinical trials are needed to establish appropriate therapy and identify ways to predict and individualize therapy for the most at-risk children with uveitis.
CONCLUSION
Pediatric uveitis is an uncommon but serious disease. New treatment options have been rapidly emerging over the last decade and promise to continue this trend into the future as new technology and our understanding of the disease–immune system interface evolves. In this article, we have summarized the past, present, and predictable future of pediatric uveitis treatments. The mainstays of therapy include corticosteroids, antimetabolites, and T-cell inhibitors. In the last decade, biologic therapies have revolutionized our armory for treating pediatric uveitis, and novel corticosteroid delivery devices have enhanced our ability to provide longer-lasting local disease control. Further research is needed to ensure that development of novel treatments can continue to grow and maintains the highest level of safety and efficacy.
KEY POINTS.
Pediatric uveitis is an uncommon but potentially blinding condition with a higher rate of complications and vision loss than adults.
Treatment should be a sufficiently aggressive anti-inflammatory therapy to quiet inflammation quickly and then maintenance of quiescence for a period of 2–3 years, after which tapered discontinuation of immunosuppression can be considered.
Methotrexate remains the first-line immunomodulatory agent in pediatric uveitis.
Biologic therapy, particularly the anti-TNF agents, is the newer generation of gold standard therapy and is commonly used in methotrexate failures.
Many novel therapies for pediatric uveitis are under investigation; promising future interventions include biologics targeting of IL-1, IL-2, IL-6, as well as CD 20 and CD 80/86.
Footnotes
Conflicts of interest
The authors of this article have no financial or proprietary interests to disclose.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 517).
- 1.Levy-Clarke GA, Nussenblatt RB, Smith JA. Management of chronic pediatric uveitis. Curr Opin Ophthalmol. 2005;16:281–288. doi: 10.1097/01.icu.0000177414.79030.32. [DOI] [PubMed] [Google Scholar]
- 2.Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116:1544–1551. doi: 10.1016/j.ophtha.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111:2299–2306. doi: 10.1016/j.ophtha.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated uveitis. Ophthalmology. 1997;104:236–244. doi: 10.1016/s0161-6420(97)30329-7. [DOI] [PubMed] [Google Scholar]
- 5.Edelsten C, Lee V, Bentley CR, et al. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol. 2002;86:51–56. doi: 10.1136/bjo.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne JE, Woreta F, Kedhar SR, et al. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143:840–846. doi: 10.1016/j.ajo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 7▪.Gregory AC, 2nd, Kempen JH, Daniel E, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology. 2013;120:186–192. doi: 10.1016/j.ophtha.2012.07.052. The first study of its kind to retrospectively examine a large number (n = 327) of patients with JIA-associated uveitis and determine the incidence of and risk factors for vision loss and ocular complications. This study demonstrated the correlation between increasing uveitis activity and increased risk of vision loss, and that the use of immunomodulatory therapy was associated with reduced risk of vision loss. The study highlights the importance of control of inflammation and use of immunosuppression in improving the outcomes in JIA-related uveitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Incorporated, B.L, editor. Retisert [package insert] Rochester, NY: Bausch and Lomb Inc; 2012. [Google Scholar]
- 9.Ozurdex [package insert] Irvine, CA, USA: Allergan Inc; 2012. [Google Scholar]
- 10.Gomes Bittencourt M, Sepah YJ, Do DV, et al. New treatment options for noninfectious uveitis. Dev Ophthalmol. 2012;51:134–161. doi: 10.1159/000336338. [DOI] [PubMed] [Google Scholar]
- 11.Angeles-Han S, Yeh S. Prevention and management of cataracts in children with juvenile idiopathic arthritis-associated uveitis. Curr Rheumatol Rep. 2012;14:142–149. doi: 10.1007/s11926-011-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelcorn ED. Infectious causes of posterior uveitis. Can J Ophthalmol. 2013;48:31–39. doi: 10.1016/j.jcjo.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Madigan WP, Raymond WR, Wroblewski KJ, et al. A review of pediatric uveitis: Part I. Infectious causes and the masquerade syndromes. J Pediatr Ophthalmol Strabismus. 2008;45:140–149. doi: 10.3928/01913913-20080501-16. [DOI] [PubMed] [Google Scholar]
- 14.Van Gelder RN. Ocular pathogens for the twenty-first century. Am J Ophthalmol. 2010;150:595–597. doi: 10.1016/j.ajo.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Gelder RN. Has the polymerase chain reaction come of age for ophthalmology? Am J Ophthalmol. 2009;147:5–7. doi: 10.1016/j.ajo.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tugal-Tutkun I. Pediatric uveitis. J Ophthal Vision Res. 2011;6:259–269. [PMC free article] [PubMed] [Google Scholar]
- 17.Foster CS, Davanzo R, Flynn TE, et al. Durezol (Difluprednate Ophthalmic Emulsion 0. 05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. 2010;26:475–483. doi: 10.1089/jop.2010.0059. [DOI] [PubMed] [Google Scholar]
- 18.Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. 2012;153:932–938. doi: 10.1016/j.ajo.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Nozik RA. Periocular injection of steroids. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:695–705. [PubMed] [Google Scholar]
- 20.Kim SJ. Diagnosis and management of noninfectious pediatric uveitis. Int Ophthalmol Clin. 2011;51:129–145. doi: 10.1097/IIO.0b013e318200e01b. [DOI] [PubMed] [Google Scholar]
- 21.Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010;117:1436–1441. doi: 10.1016/j.ophtha.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson T, Nussenblatt RB, Sen HN. Emerging drugs for uveitis. Exp Opin Emerg Drugs. 2011;16:309–322. doi: 10.1517/14728214.2011.537824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology. 2008;47:249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 24.Espinosa M, Gottlieb BS. Juvenile idiopathic arthritis. Pediatr Rev. 2012;33:303–313. doi: 10.1542/pir.33-7-303. [DOI] [PubMed] [Google Scholar]
- 25.Goebel JC, Roesel M, Heinz C, et al. Azathioprine as a treatment option for uveitis in patients with juvenile idiopathic arthritis. Br J Ophthalmol. 2011;95:209–213. doi: 10.1136/bjo.2009.173542. [DOI] [PubMed] [Google Scholar]
- 26.Sobrin L, Christen W, Foster CS. Mycophenolate mofetil after methotrexate failure or intolerance in the treatment of scleritis and uveitis. Ophthalmology. 2008;115:1416–1421. 1421 e1411. doi: 10.1016/j.ophtha.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Nussenblatt RB, Palestine AG, Chan CC, et al. Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis. Am J Ophthalmol. 1991;112:138–146. doi: 10.1016/s0002-9394(14)76692-9. [DOI] [PubMed] [Google Scholar]
- 28.Tappeiner C, Roesel M, Heinz C, et al. Limited value of cyclosporine A for the treatment of patients with uveitis associated with juvenile idiopathic arthritis. Eye. 2009;23:1192–1198. doi: 10.1038/eye.2008.174. [DOI] [PubMed] [Google Scholar]
- 29.Kilmartin DJ, Forrester JV, Dick AD. Cyclosporin A therapy in refractory noninfectious childhood uveitis. Br J Ophthalmol. 1998;82:737–742. doi: 10.1136/bjo.82.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪.Patel CC, Mandava N, Oliver SC, et al. Treatment of intractable posterior uveitis in pediatric patients with the fluocinolone acetonide intravitreal implant (Retisert) Retina. 2012;32:537–542. doi: 10.1097/IAE.0b013e31822058bb. This study retrospectively evaluated Retisert implant and found it can be used effectively for control of posterior segment inflammation in pediatric patients (n = 6 eyes). As in adult patients, concerns for development of cataract and secondary glaucoma remain. [DOI] [PubMed] [Google Scholar]
- 31.Gueudry J, LeHoang P, Bodaghi B. Anti-tumor necrosis factor-alpha agents in noninfectious uveitis. Dev Ophthalmol. 2012;51:63–78. doi: 10.1159/000336187. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Horneff G. Update on biologicals for treatment of juvenile idiopathic arthritis. Exp Opin Biol Ther. 2013;13:361–376. doi: 10.1517/14712598.2013.735657. This article reviews recent clinical trials and gives an overview of the current treatment options using biologics in JIA. [DOI] [PubMed] [Google Scholar]
- 33.Remicade Full Prescribing Information. Janssen Biotech, Inc; Horsham, PA: 2013. [Google Scholar]
- 34.Humira Full Prescribing Information. AbbVie, Inc; North Chicago, IL: 2013. [Google Scholar]
- 35.Simponi Full Prescribing Information. Janssen Biotech Inc; Horsham, PA: 2013. [Google Scholar]
- 36.Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 37▪▪.Zannin ME, Birolo C, Gerloni VM, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the Italian Registry. J Rheumatol. 2013;40:74–79. doi: 10.3899/jrheum.120583. This relatively large study (n = 91) evaluated the safety and efficacy of adalimumab and infliximab for the treatment of JIA-related anterior uveitis in patients with at least 1 year of follow-up. They found adalimumab and infliximab to be effective and well tolerated for the treatment of refractory JIA-related uveitis. [DOI] [PubMed] [Google Scholar]
- 38▪▪.Magli A, Forte R, Navarro P, et al. Adalimumab for juvenile idiopathic arthritis associated uveitis. Graefes Arch Clin Exp Ophthalmol. 2013;251:1601–1606. doi: 10.1007/s00417-013-2275-x. This study looked prospectively at pediatric patients (n = 21) with JIA uveitis started on adalimumab and followed for an average of 18 months. These patients were found to have a statistically significant reduction in number of uveitis relapses while on adalimumab compared with their relapse pattern prior to starting adalimumab. [DOI] [PubMed] [Google Scholar]
- 39▪▪.Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119:1575–1581. doi: 10.1016/j.ophtha.2012.02.018. This prospective case series followed a large number (n = 131) of pediatric and adult patients with refractory uveitis for 6 months and found that adalimumab was well tolerated and effectively controlled inflammation. [DOI] [PubMed] [Google Scholar]
- 40.Simonini G, Taddio A, Cattalini M, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res. 2011;63:612–618. doi: 10.1002/acr.20404. [DOI] [PubMed] [Google Scholar]
- 41.Mesquida MM, Victoria Hernandez MMP, Llorenc VM, et al. Behcet disease-associated uveitis successfully treated with golimumab. Ocul Immunol Inflamm. 2012;21:160–162. doi: 10.3109/09273948.2012.741744. [DOI] [PubMed] [Google Scholar]
- 42.Cordero-Coma M, Salom D, Diaz-Llopis M, et al. Golimumab for uveitis. Ophthalmology. 2011;118:1892, e1893–1894. doi: 10.1016/j.ophtha.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 43.de Bie CI, Escher JC, de Ridder L. Antitumor necrosis factor treatment for pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:985–1002. doi: 10.1002/ibd.21871. [DOI] [PubMed] [Google Scholar]
- 44.Smith WM, Sen HN, Nussenblatt RB. Noncorticosteroid immune therapy for ocular inflammation. In: Tasman W, Jaeger EA, editors. Duane’s Foundations of Clinical Ophthalmology. 2012. [Google Scholar]
- 45.Sen HN, Levy-Clarke G, Faia LJ, et al. High-dose daclizumab for the treatment of juvenile idiopathic arthritis-associated active anterior uveitis. Am J Ophthalmol. 2009;148:696–703. e691. doi: 10.1016/j.ajo.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caorsi R, Federici S, Gattorno M. Biologic drugs in autoinflammatory syndromes. Autoimmun Rev. 2012;12:81–86. doi: 10.1016/j.autrev.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Bresnihan B, Alvaro-Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 48.Ilaris Full prescribing information. Novartis Pharmaceuticals Corp; East Han-over, NJ, USA: 2013. [Google Scholar]
- 49.Simonini G, Xu Z, Caputo R, et al. Clinical and transcriptional response to the long-acting interleukin-1 blocker canakinumab in Blau syndrome-related uveitis. Arthritis Rheum. 2013;65:513–518. doi: 10.1002/art.37776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raiji VR, Miller MM, Jung LK. Uveitis in Blau syndrome from a de novo mutation of the NOD2/CARD15 gene. J AAPOS. 2011;15:205–207. doi: 10.1016/j.jaapos.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Muselier A, Bielefeld P, Bidot S, et al. Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocul Immunol Inflamm. 2011;19:382–383. doi: 10.3109/09273948.2011.606593. [DOI] [PubMed] [Google Scholar]
- 52.Hirano T, Ohguro N, Hohki S, et al. A case of Behcet’s disease treated with a humanized antiinterleukin-6 receptor antibody, tocilizumab. Mod Rheumatol. 2012;22:298–302. doi: 10.1007/s10165-011-0497-5. [DOI] [PubMed] [Google Scholar]
- 53▪.Tappeiner C, Heinz C, Ganser G, Heiligenhaus A. Is tocilizumab an effective option for treatment of refractory uveitis associated with juvenile idiopathic arthritis? J Rheumatol. 2012;39:1294–1295. doi: 10.3899/jrheum.120010. In this small case series, tocilizumab treatment achieved control of uveitis in two of three patients in whom disease had been refractory to previous immunomodulatory therapy, including at least one TNF-α inhibitor, suggesting that the IL-6 pathway is worthy of further investigation as a uveitis treatment target. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang Y, Xu Z, de Vries DE, et al. Pharmacokinetics and safety of sirukumab following a single subcutaneous administration to healthy Japanese and Caucasian subjects. Int J Clin Pharmacol Ther. 2013;51:187–199. doi: 10.5414/CP201785. [DOI] [PubMed] [Google Scholar]
- 55.Heiligenhaus A, Miserocchi E, Heinz C, et al. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab) Rheumatology. 2011;50:1390–1394. doi: 10.1093/rheumatology/ker107. [DOI] [PubMed] [Google Scholar]
- 56.Saadoun D, Bodaghi B, Bienvenu B, et al. Biotherapies in inflammatory ocular disorders: interferons, immunoglobulins, monoclonal antibodies. Autoimmun Rev. 2013;12:774–783. doi: 10.1016/j.autrev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Angeles-Han S, Flynn T, Lehman T. Abatacept for refractory juvenile idiopathic arthritis-associated uveitis: a case report. J Rheumatol. 2008;35:1897–1898. [PubMed] [Google Scholar]
- 58.Zulian F, Balzarin M, Falcini F, et al. Abatacept for severe antitumor necrosis factor alpha refractory juvenile idiopathic arthritis-related uveitis. Arthritis Care Res. 2010;62:821–825. doi: 10.1002/acr.20115. [DOI] [PubMed] [Google Scholar]
- 59.Kenawy N, Cleary G, Mewar D, et al. Abatacept: a potential therapy in refractory cases of juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;249:297–300. doi: 10.1007/s00417-010-1523-6. [DOI] [PubMed] [Google Scholar]
- 60.Gallego-Pinazo R, Dolz-Marco R, Martinez-Castillo S, et al. Update on the principles and novel local and systemic therapies for the treatment of non-infectious uveitis. Inflamm Allergy Drug Targets. 2013;12:38–45. doi: 10.2174/1871528111312010006. [DOI] [PubMed] [Google Scholar]
- 61.Lowder C, Belfort R, Jr, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 62▪▪.Taylor SR, Tomkins-Netzer O, Joshi L, et al. Dexamethasone implant in pediatric uveitis. Ophthalmology. 2012;119:2412–12412. e2412. doi: 10.1016/j.ophtha.2012.07.025. This retrospective series provides the first published report of Ozurdex implant use in children (n = 14 eyes) [DOI] [PubMed] [Google Scholar]
- 63▪.Arcinue CA, Ceron OM, Foster CS. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J Ocul Pharmacol Ther. 2013;29:5012–5017. doi: 10.1089/jop.2012.0180. This comparative retrospective study evaluating dexamethasone (Ozurdex; n = 11) and the fluocinolone acetonide (Retisert) implant (n = 16) found them comparable in preventing recurrence of noninfectious uveitis and in improving inflammation and vision. Higher rates of cataract progression and the need for glaucoma medications, laser, and surgery with the Retisert implant were found. [DOI] [PubMed] [Google Scholar]