Abstract
Purpose: The objective is to demonstrate the clinicopathological characteristics of patients with unexpected node-positive lung adenocarcinoma and to analyze predictive factors of unexpected disease.
Methods: We reviewed 225 patients with lung adenocarcinoma who underwent curative-intent operation between January 2008 and December 2014. Unexpected node-positive diseases were defined as cases with hilar or mediastinal lymph nodes metastasis in spite of both negative significant enlargement of lymph nodes on preoperative chest computed tomography (CT) and negative fluorodeoxyglucose (FDG) uptake in lymph nodes on preoperative positron emission tomography (PET)/CT. We retrospectively analyzed clinical features of these patients and evaluated associated factors for unexpected diseases.
Results: There were 41 patients (18%) with unexpected node-positive disease, consisting of 16 (39%) unexpected pN1 and 25 (61%) unexpected pN2 diseases. The most common predominant subtype was papillary (22 patients; 54%), and 17 patients (41%) had micropapillary component in the tumors. Younger age (p <0.01), left side (p <0.01), larger tumor size (p <0.01), and having a micropapillary component (p <0.01) were significant associated factors of unexpected diseases in multivariate analysis.
Conclusion: Histological findings of the primary tumor are often important because they can provide predictive information for lymph nodes status. Having a micropapillary component was one of the significant predictors of unexpected node-positive diseases.
Keywords: lung adenocarcinoma, unexpected lymph node metastasis, micropapillary component
Introduction
For the patients with non-small cell lung cancer (NSCLC), preoperative staging of their disease is necessary for appropriate treatment allocation. Enhanced computed tomography (CT) of the chest is a widely accepted radiographic examination for preoperative nodal staging in NSCLC patients. Nowadays, F-18-2-deoxyfluoro-D-glucose (FDG) positron emission tomography/CT (PET/CT) is also generally performed as a standard non-invasive imaging examination for nodal and systemic evaluation of NSCLC. Although PET/CT seems to be able to provide accurate information of nodal status in NSCLC patients, the fact remains that there are a lot of surgical cases unexpectedly proven to have hilar and mediastinal lymph nodes metastasis by the histological evaluation after operation. The difference between preoperative and postoperative evaluation of nodal status might lead to contributions of misdirected or insufficient surgical treatment for NSCLC patients. Thus, it would be of great significance for us to recognize the clinicopathological features of NSCLC cases with unexpected lymph node metastasis.
In the past, some authors had reported the problems of false-negative assessments of mediastinal lymph nodes by PET/CT in NSCLC cases.1–8) Some of them evaluated histological findings of metastatic lymph nodes in detail, and they investigated causative factors for false-negative PET scans. However, to our knowledge, few studies have investigated the association between PET false-negative, unexpectedly proven lymph node metastasis and the histological features of primary tumors. In this retrospective study, we demonstrated clinicopathological characteristics of patients with unexpected node-positive NSCLC, paying attention to the detailed histological findings of primary tumors with unexpected lymph node metastasis. In addition, we analyzed associated factors of unexpected lymph node metastasis.
Materials and Methods
Patients
In this retrospective study, we analyzed 225 patients who were treated with surgery and diagnosed as having primary lung adenocarcinoma between January 2008 and December 2014. All patients underwent preoperative contrast-enhanced CT of the chest and upper abdomen. In addition, PET/CT was also performed for evaluating the lymph nodes status and systemic survey before the operation. Lymph nodes that were larger than 1.0 cm in the short axis on chest CT and with FDG uptake on PET/CT were clinically suspected as lymph node metastasis positive. Endobronchial ultrasonography-guided transbronchial needle aspiration (EBUS-TBNA) was performed for some cases with these lymph nodes; however, it was not routinely performed during the period of this study. Mediastinoscopy was not performed during this study. Surgical procedures included pneumonectomy, bilobectomy, lobectomy, and segmentectomy with systematic or lobe-specific selective mediastinal lymph node dissection.
Unexpected diseases were defined as surgical cases which were proved to have hilar or mediastinal lymph nodes metastasis postoperatively in spite of both negative significant enlargement of hilar or mediastinal lymph nodes on preoperative chest CT and negative FDG uptake in hilar or mediastinal lymph nodes on preoperative PET/CT. On the other hand, expected diseases were defined as cases which had the same lymph node status between preoperative and postoperative evaluations. In this study, six cases which were proved not to have lymph node metastasis postoperatively in spite of positive-FDG uptake in these lymph nodes on preoperative PET/CT were excluded. Because clinical and pathological stage was determined according to the 6th Edition Tumor-Node-Metastasis (TNM) classification in the initial period of this study, we re-staged all cases according to the 7th Edition TNM classification for the present study.
All surgical specimens were fixed with 10% formalin and embedded in paraffin. Serial 4-µm sections were stained using the haematoxylin–eosin (HE) stain. Histological analysis of primary tumors and lymph nodes was made by HE stain. Primary tumors were classified into more specific types such as adenocarcinoma, squamous cell carcinoma, large cell carcinoma according to the last World Health Organization (WHO) classification. Adenocarcinomas were additionally classified based on predominant subtypes following the most recent recommended classification.9) Furthermore, the presence of micropapillary component was evaluated and recorded according to its occupancy as a specific subtype. In this study, we determined that micropapillary component was positive when it occupied at least 1% of the entire tumor.10)
We retrospectively reviewed the medical records of each patient group (expected and unexpected diseases) for clinicopathological information as follows: age, gender, smoking status (never or ever smoker), tumor location (right upper, middle, lower lobe, and left upper and lower lobe), clinical T factor, operative procedure (lobectomy or segmentectomy), tumor size, histological type, pathological T factor and pathological stage, pleural invasion (negative or positive), lymphovascular invasion (negative or positive), predominant subtype, and micropapillary component (negative or positive). Additionally, we evaluated significant associated factors for unexpected disease among these clinicopathological factors by univariate and multivariate analyses. Data collection and analyses were approved, and the need to obtain written informed consent was waived by the Institutional Review Board in January 2014.
Statistical analysis
All statistical analyses were performed using SPSS ver. 22 (IBM, Chicago IL). Categoric data were expressed as counts and proportions. Two-category comparison was performed by the Pearson’s chi-square test and Fisher’s exact test for quantitative data. Univariate and multivariate analyses using logistic regression model were performed to identify predictive factors of unexpected diseases. A p value <0.05 was considered to be significant in statistical analyses.
Results
Clinicopathological characteristics of patients
Table 1 shows the clinicopathological characteristics of patients. This study included 116 men and 109 women with a median age of 67 years (range: 25–85 years). There were 184 patients (82%) with expected disease group and 41 patients (18%) with unexpected disease, consisting of 17 (41%) unexpected pN1 and 24 (59%) unexpected pN2 diseases (Fig. 1). In unexpected disease group, there were 19 males and 22 females, and 22 ever smokers and 19 never smokers. Operative procedure was pneumonectomy in 2, lobectomy in 36, and segmentectomy in 3. The maximum tumor size was ranged from 18 to 65 mm, with a median size of 32 mm. Pathological T factors were pT1a in 5, pT1b in 6, pT2a in 22, pT2b in 6, and pT3 or 4 in 2. Pleural and lymphovascular invasion were positive for 14 (34%) and 39 (100%), respectively. The most common predominant subtype was papillary (22 patients; 54%), followed by acinar (10 patients; 24%), solid with mucin (4 patients; 10%), and micropapillary (2 patients; 5%). Of these patients, 18 patients (44%) had micropapillary component in varying proportions in central or peripheral areas of their primary tumors. On the other hand, only 7 patients (4%) had micropapillary component in their primary tumors among 184 adenocarcinoma patients with expected diseases. There were statistical significances in age, clinical, and pathological T factor, pleural and lymphovascular invasion between expected and unexpected groups (Table 1).
Table 1. Clinicopathological characteristics of patients.
| All patients | Unexpected disease | Expected disease | p value | |||||
|---|---|---|---|---|---|---|---|---|
| N = 225 | (%) | N = 41 | (%) | N = 184 | (%) | |||
| Age (years) | Median (Range) | 68 | (25–85) | 65 | (33–78) | 69 | (25–85) | <0.01* |
| Gender | Male | 115 | (51) | 19 | (46) | 96 | (52) | 0.60 |
| Female | 110 | (49) | 22 | (54) | 88 | (48) | ||
| Smoking status | Ever | 112 | (50) | 22 | (54) | 90 | (49) | 0.61 |
| Never | 113 | (50) | 19 | (46) | 94 | (51) | ||
| Tumor location | Right upper lobe | 78 | (35) | 9 | (22) | 69 | (38) | 0.09 |
| Right middle lobe | 17 | (8) | 2 | (5) | 15 | (8) | ||
| Right lower lobe | 48 | (21) | 8 | (19) | 40 | (21) | ||
| Left upper lobe | 55 | (24) | 13 | (32) | 42 | (23) | ||
| Left lower lobe | 27 | (12) | 9 | (22) | 18 | (10) | ||
| Clinical T factor | T1a | 86 | (38) | 4 | (10) | 82 | (44) | |
| T1b | 79 | (35) | 19 | (46) | 60 | (33) | <0.01 | |
| T2a | 49 | (22) | 16 | (39) | 33 | (18) | ||
| T2b or greater | 11 | (5) | 2 | (5) | 9 | (5) | ||
| Operative procedure | Lobectomy or greater | 202 | (90) | 38 | (93) | 164 | (89) | 0.78 |
| Segmentectomy | 23 | (10) | 3 | (7) | 20 | (11) | ||
| Tumor size (mm) | Median (Range) | 24 | (6–99) | 33 | (15–65) | 22 | (6–99) | <0.01* |
| Pathological T factor | T1a | 69 | (31) | 5 | (12) | 64 | (35) | |
| T1b | 72 | (32) | 6 | (14) | 66 | (36) | <0.01 | |
| T2a | 64 | (28) | 22 | (54) | 42 | (23) | ||
| T2b or greater | 20 | (9) | 8 | (20) | 12 | (7) | ||
| Pleural invasion | Negative | 182 | (81) | 14 | (34) | 155 | (84) | 0.01 |
| Positive | 43 | (19) | 27 | (66) | 29 | (16) | ||
| Lymphovascular invasion | Negative | 100 | (44) | 2 | (5) | 98 | (53) | <0.01 |
| Positive | 125 | (56) | 39 | (95) | 86 | (47) | ||
*Mann–Whitney U-test.
Fig. 1. Changing of clinical and pathological lymph node status in patients with expected and unexpected diseases.
Maximum diameters of lymph node metastatic focus
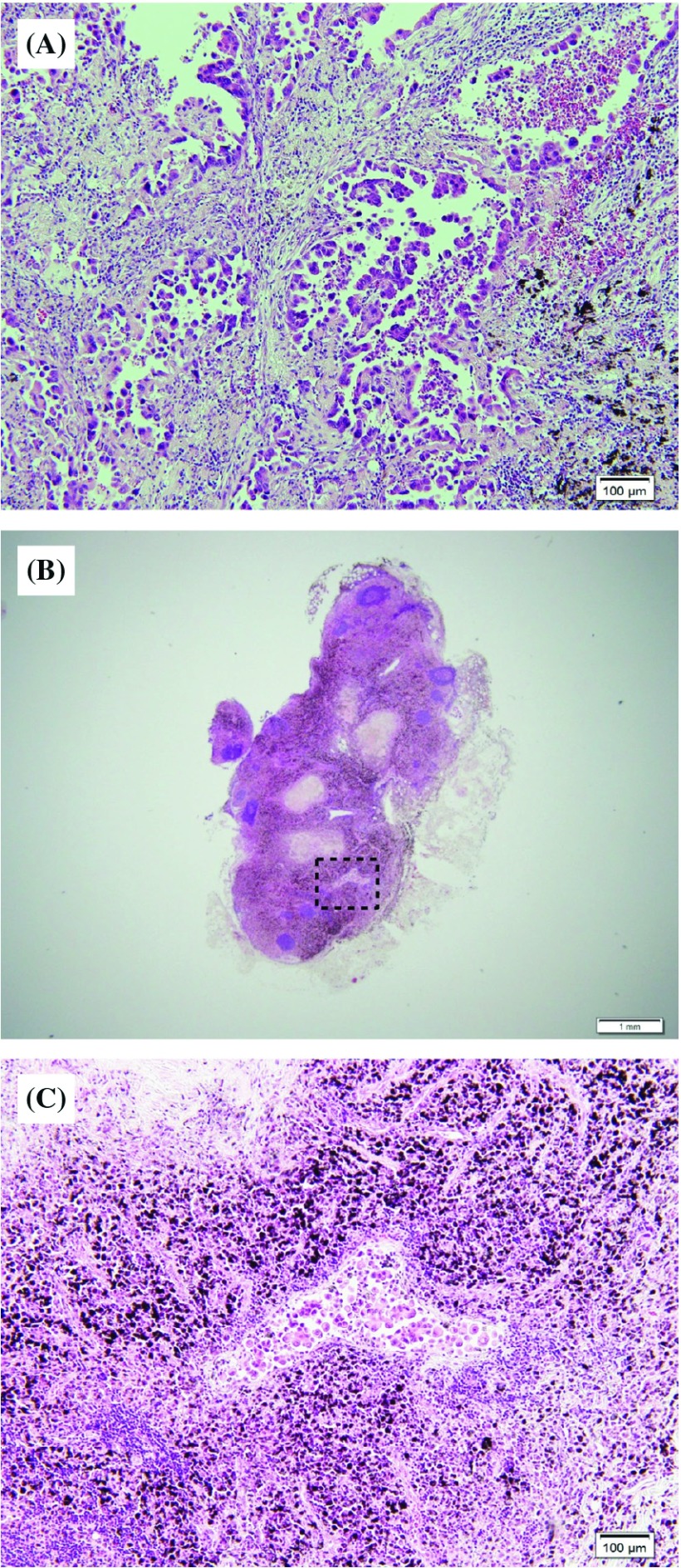
Maximum diameter of lymph node metastatic focus in cases with unexpected diseases ranged from 0.4 to 19.0 mm, with a mean value of 5.7 ± 3.8 mm, whereas those in cases with expected diseases ranged from 1.0 to 23.0 mm, with a mean value of 12.9 ± 7.2 mm. There was a significant difference between these two groups (p <0.01; Mann–Whitney U-test). Figure 2 shows the representative findings of primary tumor and lymph node metastatic focus in the case of unexpected disease. There was a very small metastatic focus within the lymph node, which was present as micrometastasis (Fig. 2).
Fig. 2. Representative pathological findings of unexpected lymph node metastasis. (A) Primary tumor had micropapillary components. (B) Metastatic mediastinal lymph node. (C) Micrometastatic foci in the lymph node.
Univariate and multivariate analyses for associated factors of unexpected diseases
Among clinicopathological factors, younger age (p = 0.03), left tumor laterality (p = 0.01), larger tumor size (p <0.01), positive pleural invasion (p = 0.01), and having a micropapillary component (p <0.01) were significant associated factors for unexpected lymph node metastasis in univariate analysis. In multivariate analysis, younger age (p <0.01), left tumor laterality (p <0.01), larger tumor size (p <0.01), and having a micropapillary component (p <0.01) were significant associated factors of unexpected diseases (Table 2).
Table 2. Univariate and multivariate analyses for associated factors of unexpected diseases.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| p value | p value | Odds ratio | 95% CI | ||
| Age | ≥70 years old | Reference | |||
| <70 years old | 0.03 | <0.01 | 4.07 | 1.63–10.18 | |
| Gender | Female | ||||
| Male | 0.50 | ||||
| Smoking status | Never | ||||
| Ever | 0.58 | ||||
| Laterality | Right | Reference | |||
| Left | 0.01 | <0.01 | 3.04 | 1.33–6.97 | |
| Location | Lower lobe | ||||
| Upper or middle lobe | 0.22 | ||||
| Surgical procedure | Lobectomy | ||||
| Segmentectomy | 0.50 | ||||
| Tumor size | ≤3.0 cm | Reference | |||
| >3.0 cm | <0.01 | <0.01 | 9.78 | 3.92–24.37 | |
| Pleural invasion | Negative | ||||
| Positive | 0.01 | 0.241 | |||
| Micropapillary component | Negative | Reference | |||
| Positive | <0.01 | <0.01 | 6.19 | 2.58–14.86 | |
CI: confidence interval
Discussion
PET/CT is currently an important modality for preoperative systemic survey and evaluation of lymph nodes status because accurate preoperative staging is essential for appropriate treatment allocation both in early and locally advanced NSCLC cases. However, we sometimes experience surgical cases in which postoperative pathological stage was unexpectedly different from preoperative stage proven by PET/CT. In particular, the problem of PET/CT false-negative assessments for hilar and mediastinal lymph nodes has been observed, so it would be meaningful to investigate associated clinicopathological factors for these unexpected cases.
There are some literatures demonstrating false-negative rate of preoperative PET/CT for hilar and mediastinal staging. Gomez-Caro et al. revealed that false-negative rate for mediastinal staging using CT and PET/CT was 11% in clinical stage I lung cancer.7) Similarly, Kim et al.11) reported that 89 (16%) were found to have pathologic N1 (n = 49; 9%) and N2 (n = 40; 7%) disease among 547 patients with clinical stage I disease diagnosed by preoperative PET/CT. Although there seems to be some causes of PET/CT false-negative findings, the primary cause was micrometastasis within the lymph nodes. Nomori et al.4) reported that the size of metastatic foci within lymph nodes was 0.5–9 mm for false-negative cases and 4–8 mm for true-positive cases, and there was a significant difference between these two groups. Additionally, Ozawa et al.8) analyzed PET/CT false-negative lymph nodes in more detail, and they also concluded that the overall size of PET/CT false-negative mediastinal nodes was significantly smaller than that of true-positive nodes (39.3 ± 30.5 mm2 vs. 106.2 ± 60.1 mm2; p <0.0001). Although these reports were generally consistent with our results, there was few description of the association between histological features of the primary tumor and patterns of lymph node metastasis in these literatures and others.
Among cases of our study, having a micropapillary component in the primary tumor is one of the significant associated factors of unexpected lymph nodes metastasis. Micropapillary subtype in lung adenocarcinoma was initially reported by Amin et al. in 200212) and some investigators have reported that this subtype is an unfavorable prognostic factor even in patients with early-stage lung adenocarcinoma.10,13–16) There have been some literatures which demonstrated the association between micropapillary subtype and lymph node metastasis in adenocarcinoma cases. Miyoshi et al.13) reported that tumors with a micropapillary component showed a statistically significant difference between the clinical and pathological stage, with the metastatic foci in the lymph nodes and lung often being very small. Roh et al.17) evaluated the relationship between this component in adenocarcinoma and nodal micrometastasis by immunohistochemical method, and they demonstrated that the frequency of nodal micrometastasis in the case with a micropapillary component was significantly higher than in those without. Considering these previous reports and results of our study, it would appear that tumors with micropapillary component have a tendency to metastasize to regional lymph nodes with the formation of micrometastatic foci, which would be extremely difficult to detect on PET/CT. In recent years, other imaging modalities or intraoperative diagnostic procedures are being developed to obtain more accurate diagnosis of lymph node metastasis in lung cancer patients. Ohno et al.18) evaluated the diagnostic capability of short inversion time inversion-recovery (STIR) turbo spin-echo (SE) imaging in nodal assessment in patients with NSCLC, and they concluded that STIR turbo SE imaging is more sensitive and/or more accurate than FDG-PET/CT.18) Inoue et al.19) suggested the potential clinical utility of one-step nucleic acid amplification (OSNA) assay, which was an automated rapid molecular diagnostic method using optimal mRNA marker, for intraoperative rapid and accurate diagnosis of nodal status in lung cancer. However, if using these new diagnostic technologies, it would be quite difficult to detect micrometastatic foci of lymph nodes reliably. Similarly, if we can predict the presence of micropapillary component in the primary tumor pre- or intra-operatively, we will be able to perform appropriate treatment with adequate considerations of lymph node status. However, the prediction of detailed intratumoral subtypes is also quite difficult by whatever current imaging modalities or diagnostic technologies. We expect new and sensational accurate imaging modality which can reflect tumor histology and lymph nodes micrometastasis.
Our results showed that there were also significant differences in age and tumor size between unexpected and expected groups, and younger age, left-sided primary tumor, and larger tumor size were significantly associated with unexpected diseases in multivariate analysis. Zhang et al.20) suggested that larger tumor size and younger age at diagnosis were both predictive factors for N2 disease in CT-defined T1N0 NSCLC. In addition, Chen et al.21) demonstrated development and validation of a clinical prediction model for N2 lymph node metastasis in NSCLC, and they identified that younger age was one of the independent predictive factors for unexpected mediastinal lymph node metastasis as well as larger tumor size, central tumor location, and adenocarcinoma pathology. These results of their studies were consistent in principle with our study. On the other hand, it is arguable whether tumor laterality could be a predictor of unexpected lymph node metastasis or not. In this study, the left-sided primary tumor was one of the significant factors of unexpected diseases, whereas some studies suggested that there was no correlation between tumor laterality and unexpected lymph node involvement.7,21) Larger cohort studies are needed to clarify this issue. Although there was no description of the relationship between detailed histological subtypes of primary tumor and age at diagnosis in these studies, there might be some associations between younger age and having a micropapillary component. At any rate, we need to pay attention to the possibility of unexpected mediastinal lymph node metastasis, especially in younger adenocarcinoma patients.
There were several limitations in our study. First, the retrospective nature of this study might have introduced biased information. Second, this study was performed using patients’ data at a single institution, and the sample size was small and insufficient to reach a definitive conclusion. Third, there were some histological diagnostic biases because we evaluated the tissue samples pathologically only for the maximal cut surface, not for several cut surfaces, and all cases were diagnosed only by a single institutional pathologist.
In conclusion, we demonstrated clinicopathological characteristics of patients with unexpected pN1 and pN2 lung adenocarcinoma and to analyzed associated factors of unexpected lymph node metastasis. Having a micropapillary component was one of the significant associated factors of unexpected pN1 and pN2 diseases. We should warrant attention to histological findings of the primary tumor because they can somewhat provide predictive information for lymph nodes status even if there is no FDG uptake in regional lymph nodes on preoperative PET/CT. It is hoped that new modalities which can reflect primary tumor subtypes and lymph node metastasis more accurately will be developed.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1).Gupta NC, Tamim WJ, Graeber GG, et al. Mediastinal lymph node sampling following positron emission tomography with fluorodeoxyglucose imaging in lung cancer staging. Chest 2001; 120: 521-7. [DOI] [PubMed] [Google Scholar]
- 2).Schrevens L, Lorent N, Dooms C, et al. The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist 2004; 9: 633-43. [DOI] [PubMed] [Google Scholar]
- 3).Takamochi K, Yoshida J, Murakami K, et al. Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer 2005; 47: 235-42. [DOI] [PubMed] [Google Scholar]
- 4).Nomori H, Watanabe K, Ohtsuka T, et al. The size of metastatic foci and lymph nodes yielding false-negative and false-positive lymph node staging with positron emission tomography in patients with lung cancer. J Thorac Cardiovasc Surg 2004; 127: 1087-92. [DOI] [PubMed] [Google Scholar]
- 5).Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg 2008; 33: 104-9. [DOI] [PubMed] [Google Scholar]
- 6).Gomez-Caro A, Garcia S, Reguart N, et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur J Cardiothorac Surg 2010; 37: 1168-74. [DOI] [PubMed] [Google Scholar]
- 7).Gomez-Caro A, Boada M, Cabanas M, et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in cI stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012; 42: 93-100; discussion 100. [DOI] [PubMed] [Google Scholar]
- 8).Ozawa Y, Hara M, Sakurai K, et al. Diagnostic accuracy of (18)F-2-deoxy-fluoro-D-glucose positron emission tomography for pN2 lymph nodes in patients with lung cancer. Acta Radiol 2010; 51: 150-5. [DOI] [PubMed] [Google Scholar]
- 9).Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/ American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol 2007; 20: 638-47. [DOI] [PubMed] [Google Scholar]
- 11).Kim HK, Choi YS, Kim J, et al. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010; 140: 1288-93. [DOI] [PubMed] [Google Scholar]
- 12).Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol 2002; 26: 358-64. [DOI] [PubMed] [Google Scholar]
- 13).Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003; 27: 101-9. [DOI] [PubMed] [Google Scholar]
- 14).Hoshi R, Tsuzuku M, Horai T, et al. Micropapillary clusters in early-stage lung adenocarcinomas: a distinct cytologic sign of significantly poor prognosis. Cancer 2004; 102: 81-6. [DOI] [PubMed] [Google Scholar]
- 15).Makimoto Y, Nabeshima K, Iwasaki H, et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (</=20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi’s type C tumours). Histopathology 2005; 46: 677-84. [DOI] [PubMed] [Google Scholar]
- 16).Kawakami T, Nabeshima K, Makimoto Y, et al. Micropapillary pattern and grade of stromal invasion in pT1 adenocarcinoma of the lung: usefulness as prognostic factors. Mod Pathol 2007; 20: 514-21. [DOI] [PubMed] [Google Scholar]
- 17).Roh MS, Lee JI, Choi PJ, et al. Relationship between micropapillary component and micrometastasis in the regional lymph nodes of patients with stage I lung adenocarcinoma. Histopathology 2004; 45: 580-6. [DOI] [PubMed] [Google Scholar]
- 18).Ohno Y, Koyama H, Yoshikawa T, et al. N stage disease in patients with non-small cell lung cancer: efficacy of quantitative and qualitative assessment with STIR turbo spin-echo imaging, diffusion-weighted MR imaging, and fluorodeoxyglucose PET/CT. Radiology 2011; 261: 605-15. [DOI] [PubMed] [Google Scholar]
- 19).Inoue M, Hiyama K, Nakabayashi K, et al. An accurate and rapid detection of lymph node metastasis in non-small cell lung cancer patients based on one-step nucleic acid amplification assay. Lung Cancer 2012; 78: 212-8. [DOI] [PubMed] [Google Scholar]
- 20).Zhang Y, Sun Y, Xiang J, et al. A prediction model for N2 disease in T1 non-small cell lung cancer. J Thorac Cardiovasc Surg 2012; 144: 1360-4. [DOI] [PubMed] [Google Scholar]
- 21).Chen K, Yang F, Jiang G, et al. Development and validation of a clinical prediction model for N2 lymph node metastasis in non-small cell lung cancer. Ann Thorac Surg 2013; 96: 1761-8. [DOI] [PubMed] [Google Scholar]