Abstract
In recent years, the use of mammalian target of rapamycin inhibitors has gained traction in their use as alternative or adjunct immunosuppressants in the post-liver transplantation (LT) setting. The efficacy of everolimus (EVR) in de novo LT is established and a reasonable time to initiate EVR is 30 d from LT surgery. Initiating EVR early post-LT allows for calcineurin inhibitor (CNI) reduction, thus reducing nephrotoxicity in LT recipients. However, data is inadequate on the appropriate timing for conversion from CNI to EVR maintenance in order to achieve optimal renoprotective effect without compromising drug efficacy. Adverse effects of proteinuria, hypercholesterolemia and hyperlipidemia are significantly higher as compared to standard CNI and long-term implications on graft and patient survival in LT is still unclear. Future research to explore strategies to minimise EVR adverse effects will be crucial for the success of EVR as an important alternative or adjunct immunosuppressive therapy in LT.
Keywords: Everolimus, Mammalian target of rapamycin inhibitor, Immunosuppression, Liver transplantation, Nephrotoxicity
Core tip: Everolimus is the most recently approved immunosuppressant for use in liver transplantation (LT). Its renoprotective effect is an attractive option for LT recipients who have calcineurin inhibitor-induced nephrotoxicity. This review examines through data published, discovers gaps of evidences and discusses the place in therapy for everolimus (EVR) in LT. At the end of review, it summarises how EVR can benefit LT recipients as well as the caveat in using EVR.
INTRODUCTION
Since the first liver transplantation (LT) surgery in 1963, surgical techniques and immunosuppression therapy have evolved much and improved patient outcomes. Based on Organ Procurement Transplantation Network/Scientific Registry of Transplant Recipients (OPTN/SRTR) data in 2013, the 5-year graft survival rate in LT is as high as 76%[1]. In most transplant centers, LT immunosuppressive regimes include calcineurin inhibitors (CNI), antimetabolites, steroid with or without induction therapy[2]. For the past few decades, CNIs have been the cornerstone of immunosuppressant regimens for LT recipients. The overall patient survival at 1-, 5- and 10-years for LT with tacrolimus (FK) were in range of 81%-84%, 70%-72% and 57%-68% respectively[3,4]. Nonetheless, CNIs, both FK and cyclosporine (CsA), increase the risk of nephrotoxicity, diabetes, hypertension and neurotoxicity[2]. Ojo et al[5] reported as high as 18% of LT recipients developed renal impairment within 5 years post-LT. Therefore much research has been focused on finding strategies or alternatives to avoid or minimize nephrotoxicity in the past 10 years and one of the more recent drug classes to be used are the mammalian target of rapamycin (mTOR) inhibitors [sirolimus, everolimus (EVR)].
EVR was approved for the prevention of graft rejection in LT when used in combination with both FK and steroid in Europe (October 2012) and in the United States (February 2013).
PHARMACOLOGICAL PROPERTIES OF EVR
EVR is an mTOR inhibitor and has antiproliferative properties. It reduces protein synthesis and cell proliferation by binding to FK binding proten-12 to form a complex that inhibits activation of the mTOR serine threonine kinase activity (Figure 1). It also has antiangiogenic effects by inhibiting expression of hypoxia inducible factor and vascular endothelial growth factor. In addition, mTOR may have additional importance in neuroendocrine cells and EVR has been shown to block the action of IGF-1 in neuroendocrine cells[6].
Figure 1.
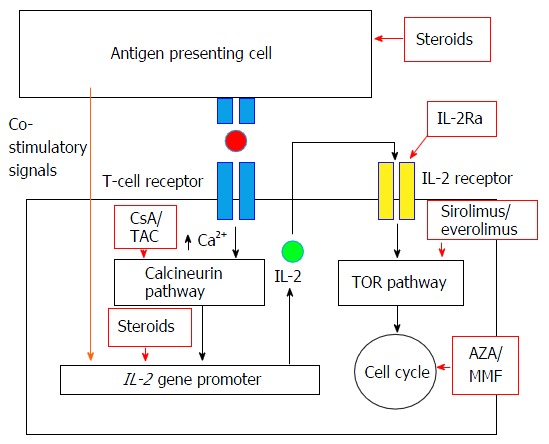
Mechanism of action of efficacy of everolimus and other immunosuppressants in solid organ transplantation (permission from Moini et al[2], World J Hepatol 2015). AZA: Azathioprine; CsA: Cyclosporine; IL-2: Interleukin-2; IL-2Ra: Interleukin-2 receptor antagonist; MMF: Mycophenolate mofetil; TAC: Tacrolimus; TOR: Target of rapamycin.
EVR is a derivative of sirolimus, differing by one extra hydroxyethyl group at position 40 (Figure 2). Based on pharmacokinetics data, its absorption is rapid and bioavailability is variable, about 16%-20% (higher than sirolimus’ 10%-14%)[7,8]. EVR requires twice daily dosing as its elimination half-life is 32 h, which is shorter than sirolimus’ half-life of 62 h. Therefore, no loading dose is required for EVR and steady state can be achieved faster, in 4 d, vs 6 d for sirolimus. EVR is extensively metabolised in the liver via cytochrome P450-3A4 (CYP3A4) and has 6 wk metabolites. Similar to sirolimus, it is a substrate of p-glycoprotein (PgP) and CYP3A4 pathways. It interacts with strong and moderate inhibitors, inducers and substrates of CYP3A4 and PgP at different intensities[9,10]. CsA increases the maximum concentration of EVR by 82%, EVR however does not influence trough level nor drug exposure (area under the curve, AUC) of CsA[8]. EVR is excreted mainly (80%) via feces and only 5% in urine[7,8]. There is no dose adjustment required in renal impairment but dose reduction is recommended for moderate and severe liver impairment. As EVR has a narrow therapeutic index and immunogenicity varies post LT, therapeutic monitoring is essential for dose titration and monitoring. The EVR trough level (C0) correlates well (correlation coefficient of 0.86-0.94) with drug exposure, i.e., AUC, and has been recommended as the standard for EVR monitoring[7,11].
Figure 2.
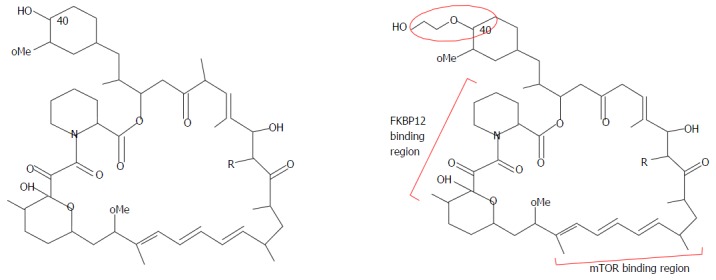
Molecular structure of sirolimus and everolimus.
Key studies on the use of EVR in LT (Tables 1 and 2)
Table 1.
Outcomes of everolimus-based immunosuppressant for de-novo liver transplantation recipients in prospective randomised controlled trial
| Ref. | Treatment group | Time (d) from transplant EVR was initiated | Key inclusion and exclusion criteria | n | Follow-up period (mo) | Efficacy | Mean improvement in eGFR (mL/min per 1.73 m2) | Safety |
| Fischer et al[13] 2012 (PROTECT Study) | EVR + eliminate CNI by month 4 (EVR C0 5-12 ng/mL, if with CsA, EVR C0 8-12 ng/mL) | from day 30 and by day 56 | Inclusion: No rejection 2 wk before study, renal function > 50 mL/min | 101 | 12 | BPAR, graft loss or death: 20.8% vs 20.4% (P = 1.0) | 7.8 (P = 0.021) | No HAT, no increased risk of delayed wound healing. Higher incidence of infections, leukopenia, hyperlipidemia, anemia, proteinuria and arterial hypertension in the EVR group |
| Control: FK or CsA | Exclusion: Severe systemic infections, total cholesterol≥ 9 mmo/L, TG > 8.5 mmol/L, significant renal dysfunction (eGFR < 50 mL/min) | 102 | ||||||
| Sterneck et al[14] 2014 (PROTECT Study, extended to 36 mo) | Same as above | From day 30 and by day 56 | 41 | 36 | BPAR, graft loss and death: 19.5% vs 2.5% (P = 0.029) at month 11 (baseline) | 9.4 (P = 0.053) | Peripheral edema and back pain were significantly higher in EVR group | |
| 40 | BPAR, graft loss and death: 4.9% vs 5.0% (P = 1.0) at month 36 | |||||||
| Sterneck et al[15] 2016 (PROTECT Study, extended to 59 mo) | Same as above | From day 30 and by day 56 | 41 | 59 | BPAR, graft loss and death: 9.8% vs 7.5% (P = 1.0) from month 11 to month 59 | 11.4 (P = 0.021) | Peripheral edema and back pain were significantly higher in EVR group | |
| 40 | ||||||||
| De Simone et al[16] 2012 (H2304 Study) | EVR + low FK (EVR C0 3-8 ng/mL and FK C0 3-5 ng/mL) | Day 30 | Inclusion: eGFR ≥ 30 mL/min, FK trough ≥ 8 ng/mL. | 245 | 12 | BPAR, graft loss or death: 6.5% in EVR group vs 9.5% in control group (P < 0.001) | 8.5 (P < 0.001) | Higher incidence of proteinuria, acute renal failure, hyperlipidemia, neutropenia, peripheral edema, stomatitis/mouth ulceration, and thrombocytopenia in the EVR group |
| FK elimination (EVR C0 3-8 ng/mL till month 4 then 6-10 ng/mL thereafter and FK elimination started at month 4 when EVR C0 6-10 ng/mL achieved | Patent hepatic artery and veins, absence of rejection | 231 | ||||||
| Control: FK (C0 8-12 ng/mL until month 4 and C0 6-10 ng/mL thereafter) | Exclusion: HCC not fulfill Milan criteria, receipt of antibody induction therapy proteinuria ≥ 1 g/24 h | 243 | ||||||
| Saliba et al[17] 2013 (H2304 Study, extended to 24 mo) | EVR + low FK (EVR C0 3-8 ng/mL and FK C0 3-5 ng/mL) | Day 30 | 245 | 24 | BPAR, graft loss or death: 10.3% in EVR group vs 12.5% in control group (P = 0.452) | 6.7 (P = 0.002) | No increased risk of wound healing. Higher incidence of proteinuria, acute renal failure, hyperlipidemia, neutropenia, peripheral edema, stomatitis/mouth ulceration, and thrombocytopenia in the EVR group | |
| 243 | ||||||||
| Fischer et al[18] 2015 (H2304 Study, extended to 36 mo) | Same as above | Day 30 | 106 | 36 | BPAR, graft loss and death: 11.5% vs 14.6% (P = 0.334) | 8.5 (P = 0.005) | Higher drop-out rate due to ADR and incidence of hyperlipidemia in EVR group | |
| 125 |
ADR: Adverse drug reaction; BPAR: Biopsy proven acute rejection; C0: Trough level; CNI: Calcineurin inhibitor; CsA: Cyclosporine; EVR: Everolimus; FK: Tacrolimus; eGFR: Based on Modification of Diet in Renal Disease (MDRD) 4.
Table 2.
Outcomes of everolimus-based immunosuppressant as maintenance for lt recipients in prospective RCT
| Ref. | Treatment group | Time (mo) from transplant surgery EVR was initiated | Key inclusion and exclusion criteria | n | Follow-up period (mo) | Efficacy | Mean improvement in CrCl (mL/min) | Safety |
| De Simone et al[19] 2009 (RESCUE Study) | EVR with CNI reduction or elimination (EVR C0 3-8 ng/mL, FK C0 3-5 ng/mL or EVR C0 6-12 ng/mL with FK elimination | 12 to 60 mo | Inclusion: CrCl ≤ 60 mL/min and ≥ 20 mL/min Exclusion: Renal dysfunction not due to CNI toxicity, proteinuria ≥ 1 g/24 h, acute rejection < 6 mo, hepatitis C infection need active antiviral therapy | 72 | 12 | BPAR, graft loss or death: 8.3% in EVR group vs 4.1% in control group | -1.1 (P = 0.463) at month 6 | Higher incidence of hyperlipidemia, mouth ulceration, increased hepatitis C virus viral titer, dry skin, eczema, and rash in the EVR group |
| Control: Standard exposure of FK or CsA | 73 |
BPAR: Biopsy proven acute rejection; C0: Trough level; CNI: Calcineurin inhibitor; CrCl: Creatinine clearance (based on Cockcroft-Gault formula); CsA: Cyclosporine; EVR: Everolimus; FK: Tacrolimus.
Several studies, both prospective and retrospective, on EVR in LT have been reported. In a phase II study, Levy et al[12] compared different dosing regimen of EVR (0.5 mg BD, 1 mg BD and 2 mg BD) to placebo. The study concluded that EVR in combination with CsA could be a safe and tolerable alternative in LT, despite the increased incidence of adverse effects. There are 3 main phase III studies in the use of EVR in LT, i.e., PROTECT, H2304 and RESCUE studies (Table 1). PROTECT, an open-label multi-center prospective randomised controlled trial (RCT) recruited 203 patients randomised to EVR plus withdrawal of CNI by month 4 post-LT vs continued standard CNI till month 11[13]. Steroid was optional in either group. The study concluded significant improvement in renal function (estimated glomerular filtration rate, i.e., eGFR improved by 7.8 mL/min) in the group with EVR, despite similar mortality rates, biopsy-proven rejection (BPAR) rates and efficacy failure rates between both groups. However, it also reported a significantly higher incidence of adverse effects mainly oral herpes, leukopenia, hypercholesterolemia, hyperlipidemia and proteinuria in the EVR-treated group as compared to standard CNI. In its subsequent study, 81 patients were further followed-up till 3 years. A significant difference in renal function continued to be seen between the EVR with CNI-withdrawal vs the control group at month 35 from randomization, mainly due to the progressive deterioration of renal function in the standard CNI group[14]. Recently, the 5-year follow-up on these same 81 patients has been published, reporting a continued improved trend in renal function in the EVR-treated group (eGFR improved by 11.4 mL/min, P = 0.021) with comparable treatment failure rates (9.8% in EVR group vs 7.5% in standard CNI group, P = 1.000) in both groups[15].
In another open-label multi-center prospective RCT, H2304, 719 patients were randomised to receive EVR (EVR C0 3-8 ng/mL) with reduced FK dosing (FK C0 3-5 ng/mL) (n = 245) or control standard FK dosing (FK C0 8-12 ng/mL till month 4 then C0 6-10 ng/mL thereafter) (n = 243) or FK elimination (EVR C0 3-8 ng/mL till month 4 then 6-10 ng/mL thereafter, FK elimination from month 4 when EVR C0 6-10 ng/mL achieved ) (n = 231) at 1 mo post liver transplant[16]. Steroid was initiated at time of transplant up till at least 6 mo from transplant while MMF was discontinued at the time of randomization. Recruitment to FK elimination group was terminated prematurely due to higher (19.5%) treated BPAR (tBPAR) episodes as compared to 6.5% and 9.5% of tBPAR in the EVR with reduced FK and control group, which clustered around the time of FK elimination at 4 mo post-randomization. At the end of both the first and second year, subjects in the EVR with reduced FK group had improved renal function significantly with comparable primary efficacy (tBPAR, graft loss and death) but a higher incidence of adverse effects (mainly hyperlipidemia, neutropenia, peripheral edema and stomatitis/mouth ulceration) than controls[16,17]. At the end of the third year, improvement in renal function was consistently significant in EVR with reduced FK group (n = 106) with comparable tBPAR rates and adverse effects as compared to the standard FK group (n = 125)[18].
The third Phase III study of interest, RESCUE, provides evidence for converting to EVR 1 year post-LT (Table 2)[19]. In this 6 mo open-label multi-center prospective RCT, 154 patient were followed-up for 12 mo. The studied group, EVR with CNI reduction or elimination was compared to standard CNI with or without MMF, azathioprine or steroid in both groups. While all concurrent immunosuppressants were kept the same in control group, MMF was discontinued at day 1 in the EVR group. Despite no graft loss, BPAR in EVR group at month 12 (4.2%) were higher than standard CNI group (1.4%). Furthermore, the improvement of renal function in the studied group was not statistically significant at 12-mo follow up.
EFFICACY OF EVR IN LT
De novo therapy
In the PROTECT study, the efficacy of EVR in LT was still doubtful with conflicting results. Initial results reported comparable composite BPAR, graft loss and death rates in the EVR-treated (20.8%) and the control (20.4%) group, up to month 11 of follow-up[13]. A similar comparable trend for its composite end-points in the extension study (from month 11 to month 35) results were reported at the end of 35 mo, despite a difference at baseline between both groups[14]. Treatment efficacy with EVR was difficult to analyse due to high discontinuation rates of drugs used in both groups due to adverse drug reactions (49.5% in EVR group and 38.2% in control CNI group). The discontinuation of CNI by end of month 4 could have compromised efficacy of immunosuppressive therapy. This similar finding was reported in the H2304 Study where efficacy failure (BPAR) in the FK elimination group was significantly higher (19.9%) as compared to control group (10.7%), P = 0.005. Hence, EVR monotherapy is not recommended in LT and EVR should instead be used in combination with CNI. EVR efficacy in de novo LT, and hence, United States FDA and Europe EMEA approval is based on results from De Simone’s landmark H2304 study (Table 1)[16]. The reported outcomes of BAPR, graft loss and death in the treatment group were non-inferior to the control group on FK alone. In the post-hoc analysis for H2304 study, incidence of tBPAR was lower in those aged < 60 years and hepatitis C virus (HCV)-negative[20].
Based on phase III studies (Table 1), EVR is approved for use 30 d from LT. However, there is emerging data on the safety and efficacy of EVR initiation within 30 d from LT. Masetti et al[21]’s prospective, single-center randomized trial described early initiation of EVR at day 10 from LT in 52 patients (EVR C0 6-10 ng/mL till day 30, then C0 8-12 ng/mL (when CsA was discontinued from day 30) till month 6 and then C0 6-10 ng/mL thereafter) vs standard CsA in 26 patients (CsA C0 225 ± 25 ng/mL till day 30, 200 ± 25 ng/mL till month 6 and 150 ± 25 ng/mL thereafter)[21]. There was no difference in BPAR nor patient survival rates in both groups. The study concluded that early withdrawal of CsA and early EVR use in de novo LT recipients significantly improved renal function (eGFR 87.7 mL/min in EVR group vs 59.9 mL/min in standard CsA group) and reduced incidence of chronic kidney disease (CKD) stage ≥ 3 (15.4% in EVR group vs 52.2% in CsA group, P = 0.005) at 1 year post-LT.
In a single-center prospective cohort study, safety of EVR use in the early post-LT was evaluated in 43 living donor LT recipients[22]. All patients received basiliximab, steroid, FK and MMF as immunosuppressive therapy where steroid was discontinued after 2 wk from transplant and FK was maintained at C0 of 8-10 ng/mL. EVR was introduced from low dose of 0.25 mg BD and titrated to 0.5 mg BD to achieve C0 of 3-5 ng/mL while FK was kept at C0 of 6-8 ng/mL. Mean time for EVR initiation was 12 ± 8 d (range: 4-20 d) from transplant where 33 patients were initiated within the 1st week, 9 patients within the 2nd week and 1 patient on day 20. EVR was continued for an average of 97 d (range: 26-190 d) from transplant. The mean follow up was 9 ± 6 mo (range: 3-15 mo) till discontinuation of EVR or death. No acute rejection episodes were reported.
In a retrospective study, Gastaca et al[23] reported 92.7% patient survival rates at 1 year post-LT for 28 patients who had EVR initiated early post-LT (median 14 d) where 85.7% was in combination with MMF or enteric-coated mycophenolate sodium and steroid. Nonetheless, more concrete data is warranted for EVR initiation within 30 d from LT.
Maintenance therapy
The efficacy data for EVR as maintenance immunosuppression in LT is sparse; with only one RCT to date (Table 2). De Simone et al[19]’s RCT reported results of conversion from CNI-based to EVR-based maintenance immunosuppression after 12 mo and up till 60 mo post-LT. Although the composite endpoint of BPAR, graft loss and death was low overall in both groups, it was double in the EVR group (8.3%) as compared to control group (4.1%)[19].
In another prospective cohort study by De Simone et al[24], 40 patients were converted to EVR at mean of 45.5 ± 31.2 mo from transplant and CNI was tapered by 50% every week and withdrawn over 4 wk with or without MMF or azathioprine and steroid. Concurrent MMF or azathioprine was discontinued at day 1 of conversion while steroid was remained unchanged in the EVR group. Indications for conversion to EVR included deterioration of renal function (90.0%), CNI-associated peripheral neuropathy (7.5%) and CNI-associated microangiopathy (2.5%). Despite a 100% patient and graft survival rate at 12 mo post-conversion, the incidence of BPAR was 15% and 4 of the patients (10%) had to be switched back to CNI for this reason.
Castroagudín et al[25] analysed impact on renal function post conversion to EVR at mean 62.4 ± 36.6 mo from LT in 21 patients with CKD. Twenty patients (95%) were able to have CNI completely withdrawn. From a baseline eGFR of 42.1 ± 8.7 mL/min, renal function improved to eGFR 49.8 ± 10.3 mL/min at the end of 360 d from conversion.
In a retrospective study, Saliba et al[26] described 240 patients who were successfully converted to EVR at median of 3 years from transplant with a low overall rejection rate (1.6%). At 12 mo post conversion, 61% of patients had CNI discontinued. Mean EVR C0 was 7.3 ng/mL and 8.1 ng/mL at month 1 and 12 post conversion respectively while mean EVR C0 was higher (8.8 ng/mL) in 40 patients who were kept on EVR monotherapy at month 12. Immunosuppression therapy was in combination with or without MMF and steroid in both groups. Renal function was markedly improved in patients who were converted within the first year from transplant (n = 68) as compared to conversion after 1 year from transplant (n = 172), calculated creatinine clearance 12.5 mL/min vs 5.5 mL/min based on Cockcroft-Gault formula.
In another retrospective study, 477 patients were recruited and 157 (33%) were converted to EVR for indication of renal dysfunction at median of 24 mo[27]. Significant improvement of renal function was observed in patients who were converted to EVR within 1 year from transplant but not in patients who were converted after 5 years from transplant. Of note, in patients who were converted in between 1-5 years from transplant, the improvement in renal function was only appreciable at month 3 and 6 but did not persist at 12 mo post conversion. Overall graft rejection rate was 5.9% which mostly occurred at 3 and 6 mo post conversion.
VALUE OF EVR IN LT
Renoprotective effect
Long-term renoprotective benefits of EVR in LT have been demonstrated in the H2304 study[16-18]. Based on the results, De Simone et al[16] showed that EVR with reduced FK dose is as efficacious as the FK standard regimen in the control group and improved patients’ eGFR by 8.5 mL/min at month 12. The improvement trend in eGFR continued to be seen at month 24 (eGFR improved by 6.7 mL/min) and at month 36 (eGFR improved by 8.5 mL/min) of follow-up (Table 1). However, it can also be argued that the H2304 study had unintentionally recruited a majority of patients (72.3%) with better baseline renal functions of eGFR ≥ 60 mL/min, with mean baseline of 80.8 mL/min in EVR group and 78.9 mL/min in control group. Similar high baseline eGFR (78.0 mL/min and 74.9 mL/min in EVR and control groups respectively) were also seen in the PROTECT study. In the RESCUE study, baseline eGFR was 51.0 mL/min and 50.3 mL/min in EVR and control groups. Clearly, results in these studies should not be generalised to LT recipients with eGFR < 50 mL/min, where similar benefits might be doubtful. This was reaffirmed with the H2304 post-hoc analysis which showed that renal improvement was not observed in patients with eGFR of 30 to < 55 mL/min[20]. This analysis suggested that EVR renoprotective effect was observed particularly in patients aged < 60 years, female gender, HCV-negative and in those with baseline eGFR of 55 to < 70 mL/min.
The FK dose in the control group of the H2304 study was maintained at target C0 of 8-12 ng/mL until month 4 and then tapered to target C0 of 6–10 ng/mL for the remainder of the study. The FK C0 in EVR group was targeted to be 3-5 ng/mL from 1 mo post-LT, though majority of patients maintained levels slightly above 5 ng/mL from month 3 onwards till month 12 of study period. Hence, the addition of EVR early post-LT allowed tapering of FK safely without an increased risk of rejection. The decrease in renal impairment was possibly contributed by the reduced CNI level. It has been proven that CNI minimization strategy improves renal function in LT recipients[28-30].
Before EVR was started in the H2304 study, majority (70%) of patients were also on mycophenolate mofetil (MMF) which was discontinued according to protocol. It would seem logical in clinical practice to have another non-CNI immunosuppressant, in combination with reduced CNI doses. In fact, the combination of MMF with reduced CNI has been a strategy which many clinicians adopt to minimize CNI nephrotoxicity[29,30]. A case-control study described 20 patients on de novo EVR plus MMF and steroids without CNI in comparison to 31 controls of FK plus MMF and steroids[31]. The eGFR in both groups were not statistically different at the end of 1- and 2-years follow-up but a 35% of rejection rate at 2 years from LT in EVR group was reported and attributed to difficulty achieving target drug levels. There is no head-to-head RCT comparing EVR-based and MMF-based LT immunosuppression regimes to date.
On the other hand, the evidence for EVR renoprotective effect in conversion after 6 mo post-LT is lacking. The RESCUE Study which showed an increased composite outcome of BPAR, graft loss and death demonstrated an improvement of eGFR by only 1.1 mL/min at the end of 1 year. In this study, the conversion to EVR occurred at mean 3.3 ± 1.7 years from transplant. In a retrospective observational study, Saliba et al[26] found the improvement in renal function to be greater when conversion to EVR was within first year post-LT (eGFR increased from 77.5 to 90.0 mL/min, P = 0.04) vs those who were converted beyond 1 year post-LT (eGFR increased from 59.1 to 64.6 mL/min, P = 0.01)[26]. The findings Castroagudín et al[25] reported in a retrospective study echoed the less remarkable renal improvement (eGFR improved by 7.7 mL/min at month 12) when conversion to EVR occurred at 5.2 ± 3.1 years from transplant.
Thus, the best time point for conversion to EVR for optimal renoprotective effect is still unclear and further studies are warranted. Although it would appear, from current available data, that earlier conversion (within 12 mo post-LT) is better than late conversion (beyond 3 years post-LT); and that renal protective effects are more prominent with mild renal impairment (eGFR > 60 mL/min) rather than with moderate-severe renal impairment (eGFR < 55 mL/min).
Alternative for CNI-induced neurotoxicity
Bilbao et al[32] reported a retrospective analysis on the use of EVR in 10 patients who experienced FK-neurotoxicity requiring the discontinuation of FK in the first 3 mo post-LT. Seven of the patients were converted to everolimus in the first month post-LT and the remaining 3 were converted in the second or third month.
However, within 80 d post conversion, graft rejection occurred in 4 of the 10 patients, all of whom were on triple immunosuppression (i.e., EVR plus MMF plus steroids) at the time of graft rejection. All 4 patients subsequently had CNI (3 with FK, 1 with CsA) re-introduced, without recurrence of neurotoxicity. The findings suggest EVR use enables a temporary withdrawal of CNI in managing CNI-induced neurotoxicity. Re-introduction of CNI may be prudent after resolution of neurotoxicity in view of high rejection rates, especially within first 3 mo post-transplant, when EVR is not used in combination with CNI. Furthermore, in patients with acute rejection, the introduction of CsA or re-introduction of FK may be possible because the risk of further neurologic complications may be low.
Prevention of hepatocellular carcinoma recurrence
EVR has proven efficacy against breast cancer, renal cell carcinoma, neuroendocrine tumours and subependymal giant cell astrocytoma[33]. There is no data on de novo hepatocellular carcinoma (HCC). The HCC recurrence rate post-LT is 8%-20%, with most occurring within the first 2 years post-LT[34,35]. There is no RCT on EVR for the prevention of HCC recurrence. In Jeng et al[22]’s single-centre prospective non-randomised study, HCC recurred in 7% of the patients using EVR. In retrospective studies, it has been observed that EVR has no HCC recurrence post-LT during a mean follow-up of 11.2 ± 6.8 mo in 44 patients and 48 mo (range: 11-76 mo) in 21 patients respectively[26,36]. In a systematic review, LT recipients who were on mTOR inhibitors (sirolimus or EVR) had lower HCC recurrence rates[37]. However, the follow up period varied widely among the groups on CNI (42 mo), sirolimus (30 mo) and EVR (19 mo). No mortality data was presented in this review.
Hepatitis C and liver fibrosis
In an open-label multi-center randomised study, conversion to EVR delayed histological fibrosis progression in 43 LT recipients with HCV recurrence as compared to FK-based immunosuppressive therapy[38]. However, this potential benefit was not observed in the extended H2304 study, where no significant difference in histological fibrosis scores between the EVR and control groups was reported at the end of 3 years of follow-up[16]. Hence, more studies are warranted to confirm EVR benefit in delaying liver fibrosis progression of hepatitis C.
ADVERSE EFFECTS OF EVR
The most common adverse effects of EVR use in LT recipients are infections (50.6%), hyperlipidaemia (23.7%), hypertension (18.0%), peripheral oedema (17.6%), leukopenia (14.3%), and wound healing impairment (11.0%)[8,9]. In a phase II study, the incidence of adverse effects was higher in patients with higher daily EVR doses, especially > 4 mg/d[11]. In de novo LT, the discontinuation of EVR due to adverse effects was higher in the EVR group, 25.7%, which was nearly double of the control (14.1%) in this study[16]. The common adverse effects that led to EVR discontinuation were proteinuria, delayed wound healing, pancytopenia, leukopenia and thrombocytopenia.
In maintenance therapy, 22% of patients discontinued EVR due to adverse effects while no patients in the control group discontinued study medication[18]. The adverse effects that led to EVR discontinuation included leukopenia, proteinuria, thrombotic microangiopathy, elevation in hepatic enzymes, increased HCV viral load, hypertriglyceridemia, renal impairment, interstitial lung disease, pneumonitis, pulmonary fibrosis and stomatitis. In a study on 94 patients converting to EVR at mean of 5 years from transplant, as many as 70% of patients experienced adverse reaction and 16% required EVR to be discontinued despite mean EVR C0 level being at only 6 ng/mL[39].
Hepatic artery thrombosis
In February 2013, the United States Food and Drug Administration included a warning of hepatic artery thrombosis (HAT) in the EVR product insert. Most of the reported cases of HAT in the presence of mTOR inhibition occurred within the first 30 d from transplant surgery, leading to graft loss or death. Therefore, in most EVR studies, randomization was started only 30 d after transplant surgery.
One case of HAT was reported in the H2304 study, occurring in a subject who had a prior history of HAT before randomization. There were no reports of HAT in the PROTECT nor RESCUE studies. In the RESCUE study, although an adverse effect of thrombotic microangiopathy was reported, there were no specific details of its incidence nor eventual outcomes. Combination of EVR and CNI has also been reported to increase the risk of thrombotic microangiopathy elsewhere[40].
In Masetti et al[21]’s study on the early use of EVR within the first 30 d of LT, no HAT was reported with EVR use. This is in contrast to the control CsA group which had 2 (7.6%) patients with HAT and 2 (7.6%) patient with hepatic artery stenosis. Although there was a significant higher rate of hepatic stenosis and thrombosis in CsA group, it is important to note number of patients in CsA group (n = 26) was just half of patients in EVR group (n = 52).
In another prospective cohort study, no HAT was reported with EVR use in 43 patients in the early (33 patients within week 1, 9 patients within week 2 and 1 at day 20 from transplant) post-LT period[22]. Similarly, in a retrospective study, no HAT was observed in 28 patients when EVR was initiated at median of 14 (range 4-24) d[23].
Impaired wound healing
Wound healing is an important care issue for post-transplant surgery. Sirolimus has as high as 36% incidence rate of impaired wound healing[41]. Furthermore, it was reported that mTOR inhibitor is an independent risk factor for incisional hernia in LT[42]. Impairment of wound healing between EVR and control group was presented in the PROTECT study (3.0% vs 4.9% at 11 mo) and H2304 study (11.0% vs 7.9% at 1 year, RR = 1.40, 95%CI: 0.80, 2.45; 11.0% vs 8.3% at 2 year, P = 0.36)[13,16,17]. Both studies also reported an increase of incisional hernia with EVR exposure, although the difference did not reach statistical significance in either study[14,17]. Similarly, Masetti et al[21] reported a non-significant increase in incisional hernia in the EVR group. The findings in these studies were similar to the not statistically significant increase of wound healing impairment in renal transplant patients using EVR as compared to standard CNI reported by Nashan et al[43].
Generally, impaired wound healing rates with EVR use ranges from 11%-35%[41]. Despite the lack of statistical significance, further analyses or studies to guide the optimal time for initiation of EVR in LT are warranted, especially when EVR is used in combination with other immunosuppressants that may delay healing process as well.
Infection
The risk of infection is of concern in post-transplant care and the higher incidence of infection with EVR should not be overlooked. In the H2304 study, the overall incidence of any infection at 1 year was not statistically significant[16]. However, there was an increase for any serious infection (13.9% in EVR group vs 7.9% in control, RR = 1.76, 95%CI: 1.03, 3.00) which included pneumonia and hepatitis C. The overall incidence of any infection was also comparable between EVR and control groups in the 2-year and 3-year follow up (56.3% vs 51.7% and 70.8% vs 64.0% respectively) period, without a significant difference in the rates of serious infections.
In the PROTECT study, Kaplan-Meier survival plot showed the occurrence of any infection was higher in the EVR group as compared to standard CNI group (79.5% vs 68.3%, P = 0.050) at 11 mo from randomization particularly oral herpes, sinusitis and wound infection[13]. In the RESCUE study, 31.9% of patients in EVR group vs 21.9% in standard CNI group experienced infections which included stomatitis, herpes simplex, bronchitis and urinary tract infections[19]. Of note, it also reported a significant increase in HCV viral load in their EVR group (6.9%, P = 0.028) as compared to none in the control group. Although statistical difference was unknown, the authors also reported 15.3% (EVR group) in contrast to 1.4% (standard CNI group) of infections being related to studied drug.
Incidence of infection was the same in both EVR and control groups, 46.2% in Masetti’s study[21]. In a retrospective observational study, infection was 60.7% with de novo EVR use in 28 patients. On the other hand, only 1 case of infection was reported in a single-center prospective study[22]. There was no clear definition of infection and the disparity could possibly be due to different definition among various studies.
Stomatitis
Stomatitis incidence was significantly higher (10.6%, 26.4%) in EVR group as compared to standard CNI group (1.2%, 0%) at 1-year and 2 year follow up of the H2304 (P < 0.001) and RESCUE (P < 0.010) studies[17,19]. Stomatitis has also been reported as one of the common adverse effects when EVR was used as maintenance immunosuppressive therapy[22,24]. Management strategies for stomatitis include the use of local anesthetic, intralesional and topical steroid to control stomatitis and reduce pain[41,44,45]. EVR has also been used as an alternative for renal transplant recipients who experienced sirolimus-induced stomatitis[46].
In general, mTOR inhibitor-associated stomatitis is generally not severe (< 5% is Grade 3 or 4)[41]. However, if nutrition status is compromised due to poor oral intake secondary to stomatitis, dose reduction or even withdrawal may be warranted.
Peripheral edema
mTOR inhibitor adverse effect of peripheral edema may be related to its anti-lymphangiogenetic effect, leading to lymphedema and capillary leak which may not be reversible[41]. In all 3 main phase III studies, peripheral edema was reported to be significantly higher in EVR group in comparison to control group. In the PROTECT study, peripheral edema was consistently higher in EVR group (26.8%) vs in standard CNI group (12.5%), P = 0.162 at month 11[13]. The incidence of peripheral edema continued to increase in the extension study period from month 11-35 (22% in EVR group vs 5% in standard CNI group, P = 0.048) and from month 11-59 (31.7% in EVR group vs 7.5% in standard CNI group, P = 0.011)[14,15]. In H2304 study, 17.6% and 22.4% in EVR group as compared to 10.8% and 14.9% in the standard CNI group experienced peripheral edema at 1-year (RR = 1.63, 95%CI: 1.03, 2.56) and at 2-year (P = 0.036) respectively[16,17]. Similar trend was observed in the RESCUE study, with the incidence of peripheral oedema 5.6% in EVR group and 1.4% in the standard CNI group[19]. Nonetheless, peripheral edema was not reported as one of the adverse effects that led to drug discontinuation in all above studies.
Proteinuria
It is unclear how mTOR inhibitors influence glomerulus permeability and cause proteinuria[41]. Nonetheless, as proteinuria is an indicator of kidney injury and strong predicator for cardiovascular events, this adverse effect warrants clinical attention. Patients with proteinuria (≥ 1 g/24 h) were excluded in H2304 and RESCUE study[15,18]. At the end of 1 year of H2304 study, 2.9% in EVR group developed proteinuria as compared to 0.4% in standard CNI group (RR = 6.89, 95%CI: 0.85, 55.54)[16]. In the subsequent follow up year, proteinuria was the most frequent adverse event that resulting in discontinuation of EVR (3.3%) in contrast to standard CNI group (0.4%)[17]. In the RESCUE study, 2 out of 16 patients required discontinuation of EVR due to proteinuria, while no patients required drug discontinuation in the standard CNI group for the same reason[19]. Similarly, incidence of proteinuria was significantly higher in EVR group (9.9%) as compared to the standard CNI group (2.0%) at month 11 in the PROTECT study[13]. A similar trend in their extension study up to month 59 was seen, albeit without statistical difference[15].
The characteristic and long-term outcomes of patients experiencing proteinuria, which could possibly guide patient selection and risk-benefit consideration to use EVR in LT, are lacking.
Hyperlipidemia
Hyperlipidemia is one of the most common adverse effects of mTOR inhibitors[41]. From phase II studies, a trend of dose-dependent hyperlipidemia was observed[11]. Masetti et al[21] reported significant increase in the incidence of hyperlipidemia but not hypertriglyceridemia with EVR use. In H2304, 23.3% in EVR group vs 17.8% in standard CNI group required lipid-lowing therapy (P = 0.944) at the end of 1 year and the incidence of hyperlipidemia was significantly higher (26.9% in EVR group vs 11.6% in standard CNI, P < 0.001) at the end of 2 years[16,17]. In the PROTECT study, EVR use was associated with an increased incidence of hyperlipidemia as compared to controls (11.9% vs 2.0%, P < 0.05) at month 11[13].
Although cardiovascular risk in LT is lower than renal and cardiac transplant, cardiovascular disease is still one of the leading causes of morbidity[47]. Undoubtedly, there is a range of effective lipid-lowing therapy in managing hyperlipidemia, and it is prudent to always weigh cardiovascular risks over the benefits before initiation or conversion to EVR.
RECOMMENDATION
A working group has recently consolidated recommendations for EVR use in LT based on consensus and experiences[48]. It provides some guidance while more outcome data is warranted to establish a comprehensive guideline for EVR use in LT. Based on current available data discussed in this review, EVR is an appropriate immunosuppressant for LT recipients as listed in Table 3.
Table 3.
Recommendation for everolimus use in liver transplantation recipients
| Indication and regimen | Renoprotective benefit |
| EVR in combination with CNI to allow CNI dose reduction | |
| Management of CNI neurotoxicity | |
| EVR allows temporary withdrawal of CNI till resolution of neurotoxicity | |
| Patients | LT recipients with renal function > 60 mL/min |
| LT recipients proteinuria < 1 g/24 h | |
| Timing | De novo therapy: Initiate EVR at 1 mo from transplant |
| Maintenance therapy: Introduce EVR within 1 yr from transplant | |
| CNI neurotoxicity: Stop CNI and initiate EVR immediately |
CNI: Calcineurin inhibitor; EVR: Everolimus; LT: Liver transplantation.
The increased risk of adverse effects could off-set the benefit of EVR particularly in preserving renal function. Although it has been mentioned that dose reduction was exercised in managing EVR adverse effects, but there were no details on the methods or outcomes[24,41,49]. Patient selection and strategies to reduce and minimise adverse effects will be key in determining the success of EVR use in LT.
CONCLUSION
EVR could be a viable alternative immunosuppressant in LT recipients who are at risk of renal impairment. Initiating EVR early (from 30 d post-LT and before eGFR < 55 mL/min) post-transplant allows CNI reduction and thus reduces CNI nephrotoxicity. Future research to strengthen EVR initiation, switch, or combination strategies and cost-effectiveness analyses would be important.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Peer-review started: January 18, 2017
First decision: May 9, 2017
Article in press: June 13, 2017
P- Reviewer: Carter WG, Demonacos C S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Contributor Information
Mei-Ling Yee, Department of Pharmacy, Singapore General Hospital, Singapore 169608, Singapore. yee.mei.ling@sgh.com.sg.
Hui-Hui Tan, Department of Gastroenterology and Hepatology, Singapore General Hospital, Singapore 169608, Singapore.
References
- 1.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015;15 Suppl 2:1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 2.Moini M, Schilsky ML, Tichy EM. Review on immunosuppression in liver transplantation. World J Hepatol. 2015;7:1355–1368. doi: 10.4254/wjh.v7.i10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A, Singhal A, Fontes P, Mazariegos G, DeVera ME, Cacciarelli T, Lopez RC, Sindhi R, Humar A, Marsh JW. One thousand consecutive primary liver transplants under tacrolimus immunosuppression: a 17- to 20-year longitudinal follow-up. Transplantation. 2011;91:1025–1030. doi: 10.1097/TP.0b013e3182129215. [DOI] [PubMed] [Google Scholar]
- 4.Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, Saab S, Han S, Durazo F, Weaver M, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905–916; discussion 916-918. doi: 10.1097/01.sla.0000164077.77912.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 6.von Wichert G, Jehle PM, Hoeflich A, Koschnick S, Dralle H, Wolf E, Wiedenmann B, Boehm BO, Adler G, Seufferlein T. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573–4581. [PubMed] [Google Scholar]
- 7.Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet. 2004;43:83–95. doi: 10.2165/00003088-200443020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Gabardi S, Baroletti SA. Everolimus: a proliferation signal inhibitor with clinical applications in organ transplantation, oncology, and cardiology. Pharmacotherapy. 2010;30:1044–1056. doi: 10.1592/phco.30.10.1044. [DOI] [PubMed] [Google Scholar]
- 9.Certican (Everolimus) Product Insert. Novartis, Mar 2013. [Google Scholar]
- 10.Niioka T, Kagaya H, Saito M, Inoue T, Numakura K, Yamamoto R, Akamine Y, Habuchi T, Satoh S, Miura M. Influence of everolimus on the pharmacokinetics of tacrolimus in Japanese renal transplant patients. Int J Urol. 2016;23:484–490. doi: 10.1111/iju.13081. [DOI] [PubMed] [Google Scholar]
- 11.Shipkova M, Hesselink DA, Holt DW, Billaud EM, van Gelder T, Kunicki PK, Brunet M, Budde K, Barten MJ, De Simone P, et al. Therapeutic Drug Monitoring of Everolimus: A Consensus Report. Ther Drug Monit. 2016;38:143–169. doi: 10.1097/FTD.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 12.Levy G, Schmidli H, Punch J, Tuttle-Newhall E, Mayer D, Neuhaus P, Samuel D, Nashan B, Klempnauer J, Langnas A, et al. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transpl. 2006;12:1640–1648. doi: 10.1002/lt.20707. [DOI] [PubMed] [Google Scholar]
- 13.Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, Settmacher U, Heyne N, Clavien PA, Muehlbacher F, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012;12:1855–1865. doi: 10.1111/j.1600-6143.2012.04049.x. [DOI] [PubMed] [Google Scholar]
- 14.Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, Schemmer P, Fischer L, Klein CG, Nadalin S, et al. Everolimus and early calcineurin inhibitor withdrawal: 3-year results from a randomized trial in liver transplantation. Am J Transplant. 2014;14:701–710. doi: 10.1111/ajt.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, Schemmer P, Fischer L, Klein CG, Nadalin S, et al. Long-term follow-up of five yr shows superior renal function with everolimus plus early calcineurin inhibitor withdrawal in the PROTECT randomized liver transplantation study. Clin Transplant. 2016;30:741–748. doi: 10.1111/ctr.12744. [DOI] [PubMed] [Google Scholar]
- 16.De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, Jonas S, Sudan D, Fung J, Fischer L, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008–3020. doi: 10.1111/j.1600-6143.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Jonas S, Sudan D, Fischer L, Duvoux C, et al. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013;13:1734–1745. doi: 10.1111/ajt.12280. [DOI] [PubMed] [Google Scholar]
- 18.Fischer L, Saliba F, Kaiser GM, De Carlis L, Metselaar HJ, De Simone P, Duvoux C, Nevens F, Fung JJ, Dong G, et al. Three-year Outcomes in De Novo Liver Transplant Patients Receiving Everolimus With Reduced Tacrolimus: Follow-Up Results From a Randomized, Multicenter Study. Transplantation. 2015;99:1455–1462. doi: 10.1097/TP.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 19.De Simone P, Metselaar HJ, Fischer L, Dumortier J, Boudjema K, Hardwigsen J, Rostaing L, De Carlis L, Saliba F, Nevens F. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trial. Liver Transpl. 2009;15:1262–1269. doi: 10.1002/lt.21827. [DOI] [PubMed] [Google Scholar]
- 20.De Simone P, Saliba F, Dong G, Escrig C, Fischer L. Do patient characteristics influence efficacy and renal outcomes in liver transplant patients receiving everolimus? Clin Transplant. 2016;30:279–288. doi: 10.1111/ctr.12687. [DOI] [PubMed] [Google Scholar]
- 21.Masetti M, Montalti R, Rompianesi G, Codeluppi M, Gerring R, Romano A, Begliomini B, Di Benedetto F, Gerunda GE. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant. 2010;10:2252–2262. doi: 10.1111/j.1600-6143.2010.03128.x. [DOI] [PubMed] [Google Scholar]
- 22.Jeng LB, Thorat A, Hsieh YW, Yang HR, Yeh CC, Chen TH, Hsu SC, Hsu CH. Experience of using everolimus in the early stage of living donor liver transplantation. Transplant Proc. 2014;46:744–748. doi: 10.1016/j.transproceed.2013.11.068. [DOI] [PubMed] [Google Scholar]
- 23.Gastaca M, Bilbao I, Jimenez M, Bustamante J, Dopazo C, Gonzalez R, Charco R, Santoyo J, Ortiz de Urbina J. Safety and Efficacy of Early Everolimus When Calcineurin Inhibitors Are Not Recommended in Orthotopic Liver Transplantation. Transplant Proc. 2016;48:2506–2509. doi: 10.1016/j.transproceed.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 24.De Simone P, Carrai P, Precisi A, Petruccelli S, Baldoni L, Balzano E, Ducci J, Caneschi F, Coletti L, Campani D, et al. Conversion to everolimus monotherapy in maintenance liver transplantation: feasibility, safety, and impact on renal function. Transpl Int. 2009;22:279–286. doi: 10.1111/j.1432-2277.2008.00768.x. [DOI] [PubMed] [Google Scholar]
- 25.Castroagudín JF, Molina E, Romero R, Otero E, Tomé S, Varo E. Improvement of renal function after the switch from a calcineurin inhibitor to everolimus in liver transplant recipients with chronic renal dysfunction. Liver Transpl. 2009;15:1792–1797. doi: 10.1002/lt.21920. [DOI] [PubMed] [Google Scholar]
- 26.Saliba F, Dharancy S, Lorho R, Conti F, Radenne S, Neau-Cransac M, Hurtova M, Hardwigsen J, Calmus Y, Dumortier J. Conversion to everolimus in maintenance liver transplant patients: a multicenter, retrospective analysis. Liver Transpl. 2011;17:905–913. doi: 10.1002/lt.22292. [DOI] [PubMed] [Google Scholar]
- 27.Bilbao I, Salcedo M, Gómez MA, Jimenez C, Castroagudín J, Fabregat J, Almohalla C, Herrero I, Cuervas-Mons V, Otero A, et al. Renal function improvement in liver transplant recipients after early everolimus conversion: A clinical practice cohort study in Spain. Liver Transpl. 2015;21:1056–1065. doi: 10.1002/lt.24172. [DOI] [PubMed] [Google Scholar]
- 28.Farkas SA, Schnitzbauer AA, Kirchner G, Obed A, Banas B, Schlitt HJ. Calcineurin inhibitor minimization protocols in liver transplantation. Transpl Int. 2009;22:49–60. doi: 10.1111/j.1432-2277.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 29.Pageaux GP, Rostaing L, Calmus Y, Duvoux C, Vanlemmens C, Hardgwissen J, Bernard PH, Barbotte E, Vercambre L, Bismuth M, et al. Mycophenolate mofetil in combination with reduction of calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Liver Transpl. 2006;12:1755–1760. doi: 10.1002/lt.20903. [DOI] [PubMed] [Google Scholar]
- 30.Hao JC, Wang WT, Yan LN, Li B, Wen TF, Yang JY, Xu MQ, Zhao JC, Wei YG. Effect of low-dose tacrolimus with mycophenolate mofetil on renal function following liver transplantation. World J Gastroenterol. 2014;20:11356–11362. doi: 10.3748/wjg.v20.i32.11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiménez-Pérez M, González Grande R, Rando Muñoz FJ, de la Cruz Lombardo J, Muñoz Suárez MA, Fernández Aguilar JL, Pérez Daga JA, Santoyo-Santoyo J, Manteca González R, Rodrigo López JM. Everolimus plus mycophenolate mofetil as initial immunosuppression in liver transplantation. Transplant Proc. 2015;47:90–92. doi: 10.1016/j.transproceed.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Bilbao I, Dopazo C, Castells L, Lazaro J, Caralt M, Sapisochin G, Charco R. Immunosuppression based on everolimus in liver transplant recipients with severe early post-transplantation neurotoxicity. Transplant Proc. 2014;46:3104–3107. doi: 10.1016/j.transproceed.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Afinitor (Everolimus) prescribing information. Novartis, Jul 2015 [Google Scholar]
- 34.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dugum MF, Zein NN. Liver Transplantation for Hepatocellular Carcinoma. Clinical Liver Disease. 2016;7:36–39. doi: 10.1002/cld.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cholongitas E, Goulis I, Theocaridou E, Antoniadis N, Fouzas I, Giakoustidis D, Imvrios G, Giouleme O, Papanikolaou V, Akriviadis E, et al. Everolimus-based immunosuppression in liver transplant recipients: A single-center experience. Hepatol Int. 2014;8:137–145. doi: 10.1007/s12072-013-9492-6. [DOI] [PubMed] [Google Scholar]
- 37.Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014;27:1039–1049. doi: 10.1111/tri.12372. [DOI] [PubMed] [Google Scholar]
- 38.Villamil FG, Gadano AC, Zingale F, Perez R, Gil O, Yantorno S, Mastai R, Cairo FO, Otero AB, Dong G, et al. Fibrosis progression in maintenance liver transplant patients with hepatitis C recurrence: a randomised study of everolimus vs. calcineurin inhibitors. Liver Int. 2014;34:1513–1521. doi: 10.1111/liv.12416. [DOI] [PubMed] [Google Scholar]
- 39.Vallin M, Guillaud O, Morard I, Gagnieu MC, Mentha G, Adham M, Morelon E, Boillot O, Giostra E, Dumortier J. Tolerability of everolimus-based immunosuppression in maintenance liver transplant recipients. Clin Transplant. 2011;25:660–669. doi: 10.1111/j.1399-0012.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- 40.Ventura-Aguiar P, Campistol JM, Diekmann F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opin Drug Saf. 2016;15:303–319. doi: 10.1517/14740338.2016.1132698. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando) 2014;28:126–133. doi: 10.1016/j.trre.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Montalti R, Mimmo A, Rompianesi G, Serra V, Cautero N, Ballarin R, De Ruvo N, Cunningham Gerring R, Enrico Gerunda G, Di Benedetto F. Early use of mammalian target of rapamycin inhibitors is an independent risk factor for incisional hernia development after liver transplantation. Liver Transpl. 2012;18:188–194. doi: 10.1002/lt.22445. [DOI] [PubMed] [Google Scholar]
- 43.Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94:547–561. doi: 10.1097/TP.0b013e3182551021. [DOI] [PubMed] [Google Scholar]
- 44.Ji YD, Aboalela A, Villa A. Everolimus-associated stomatitis in a patient who had renal transplant. BMJ Case Rep. 2016;2016:bcr2016217513. doi: 10.1136/bcr-2016-217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermeulen T, Rodrigus IE, Vrints CJ, Conraads V. Severe stomatitis complicating immune-suppressive switch after cardiac transplantation. Acta Chir Belg. 2010;110:339–341. doi: 10.1080/00015458.2010.11680629. [DOI] [PubMed] [Google Scholar]
- 46.Ram R, Swarnalatha G, Neela P, Dakshinamurty KV. Sirolimus-induced aphthous ulcers which disappeared with conversion to everolimus. Saudi J Kidney Dis Transpl. 2008;19:819–820. [PubMed] [Google Scholar]
- 47.Luca L, Westbrook R, Tsochatzis EA. Metabolic and cardiovascular complications in the liver transplant recipient. Ann Gastroenterol. 2015;28:183–192. [PMC free article] [PubMed] [Google Scholar]
- 48.De Simone P, Fagiuoli S, Cescon M, De Carlis L, Tisone G, Volpes R, Cillo U. Use of Everolimus in Liver Transplantation: Recommendations From a Working Group. Transplantation. 2017;101:239–251. doi: 10.1097/TP.0000000000001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumortier J, Dharancy S, Calmus Y, Duvoux C, Durand F, Salamé E, Saliba F. Use of everolimus in liver transplantation: The French experience. Transplant Rev (Orlando) 2016;30:161–170. doi: 10.1016/j.trre.2015.12.003. [DOI] [PubMed] [Google Scholar]


