Abstract
AIM
To evaluate the effect of local surgical adhesive glue (albumin/glutaraldehyde-Bioglue) on the healing of colonic anastomoses in rats.
METHODS
Forty Albino-Wistar male rats were randomly divided into two groups, with two subgroups of ten animals each. In the control group, an end-to-end colonic anastomosis was performed after segmental resection. In the Bioglue group, the anastomosis was protected with extraluminar application of adhesive glue containing albumin and glutaraldehyde. Half of the rats were sacrificed on the fourth and the rest on the eighth postoperative day. Anastomoses were resected and macroscopically examined. Bursting pressures were calculated and histological features were graded. Other parameters of healing, such as hydroxyproline and collagenase concentrations, were evaluated. The experimental data were summarized and computed from the results of a one-way ANOVA. Fisher’s exact test was applied to compare percentages.
RESULTS
Bursting pressures, adhesion formation, inflammatory cell infiltration, and collagen deposition were significantly higher on the fourth postoperative day in the albumin/glutaraldehyde group than in the control group. Furthermore, albumin/glutaraldehyde significantly increased adhesion formation, inflammatory cell infiltration, neoangiogenesis, and collagen deposition on the eighth postoperative day. There was no difference in fibroblast activity or hydroxyproline and collagenase concentrations.
CONCLUSION
Albumin/glutaraldehyde, when applied on colonic anastomoses, promotes their healing in rats. Therefore, the application of protective local agents in colonic anastomoses leads to better outcomes.
Keywords: Adhesions, Bursting pressure, Bioglue™, Albumin/glutaraldehyde, Collagen, Colonic anastomosis
Core tip: The present study was designed to investigate the effect of local surgical adhesive glue composed of albumin/glutaraldehyde in the healing of colonic anastomoses in rats. The application of adhesive glue promotes the healing of colonic anastomoses in rats as it significantly increases the bursting pressure in the early period, but it also causes more adhesions and an enhanced inflammatory reaction.
INTRODUCTION
Anastomosis dehiscence is a serious postoperative complication, and the risk of anastomotic leakage is higher in large intestine surgery compared with other gastrointestinal anastomoses[1,2]. Different techniques of using additive materials such as omentum and several types of fibrin sealants to cover the anastomosis have been proposed[3,4], and in experimental studies, various protective methods such as endoluminar latex prostheses, stents, biofragmental rings, and local application of bioadhesives have been used, with promising results[5-9]. Tissue glues and fibrin adhesives, which are biodegradable and biocompatible, have been used to seal suture lines for haemostasis and/or to strengthen and reinforce fragile tissues by tissue adherence and replacement or support of sutures by biomaterial gluing[9,10]. The goal is to reduce the incidence of dehiscence by increasing the strength of anastomoses, covering the anastomotic line, and stimulating healing[11]. The possibility of direct influence of extraluminar contents and the substance’s biological compatibility, as well as the adhesive and tension strength of the glue, has as a result in the critical healing phase: an increase in the force needed for anastomosis bursting[12].
Local application of albumin/glutaraldehyde (Bioglue™), a haemostatic and adhesive agent, has received approval for many vascular, pulmonary and soft tissue repairs, and its use is already established in cardiothoracic surgery[13,14]. So far, however, there have been no published experiments using this glue in colonic anastomosis. The aim of this experimental study was to investigate the effects of surgical adhesive glue during the healing process of colonic anastomosis in rats.
MATERIALS AND METHODS
Laboratory animals
The animal protocol was designed to minimize pain and discomfort to the animals. Forty male Wistar rats weighing 200-300 g were used in this study. The research protocol was approved by the Ethical Committee of the Department of Veterinary Services of the Prefecture of Thessaloniki (S.N.: 13/11872/11-09-08). Principles of laboratory animal care were followed. Animals were housed individually and had unrestricted access to the standard laboratory diet and water pre- and postoperatively. They were kept in our laboratory for seven-ten days before the experiment on a 12-h light and dark cycle and did not receive any course of chemoprophylaxis. At the sacrifice, all animals were euthanized by intracardiac administration of KCL 10% for tissue collection.
Anaesthesia, operative technique and experimental groups
The rats were weighed on the day of operation as well as before the sacrifice, and changes in weight were recorded. Operations were performed through a 3-cm midline incision under intraperitoneal thiopental anaesthesia (40 mg/kg bodyweight). After resection of a 1-cm segment of the colon and 5 cm from the rectum, an end-to-end anastomosis was created using a single layer of eight interrupted extramucosal 6-0 polypropylene sutures. Rats were randomly assigned to two groups of 20 animals. In the CONTROL group, an end-to-end anastomosis was created (Figure 1A). In the BIOGLUE group, after creating the anastomosis, albumin/glutaraldehyde was applied around it (Figure 1B). The glue was applied on the colon’s edges with an applicator. Holding the applicator was sufficient to place the liquid adhesive on the cut edges of the colon, taking precautions to avoid dispersion of the glue in the peritoneal cavity. The abdominal muscle layer and the skin were closed in one layer, using three sutures (3/0 silk). Each group was subdivided in two subgroups of ten animals each, with half of the animals being sacrificed on the fourth postoperative day (CONTROL4 and BIOGLUE4) and the other half being sacrificed on the eighth postoperative day (CONTROL8 and BIOGLUE8)
Figure 1.
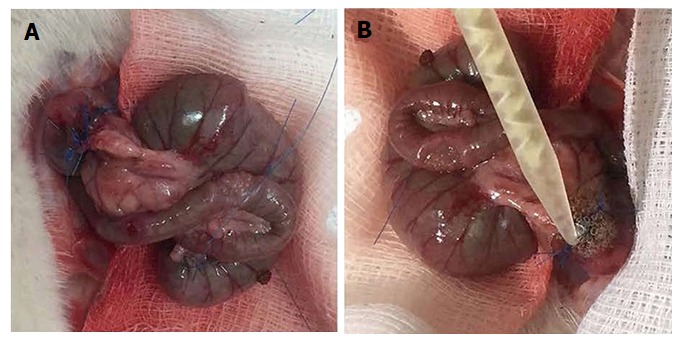
Control and Bioglue group. A: Creation of end-to-end anastomosis using a single layer of eight interrupted extramucosal 6-0 polypropylene sutures; B: Application of the Bioglue on the colon edges after taking precautions to avoid dispersion of the glue in the peritoneal cavity.
Macroscopic examination
On the day of sacrifice, the animals were anaesthetized again, and the anastomotic segments were isolated during relaparotomy. The anastomoses were examined macroscopically. Integrity of the anastomosis, existence of perianastomotic abscess or peritonitis, and adhesion formation were recorded. The evaluation was performed according to the scale of van der Ham et al[15], as has been described elsewhere. Briefly, anastomosis was given score 0 when no adhesions occurred; score 1 represented minimal adhesions mainly between the anastomosis and the omentum; score 2 corresponded to moderate adhesions, i.e., between the omentum and the anastomotic site or between the anastomosis and a loop of small intestine; finally, score 3 represented severe and extensive adhesions, including abscess formation.
Bursting pressure
Bursting pressure was measured ex vivo. The anastomosis was removed along with a 2.5 cm segment of the colon on either side en bloc with the formed adhesions and cleared of stools. The proximal end was ligated using a 3/0 silk suture, and a catheter was secured into the distal end and fixed to the bursting pressure apparatus as described elsewhere[16-18]. Through this catheter, the bowel was infused with a continuous flow of physiological saline at a rate of 1 mL/min. The bursting pressure was defined as the pressure at which leakage of saline or gross rupture was noted and recorded in mmHg. The site of leakage during the bursting pressure measurement was also recorded, since in some rats, rupture occurred at the anastomotic site, and in others far from it.
Histological assessment
After the ex vivo measurement of bursting pressure, the anastomotic segment of the colon was cleared of the surrounding mesentery and fat and rinsed with saline. The anastomosis was resected along with a 0.5 cm segment of the colon on either side and divided into two parts vertically. The first segment was placed in 4% formaldehyde solution for histopathological examination and stained with haematoxylin and eosin. The anastomosis was examined under a light microscope and graded histologically in a blind fashion, using a 0-4 Ehrlich and Hunt numerical scale as modified by Phillips et al[19]. The evaluated parameters were inflammatory cell infiltration (white blood cell count), neoangiogenesis (new blood vessel formation), fibroblast activity, and collagen deposition[20]. Each studied parameter was evaluated individually using a numerical scale from 0 to 4 as follows: 0 (-) = no evidence; 1 (+) = occasional evidence; 2 (++) = light scattering; 3 (+++) = abundant evidence; and 4 (++++) = confluent fibres or cells.
Hydroxyproline
Quantification of collagen in colonic anastomosis is synonymous with quantification of hydroxyproline. The second segment of the anastomosis was weighed and then divided into two parts vertically and stored at -20 °C. Determination of hydroxyproline tissue contents was performed as described in previous experiments, with some modifications[13,21]. Briefly, after the specimens were lyophilized, a polytron homogenizer was used to homogenize the tissue sample in distilled water. The acid-soluble collagen was extracted from the tissue sample by overnight incubation with acetic acid 0.5 mol/L at 4 °C. 70 μL of standard/test sample was hydrolysed in 30 μL NaOH 10.125 mol/L for 25 min at 120 °C by autoclaving. The hydrolysed sample was then mixed with a buffered (pH 7) chloramines-T reagent (0.056 mol/L), and at room temperature the oxidation was continued for 25 min. Following the development of chromophore was achieved with the addition of Ehrlich’s reagent. The absorbance was measured at 550 nm using a Biotek μQuant™ spectrophotometer. Absorbance values were plotted against the concentration of standard hydroxyproline, and the presence of hydroxyproline in unknown tissue extracts was determined from the standard curve. The results were expressed in μg/g of tissue[21].
Collagenase I
The concentration of collagenase I was estimated in another segment of the anastomotic site by using a commercial ELISA kit (USCNLIFE, E0212r). The test sample was added to the appropriate plate well pre-coated with anti-collagenase I antibody-microtiter with a biotin-conjugated polyclonal antibody specific to collagenase I Then, avidin conjugated to ΗRP was added to the microplate well and incubated. The chromophore was then developed with the addition of TMB substrate solution, and the reaction stopped with sulphuric acid solution. The colour change was measured spectrophotometrically at 450 nm using a Stat Fax-210™ spectrophotometer (Awareness Technology Inc.). The concentration of collagenase I in unknown tissue samples was determined from the standard curve. The results were calculated as μg/g of wet tissue weight[22].
Statistical analysis
Using statistical descriptive indices of central tendency and dispersion the experimental data were summarized. Data are presented as mean ± SD. After performing a normality test, if the data presented a normal distribution we used ANOVA to compare all four groups and and the least significant difference criterion[23] as post hoc analysis to make comparisons between groups. If the data didn’t have normal distribution we used the non-parametric Kruskal-Wallis test to compare all four groups and performed the Mann-Whitney test to make comparisons between groups, with the significance level for the certain test adjusted to P = 0.0125 (0.05/4). The significance level of statistical hypothesis testing procedures concerning comparisons of means was pre-set at P < 0.05. All the statistical analyses were performed using the SPSS version 15.0 statistical package (SPSS Inc., Chicago, IL, United States) enhanced with the module Exact Tests[24,25]. The statistical methods of this study were reviewed by Dr. Haidich AB, Assistant Professor in Hygiene-Medical Statistics, Aristotle University of Thessaloniki, Greece.
RESULTS
Mortality, infection, and anastomotic dehiscence
No deaths or wound infections occurred, and no anastomotic dehiscence was noted before the day of sacrifice.
Bodyweight change
In all the experimental groups, the bodyweight decreased from the day of the experiment till the day of sacrifice. Bodyweight changes differed significantly among the subgroups (P = 0.03). In particular, the only significant difference in bodyweight change was between subgroups BIOGLUE4 and BIOGLUE8 (P = 0.017), but there were no other differences between subgroups. The bodyweight changes are presented in Figure 2.
Figure 2.
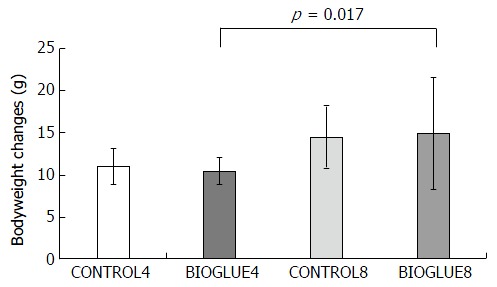
Bodyweight changes in grams in the two groups (mean ± SD). A statistically significant difference was found between subgroups BIOGLUE4 and BIOGLUE8.
Adhesion Formation
On the fourth postoperative day, in subgroup CONTROL4, 90% of animals had no adhesions, and only 10% presented with grade 1 adhesions, while in subgroup BIOGLUE4, all animals had adhesions, and the adhesion formation score was grade 2 in all animals. On the eighth postoperative day, in subgroup CONTROL8, 90% of animals had no adhesions, and only 10% presented with grade 1 adhesions, while in subgroup BIOGLUE8, all animals had adhesions, and the adhesion formation score was grade 2 in all animals (Figure 3).
Figure 3.
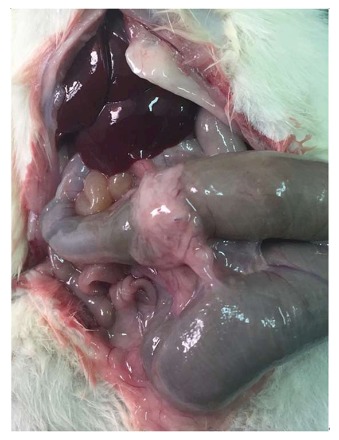
Moderate adhesion formation between the omentum and the anastomotic site in the Bioglue group.
The adhesion formation score differed significantly between groups (P < 0.001). It was significantly higher in both Bioglue subgroups than in the two control groups (P < 0.001 in both cases). The adhesion formation scores are presented in Figure 4.
Figure 4.
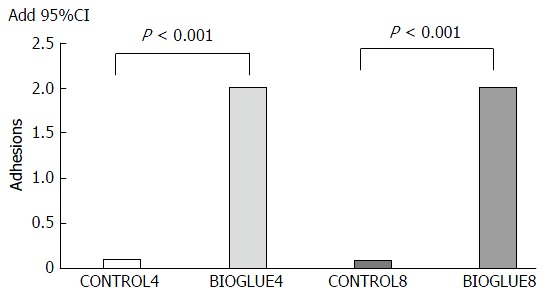
Comparative bar chart of adhesion formation (mean). The adhesion formation score was statistically significantly higher in both Bioglue subgroups compared to control.
Bursting Pressure measurement
There was a significant difference in bursting pressure among groups (P < 0.001). Bursting pressure was significantly increased in the Bioglue subgroup compared to the control subgroup on the fourth postoperative day (P = 0.017) but not on the eighth postoperative day (P = 0.545). Furthermore, bursting pressure was statistically significantly higher on the eighth postoperative day in both the Bioglue and the control groups, with P < 0.001 in both cases. The differences in bursting pressures are presented in Figure 5.
Figure 5.
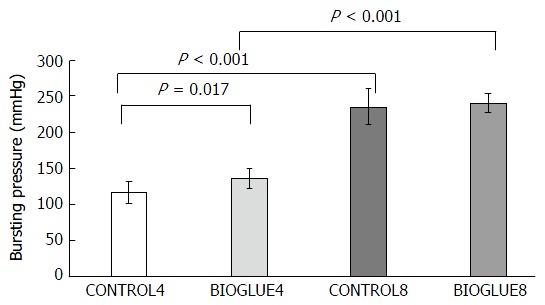
Comparative bar chart presenting bursting pressures (mmHg) (mean ± SD). A statistically significant increase in the bursting pressure was noted on the fourth postoperative day in the Bioglue subgroup compared to the control.
Regarding the site of leakage during the measurement of bursting pressure, on the fourth postoperative day in both the control (CONTROL4) and the study (BIOGLUE4) groups, the rupture occurred in 50% of the animals at the anastomosis and in 50% far from it, while on the eighth postoperative day in both subgroups (CONTROL8 and BIOGLUE8), all ruptures occurred far from the anastomotic site.
Histological assessment
The histological assessment of the anastomotic healing included measurements of inflammatory cell infiltration, neoangiogenesis, fibroblast activity, and collagen deposition. In Figure 6, histology images of various degrees of inflammation, neoangiogenesis, fibroblast activity, and collagen deposition are presented, respectively.
Figure 6.
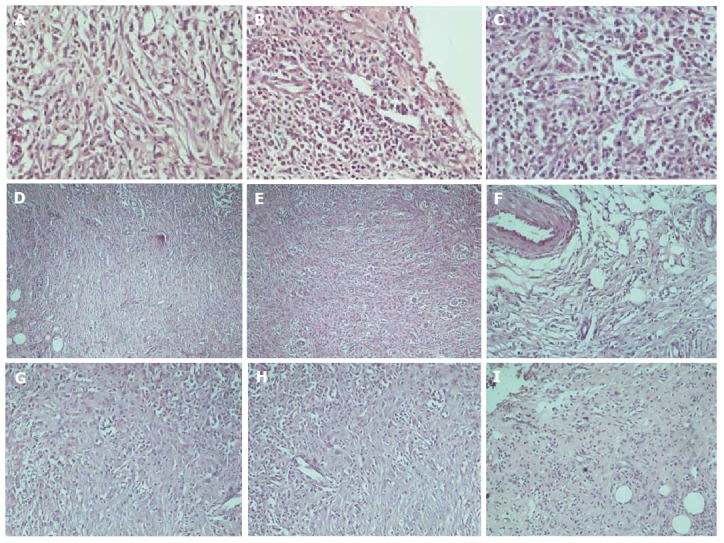
Representative haematoxylin-eosin histopathology images. A: Inflammatory cell infiltration revealing light scattering (× 400); B: Abundant evidence (× 400); C: Confluent cells (× 400); D: Neoangiogenesis revealing light scattering (A, × 100); E: Abundant evidence (× 100); F: Fibroblast activity revealing light scattering (× 100); G: Abundant evidence (× 200); H: Collagen deposition revealing light scattering (× 200); I: Abundant evidence (× 100).
Statistical analysis revealed significant changes in all histological parameters: inflammation (P < 0.001), neoangiogenesis (P < 0.001), fibroblast activity (P = 0.001), and collagen deposition (P < 0.001).
The average inflammatory cell infiltration was significantly higher in the Bioglue subgroups than in the controls for both postoperative days, with P < 0.001 in both cases. There was also a significant difference between subgroups CONTROL4 and CONTROL8 (P < 0.001) and between BIOGLUE4 and BIOGLUE8 (P = 0.001). The changes in inflammatory cell infiltration among groups are presented in a histogram in Figure 7A.
Figure 7.
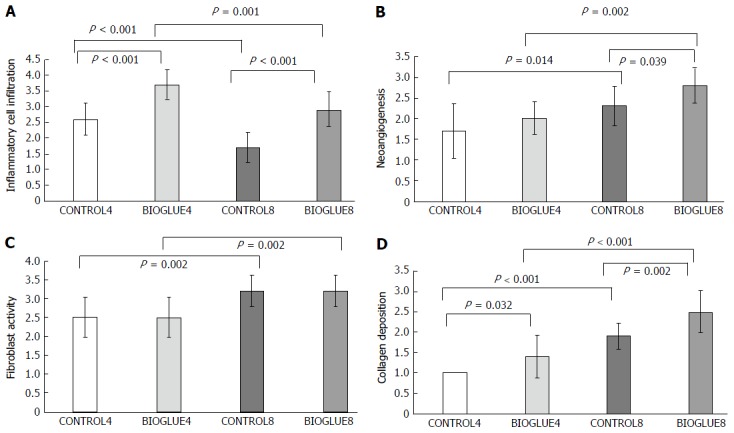
Comparative bar chart presenting the average inflammatory cell infiltration (A), new vessel formation (neoangiogenesis) (B), fibroblast activity (C) and collagen deposition (D), according to the scale of Ehrlich and Hunt, as modified by Phillips et al[19] (0-4) (mean ± SD). A: The average inflammatory cell infiltration was statistically significantly higher in the Bioglue subgroups on both the fourth and eighth days than in the controls. Also, in each subgroup, there was a statistically significant decrease in the average inflammatory cell infiltration from the fourth to the eighth day; B: The average neoangiogenesis was statistically significantly higher in the Bioglue subgroups on the eighth day than in the control. Also, in each subgroup, there was a statistically significant increase in neoangiogenesis from the fourth to the eighth day; C: The average fibroblast activity was statistically significantly higher in each subgroup from the fourth to the eighth day; D: The average collagen deposition was statistically significantly higher in the Bioglue subgroups on both the fourth and eighth days than in the control. Also, in each subgroup there was a statistically significant increase in collagen deposition from the fourth to the eighth day.
Regarding neoangiogenesis, in the Bioglue group, there was an increase on the fourth and eighth postoperative days compared to the control group, but this difference was significant only on the eighth postoperative day (CONTROL4 vs BIOGLUE4 with P = 0.2398 and CONTROL8 vs BIOGLUE8 with P = 0.039). Neoangiogenesis was also statistically significantly higher on the eighth postoperative day in both Bioglue and control groups (CONTROL4 vs CONTROL8 with P = 0.014 and BIOGLUE4 vs BIOGLUE8 with P = 0.002). Changes in neoangiogenesis between groups are plotted in Figure 7B.
The fibroblast activity was similar in the Bioglue subgroups and in the control subgroups on both postoperative days (CONTROL4 vs BIOGLUE4, P = 1, and CONTROL8 vs BIOGLUE8, P = 1). However, there was a significant difference between subgroups CONTROL4 and CONTROL8 (P = 0.002) and between BIOGLUE4 and BIOGLUE8 (P = 0.002). The changes in fibroblast activity among groups are presented in a histogram in Figure 7C.
Collagen deposition was statistically significantly lower in the control subgroups than in the Bioglue subgroups (CONTROL4 vs BIOGLUE4 and CONTROL8 vs BIOGLUE8 with P = 0.032 and P = 0.002, respectively). There was also a significant increase in collagen deposition between subgroups CONTROL4 and CONTROL8 (P < 0.001) and between BIOGLUE4 and BIOGLUE8 (P < 0.001). Changes in collagen deposition between groups are presented in Figure 7D.
Hydroxyproline and collagenase I concentration
Hydroxyproline concentration differed significantly between groups. Specifically, it was similar on the fourth and eighth postoperative days between the two groups (CONTROL4 vs BIOGLUE4 with P = 0.656 and CONTROL8 vs BIOGLUE8 with P = 0.309), but there was a significant increase in the hydroxyproline tissue content of both groups (control and Bioglue) on the eighth postoperative day compared to the fourth (BIOGLUE4 vs BIOGLUE8 and CONTROL4 vs CONTROL8, with P < 0.001 in both cases). The results are presented in Figure 8A.
Figure 8.
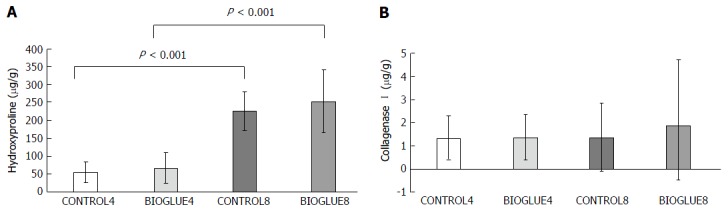
Comparative bar chart of hydroxyproline tissue contents (A), collagenase I tissue contents (B) (μg/g tissue) at the anastomotic site (mean ± SD). A: In each subgroup, there was a statistically significant increase in hydroxyproline tissue content from the fourth to the eighth day; B: No statistically significant difference was noted.
Collagenase I concentration was similar between the two groups and subgroups on the fourth and eighth postoperative days (P = 0.959). Specifically, CONTROL4 vs BIOGLUE4 (P = 0.912) and CONTROL8 vs BIOGLUE8 (P = 0.796). CONTROL4 vs CONTROL8 (P = 0.684) and BIOGLUE4 vs BIOGLUE8: (P = 0.912). The results are presented in Figure 8B.
DISCUSSION
Anastomotic leakage remains the most important cause of postoperative mortality and morbidity in colorectal surgery[26,27]. Intestinal anastomoses are complicated by leakages, even in the most experienced of hands and despite the development of new surgical techniques, suture materials, and stapling devices[28,29].
Many factors affect the healing of anastomoses, divided into general and local ones[30]. General factors include the patient’s age and diet, hypovolemia, malignancy, medications (e.g., steroids, nonsteroidal anti-inflammatory drugs, and 5-FU), immunocompetence, blood transfusion, radiotherapy, diabetes, uraemia, anaemia, jaundice, and nutrient deficiencies (vitamin C, iron, zinc, methionine, cysteine, etc.)[16,31-34]. Local factors include ischaemia at the anastomotic site, anastomotic tension, surgical technique, peritonitis, preoperative bowel preparation, infection, and emergency or elective surgery[34-36]
Bioglue surgical adhesive is a two-component surgical adhesive that confers enhanced bonding properties. It is composed of 45% purified bovine serum albumin and 10% glutaraldehyde. The advantage of Bioglue is that the bi-functional glutaraldehyde molecule covalently bonds the bovine serum albumin molecules to each other, as well as to lysine in proteins on the cell surface and in the extracellular matrix. This reaction is spontaneous, increasing tensile and shear strength. The albumin provides an extensive flexible network of bonds. When applied to the repair site, it forms a watertight mechanical seal and holds sutures securely[13]. It is necessary to dry the completed anastomosis as much as possible and to protect the area surrounding the target tissue with moist sterile gauze pads[37]. Polymerisation commences rapidly within 20 to 30 s and reaches full bonding strength in two minutes[38].
Patients with colorectal cancer usually lose weight, and malnutrition accompanying loss of bodyweight decreases the deposition of collagen and the anastomotic strength[39]. As expected following surgery, in our study, the mean postoperative bodyweight was lower both on the fourth and the eighth postoperative days in all subgroups compared to the beginning of the experiment. However, a comparison of the control and Bioglue subgroups revealed that there wasn’t any statistically significant difference between their mean bodyweights. The only significant difference was a greater decrease in bodyweight in the Bioglue group on the eighth day compared to the fourth day.
Adhesions are fibrous bands that connect the peritoneal organs to each other or the peritoneum and could possibly seal any potential micro-leakages from the anastomosis and simultaneously enhance the local blood supply[40,41]. The incidence of adhesion formation appears to be caused by many factors, such as sutures or staples that can potentially cause topical ischemia. The foreign material used can lead to an infected anastomosis followed by anastomotic leakage[42]. Furthermore, adhesion formation is dependent on the severity of the intestinal serosal trauma, fibroblast activation, metalloproteinase activity, and the efficiency of the fibrinolytic mechanism[43-45]. Adhesion formation is a serious problem to tackle when using glue for anastomosis. In the Bioglue group, there was an increase in the adhesion formation score that was statistically significant. Similar are the results of Ozel et al[46], who, in an experimental study in rats using a fibrin patch to support colonic anastomoses, found more adhesions of greater degree in the study group compared to the control. However, Kanellos et al[47] using a fibrin glue, didn’t notice any difference in adhesion formation compared to the control group in rats with colonic anastomosis, while Haukipuro et al[48] demonstrated fewer adhesions using fibrin sealant. Detweiler et al[49] proposed the combination of absorbable stents and fibrin glue to avoid adhesion formation and intestinal obstruction.
The percentage of anastomotic leakage is the most important marker reflecting the efficacy of the healing mechanisms of intestinal anastomoses and ranges from 3% to 30%[1,2,50,51]. In the present study, no anastomotic leakage was noted in either subgroup on the fourth or the eighth postoperative day, reflecting the efficacy of healing mechanisms in both groups. The mechanical strength of the anastomosis is determined by its bursting pressure. It is therefore a useful parameter to measure the healing process in the first week after anastomotic formation[52]. Bursting pressure increases progressively after the formation of the anastomosis. In our study, the bursting pressures were significantly higher in both the control group and the Bioglue group on the eighth postoperative day than the fourth. Moreover, Bioglue significantly increased bursting pressures compared to the control on the fourth postoperative day but not on the eighth. Hjortrup et al[53] found that the bursting pressure of small bowel segments with fibrin anastomoses was similar to those with sutured anastomoses. Capitán Morales et al[54] compared three groups of rats: one with anastomoses sutured with silk 5/0, one with anastomoses sutured with polyglycolic acid 5/0, and one with suture-less fibrin adhesive anastomoses. They found that the greatest bursting pressure occurred in the last series. Additionally, Akgün et al[55] in an experimental study using a fibrin sealant to protect anastomoses in rats, demonstrated an increase in bursting pressure in the study group compared to the control.
A fundamental element in the assessment of bursting pressure and the efficacy of healing mechanisms is the part of the colon that ruptures during the measurement of bursting pressure[10,56,57]. Rupture at the anastomotic site happens in mechanically weaker anastomoses or anastomoses at the early healing phase, between the third and fifth days. In contrast, rupture far from an anastomosis presents in mechanically very strong anastomoses or in anastomoses in the late healing phase, specifically in the remodelling phase[2,28]. In the current study, there were no differences in the rupture sites among the subgroups.
Healing of anastomoses is a complicated process which is divided into four different phases: coagulation, inflammation, migration and proliferation, and remodelling. Inflammation is the second phase and is characterized by infiltration of the anastomosis by inflammatory cells, especially leukocytes[58-60]. Macrophages, lymphocytes and thrombocytes produce and secrete cytokines and growth factors that regulate neoangiogenesis and collagen synthesis[60-63] and have mitogenic, chemotactic, and cell movement stimulant functions[62]. The crucial role of neutrophils in the early postoperative weakening of the anastomosis due to activation of metalloproteinases and collagen degradation is well established[64-67]. The foreign bodies such as sutures, clips, and any biological substances (glues) which are used in the anastomosis are those that keep the anastomosis intact during the healing phase[1,3,10]. In our study, inflammatory cell infiltration was statistically significantly higher in the Bioglue subgroups than in the control subgroups on both postoperative days. Ozel et al[46] noticed a significant increase in the inflammatory reaction in the fibrin patch group compared to the control, which was attributed to the increased activity of the inflammatory cells and metalloproteinases between the third and seventh postoperative days due to the presence of the fibrin patch.
After inflammation comes the proliferative phase of healing, characterized by the production of collagen by fibroblasts, which increases the strength of the anastomosis[68-70]. The fibroblasts secrete hyaluronic acid and proteoglycans, which are important for cell movement, function, and tissue resilience, and collagen, promoting anastomotic healing[71]. According to the results of our experimental study, fibroblast activity was similar between the subgroups both on the fourth and the eighth postoperative days. This finding is in contrast with Akgün et al[55], who demonstrated an increase of fibroblasts in the fibrin sealant group compared to the control.
Neoangiogenesis is the formation of new blood vessels from the endothelium of pre-existing ones and takes place in the third phase of healing, the proliferative phase, leading to the formation of granular tissue[39,72-74]. It has been shown that this phase is affected by various angiogenic factors such as platelet derived growth factor, tumour necrosis factor-α, and Vascular Endothelial Growth Factor family[61,75]. In our study, the degree of neoangiogenesis was affected by the presence of Bioglue: neoangiogenesis was statistically significantly higher in the Bioglue group only on the eighth postoperative day. Ozel et al[46] also found that neoangiogenesis was higher in the fibrin patch group than in the control.
The production and deposition of collagen, which takes place during the third (proliferation) and fourth (remodelling) phases, is a significant marker of the efficacy of the anastomotic healing process[45,65,68,76,77]. Collagen is mainly produced by fibroblasts and metabolized by certain metalloproteinases, called collagenases[68,78,79]. In our study, collagen deposition was significantly higher in the Bioglue group on both the fourth and the eighth days, a finding similar to Ozel et al[46] who noticed increased collagen production in the fibrin patch group compared to the control.
Histological analysis of anastomotic colon wall samples demonstrated the favourable influence of Bioglue on the complex process of colon anastomosis healing, reflected in the stimulation of the proliferative response, promotion of neoangiogenesis, more abundant young collagen synthesis, and reduction of the duration of the critical healing period.
The mechanical strength of healing wounds depends on fibroblast proliferation and the synthesis of collagen molecules. Collagen determines the mechanical stability and healing capacity of connective tissue. The quantitative measurement of collagen deposed in the anastomosis is accomplished by the measurement of hydroxyproline in the anastomotic tissue[45,80]. Hydroxyproline is one of the basic amino acids of collagen, and its presence is restricted exclusively to the collagen of connective tissue. Low hydroxyproline levels negatively affect the mechanism of colonic anastomosis healing[44,81]. We found that there was a statistically significant increase in hydroxyproline tissue content in both groups (control and Bioglue) on the eighth postoperative day compared to the fourth but no difference between the groups on either the fourth or the eighth day. Ozel et al[46] also demonstrated an increased hydroxyproline concentration in the fibrin patch group compared to the control.
In the third phase of healing, the collagen is synthesized and degraded by a variety of collagenase enzymes from granulocytes, macrophages, and fibroblasts. The concentration of mature collagen in the early phase of healing is decreased up to 40%, an event that correlates with a reduction in the mechanical strength of the anastomosis at this phase and with possible anastomotic dehiscence[45,77,80]. Many studies have shown that their activity is a major pathogenic factor in the postoperative decline of colonic anastomosis strength. Experimental studies have shown maximal collagenetic activity in the colon on the third postoperative day[82]. Collagenase is responsible for the degradation of mature collagen, and its concentration maximizes on the third postoperative day, when increased degradation of type III collagen and synthesis of type I collagen takes place[44,70,71,83].
Furthermore, immunohistochemical studies have shown that collagenase and other matrix metalloproteinases (MMPs) are in close vicinity to the suture line in uncomplicated anastomotic healing[71], so the measurement of collagenase concentration is a marker reflecting the balance of collagenogenesis and collagenolysis[43,84,85]. It has been previously demonstrated that the MMP levels in the anastomotic line are very different from those in adjacent tissue, where the level of MMPs was measured at up to 0.15-0.2 cm from the anastomotic line[86,87]. In the current experiment, collagenase was measured in a segment of 0.5 cm on both sides of the anastomosis in each animal. While the levels of collagenase vary at the anastomotic line and 0.5 cm from it, as tissue samples were the same in all animals, the measured collagenase activity in 1 cm of large intestine with the anastomosis in the middle reflects the total collagenase activity, which is comparable between groups. Collagenase I concentration was similar between the two groups and subgroups on the fourth and on the eighth postoperative days.
In the early period of anastomotic healing, Bioglue seems to support anastomotic integrity in rats, since it increases the bursting pressure. This effect of Bioglue has also been demonstrated in ex vivo porcine gastrojejunal anastomosis[88]. However, it also causes an inflammatory reaction which may increase the time necessary for the healing process. This may represent a major disadvantage for this biomaterial. Regarding the safety of Bioglue, our study demonstrated that the application of Bioglue on the suture line is not associated with increased mortality or dehiscence, in contrast with the study of Slieker et al[89] where the application of Bioglue on sutured colonic anastomoses in mice caused a 100% mortality rate, compared with the 40% mortality rate in the control group. Further studies are most likely required in order to assess the effects of Bioglue on anastomotic healing after direct administration into the colon, as there is only one other study assessing Bioglue’s effect in suture-less closure of colonic defects[90].
COMMENTS
Background
Anastomosis dehiscence is a serious postoperative complication in gastrointestinal surgery, and anastomotic leakage remains the most important cause of postoperative mortality and morbidity in colorectal surgery. The risk of anastomotic leakage is higher in large intestine surgery compared with other gastrointestinal anastomoses. Intestinal anastomoses are complicated by leakages, even in the best and most experienced of hands and despite the development of new surgical techniques, suture materials, and stapling devices. The efficacy of biomaterial over intestinal anastomoses is still controversial in clinical practice.
Research frontiers
The replacement or support of sutures by biomaterial gluing procedures has been investigated for many years. Different techniques using additive materials such as omentum and several types of fibrin sealants to cover the anastomosis have been proposed. Out of a large number of experimental studies, various protective methods such as endoluminar latex prostheses, stents, biofragmental rings, and local application of bioadhesives have been used, with promising results. Tissue glues have been used to seal suture lines for haemostasis and to strengthen and reinforce fragile tissues by tissue adherence. The main idea was to reduce the incidence of dehiscence by increasing the strength of anastomoses, covering the anastomotic line and stimulating healing.
Innovations and breakthroughs
The current study demonstrated the safety and efficacy of Bioglue application on a colonic anastomosis, as it promotes the healing process, especially in the early stages, and increases the bursting pressure. The increase in the mechanical strength of the colonic anastomosis in the early stages is a result of increased adhesion formation and increased collagen deposition and takes place in spite of increased inflammatory cell infiltration.
Applications
The application of biological glue on colonic anastomosis in clinical practice may reduce anastomotic dehiscence and leakage and decrease the related morbidity and mortality
Terminology
Bioglue is a two-component surgical adhesive that confers enhanced bonding properties. It is composed of 45% purified BSA and 10% glutaraldehyde. The advantage of Bioglue is that the bi-functional glutaraldehyde molecule covalently bonds the bovine serum albumin molecules to each other, as well as to lysine in proteins on the cell surface in the extracellular matrix. This reaction is spontaneous, increasing tensile and shear strength independently of the coagulation status of the patient. The albumin provides an extensive flexible network of bonds. When applied to the repair site, it forms a mechanical watertight seal and holds sutures securely.
Peer-review
An interesting paper on sealment of experimental anastomoses in a rat model.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Faculty of Surgery, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Veterinary Manager of the Prefecture of Thessaloniki [IACUC protocol number: (S.N.: 13/11872/11-09-08)].
Conflict-of-interest statement: No conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: December 30, 2016
First decision: February 13, 2017
Article in press: July 24, 2017
P- Reviewer: Krarup PM, Liu L, Nagata J S- Editor: Qi Y L- Editor: A E- Editor: Huang Y
Contributor Information
Kalliopi Despoudi, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
Ioannis Mantzoros, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
Orestis Ioannidis, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece. telonakos@hotmail.com.
Aggeliki Cheva, Department of Pathology, General Hospital “G. Papanikolaou”, 57010 Thessaloniki, Greece.
Nikolaos Antoniou, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
Dimitrios Konstantaras, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
Savvas Symeonidis, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
Manousos George Pramateftakis, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
Efstathios Kotidis, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
Stamatis Angelopoulos, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
Konstantinos Tsalis, Fourth Surgical Department, Medical School, Aristotle University of Thessaloniki, 57010 Thessaloniki, Greece.
References
- 1.Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Morel P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis. 2008;23:265–270. doi: 10.1007/s00384-007-0399-3. [DOI] [PubMed] [Google Scholar]
- 2.Rudinskaite G, Tamelis A, Saladzinskas Z, Pavalkis D. Risk factors for clinical anastomotic leakage following the resection of sigmoid and rectal cancer. Medicina (Kaunas) 2005;41:741–746. [PubMed] [Google Scholar]
- 3.Kanellos I. Progress in the treatment of colorectal cancer. Tech Coloproctol. 2004;8 Suppl 1:s1–s2. doi: 10.1007/s10151-004-0094-0. [DOI] [PubMed] [Google Scholar]
- 4.Schrock TR, Deveney CW, Dunphy JE. Factor contributing to leakage of colonic anastomoses. Ann Surg. 1973;177:513–518. doi: 10.1097/00000658-197305000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukunaga S, Karck M, Harringer W, Cremer J, Rhein C, Haverich A. The use of gelatin-resorcin-formalin glue in acute aortic dissection type A. Eur J Cardiothorac Surg. 1999;15:564–569; discussion 570. doi: 10.1016/s1010-7940(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 6.Nomori H, Horio H, Morinaga S, Suemasu K. Gelatin-resorcinol-formaldehyde-glutaraldehyde glue for sealing pulmonary air leaks during thoracoscopic operation. Ann Thorac Surg. 1999;67:212–216. doi: 10.1016/s0003-4975(98)01184-9. [DOI] [PubMed] [Google Scholar]
- 7.Fleisher AG, Evans KG, Nelems B, Finley RJ. Effect of routine fibrin glue use on the duration of air leaks after lobectomy. Ann Thorac Surg. 1990;49:133–134. doi: 10.1016/0003-4975(90)90371-c. [DOI] [PubMed] [Google Scholar]
- 8.Luukkonen P, Järvinen HJ, Haapiainen R. Early experience with biofragmentable anastomosis ring in colon surgery. Acta Chir Scand. 1990;156:795–799. [PubMed] [Google Scholar]
- 9.Hardy TG Jr, Pace WG, Maney JW, Katz AR, Kaganov AL. A biofragmentable ring for sutureless bowel anastomosis. An experimental study. Dis Colon Rectum. 1985;28:484–490. doi: 10.1007/BF02554090. [DOI] [PubMed] [Google Scholar]
- 10.Uzunköy A, Akinci OF, Coskun A, Aslan O, Kocyigit A. Effects of antiadhesive agents on the healing of intestinal anastomosis. Dis Colon Rectum. 2000;43:370–375. doi: 10.1007/BF02258304. [DOI] [PubMed] [Google Scholar]
- 11.Fini M, Giardino R, Giavaresi G, Rocca M, Aldini N. Tissue Adhesives in Experimental Intestinal Anastomoses. Fibrin Sealing in Surgical and Nonsurgical Fields. Gen Abdominal Surg Pediatr Surg. 1994;2:136–141. [Google Scholar]
- 12.Coselli JS, Bavaria JE, Fehrenbacher J, Stowe CL, Macheers SK, Gundry SR. Prospective randomised study of a protein-based tissue adhesive used as a haemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J Am Coll Surg. 2003;197:243–253. doi: 10.1016/S1072-7515(03)00376-4. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt CW, Mara S, Chrzanowski ANFJ. 1999. A novel tissue bioadhesive (BioGlue®) for thoracic aorta repair in coagulopathic sheep. 34th Congress of the European Society for Surgical Research; p. April; Bern, Switzerland. [Google Scholar]
- 14.Raanani E, Latter DA, Errett LE, Bonneau DB, Leclerc Y, Salasidis GC. Use of “BioGlue” in aortic surgical repair. Ann Thorac Surg. 2001;72:638–640. doi: 10.1016/s0003-4975(01)02663-7. [DOI] [PubMed] [Google Scholar]
- 15.van der Ham AC, Kort WJ, Weijma IM, van den Ingh HF, Jeekel H. Effect of antibiotics in fibrin sealant on healing colonic anastomoses in the rat. Br J Surg. 1992;79:525–528. doi: 10.1002/bjs.1800790617. [DOI] [PubMed] [Google Scholar]
- 16.Sapidis N, Tziouvaras C, Ioannidis O, Kalaitsidou I, Botsios D. The effect of glutamine and synbiotics on the healing of colonic anastomosis. Rev Esp Enferm Dig. 2014;106:255–262. [PubMed] [Google Scholar]
- 17.Galanopoulos G, Pramateftakis MG, Raptis D, Mantzoros I, Kanellos D, Angelopoulos S, Koliakos G, Zaraboukas T, Lazaridis C. The effects of iloprost on colonic anastomotic healing in rats. Tech Coloproctol. 2011;15 Suppl 1:S117–S120. doi: 10.1007/s10151-011-0758-5. [DOI] [PubMed] [Google Scholar]
- 18.Galanopoulos G, Raptis D, Pramateftakis MG, Mantzoros I, Kanellos I, Lazarides C. The effects of iloprost on colonic anastomotic healing in rats under obstructive ileus conditions. J Surg Res. 2014;189:22–31. doi: 10.1016/j.jss.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JD, Kim CS, Fonkalsrud EW, Zeng H, Dindar H. Effects of chronic corticosteroids and vitamin A on the healing of intestinal anastomoses. Am J Surg. 1992;163:71–77. doi: 10.1016/0002-9610(92)90255-p. [DOI] [PubMed] [Google Scholar]
- 20.Shomaf M. Histopathology of human intestinal anastomosis. East Mediterr Health J. 2003;9:413–421. [PubMed] [Google Scholar]
- 21.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 22.Raptis D, Mantzoros I, Pramateftakis MG, Despoudi K, Zaraboukas T, Koliakos G, Kanellos I, Lazarides Ch. The effects of tacrolimus on colonic anastomotic healing in rats. Int J Colorectal Dis. 2012;27:299–308. doi: 10.1007/s00384-011-1337-y. [DOI] [PubMed] [Google Scholar]
- 23.Toothaker L. Newbury Park: Sage Publications, Inc; 1993. Multiple Comparison Procedures. [Google Scholar]
- 24.Mehta C, Patel R. 1996. SPSS Exact Tests 7.0 for Windows. Chicago; p. SPSS Inc. [Google Scholar]
- 25.Fleis JL. 2nd ed. New York: John Wiley and Sons; 1981. Statistical methods for rates and proportions. [Google Scholar]
- 26.Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis. 2007;9:71–79. doi: 10.1111/j.1463-1318.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 27.Zacharakis E, Demetriades H, Kanellos D, Sapidis N, Zacharakis E, Mantzoros I, Kanellos I, Koliakos G, Zaraboukas T, Topouridou K, et al. Contribution of insulin-like growth factor I to the healing of colonic anastomoses in rats. J Invest Surg. 2007;20:9–14. doi: 10.1080/08941930601126074. [DOI] [PubMed] [Google Scholar]
- 28.Willis S, Stumpf M. [Leakages after surgery of the lower gastrointestinal tract] Chirurg. 2004;75:1071–1078. doi: 10.1007/s00104-004-0895-8. [DOI] [PubMed] [Google Scholar]
- 29.Kanellos I, Mantzoros I, Demetriades H, Kalfadis S, Sakkas L, Kelpis T, Betsis D. Sutureless colonic anastomosis in the rat: a randomized controlled study. Tech Coloproctol. 2002;6:143–146. doi: 10.1007/s101510200033. [DOI] [PubMed] [Google Scholar]
- 30.Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208:269–278. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Kanellos D, Pramateftakis MG, Mantzoros I, Zacharakis E, Raptis D, Despoudi K, Zaraboukas T, Koliakos G, Lazaridis H. The effects of the intraperitoneal administration of oxaliplatin and 5-FU on the healing of colonic anastomoses: an experimental study. Tech Coloproctol. 2011;15 Suppl 1:S111–S115. doi: 10.1007/s10151-011-0754-9. [DOI] [PubMed] [Google Scholar]
- 32.Pramateftakis MG, Kanellos D, Mantzoros I, Despoudi K, Raptis D, Angelopoulos S, Koliakos G, Zaraboukas T, Lazaridis C. Intraperitoneally administered irinotecan with 5-fluorouracil impair wound healing of colonic anastomoses in a rat model: an experimental study. Tech Coloproctol. 2011;15 Suppl 1:S121–S125. doi: 10.1007/s10151-011-0755-8. [DOI] [PubMed] [Google Scholar]
- 33.Mantzoros I, Kanellos I, Demetriades H, Christoforidis E, Kanellos D, Pramateftakis MG, Zaraboukas T, Betsis D. Effects of steroid on the healing of colonic anastomoses in the rat. Tech Coloproctol. 2004;8 Suppl 1:s180–s183. doi: 10.1007/s10151-004-0150-9. [DOI] [PubMed] [Google Scholar]
- 34.Goulder F. Bowel anastomoses: The theory, the practice and the evidence base. World J Gastrointest Surg. 2012;4:208–213. doi: 10.4240/wjgs.v4.i9.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rygl M, Novotna J, Herget J, Skaba R, Snajdauf J. Parameters of healing in approximative intestinal anastomosis. Eur J Pediatr Surg. 2009;19:25–29. doi: 10.1055/s-2008-1039010. [DOI] [PubMed] [Google Scholar]
- 36.Kanellos D, Pramateftakis MG, Demetriades H, Zacharakis E, Angelopoulos S, Mantzoros I, Kanellos I, Despoudi K, Zaraboukas T, Koliakos G, et al. Healing of colonic anastomoses after immediate postoperative intraperitoneal administration of oxaliplatin. Int J Colorectal Dis. 2008;23:1185–1191. doi: 10.1007/s00384-008-0538-5. [DOI] [PubMed] [Google Scholar]
- 37.Downing SW. What are the risks of using biologic glues? Ann Thorac Surg. 2003;75:1063; author reply 1063–1063; author reply 1064. doi: 10.1016/s0003-4975(02)03473-2. [DOI] [PubMed] [Google Scholar]
- 38.Hewitt CW, Marra SW, Kann BR, Tran HS, Puc MM, Chrzanowski FA Jr, Tran JL, Lenz SD, Cilley JH Jr, Simonetti VA, DelRossi AJ. BioGlue surgical adhesive for thoracic aortic repair during coagulopathy: efficacy and histopathology. Ann Thorac Surg. 2001;71:1609–1612. doi: 10.1016/s0003-4975(01)02424-9. [DOI] [PubMed] [Google Scholar]
- 39.Demling RH. Nutrition, anabolism, and the wound healing process: an overview. Eplasty. 2009;9:e9. [PMC free article] [PubMed] [Google Scholar]
- 40.Wasserberg N, Nunoo-Mensah JW, Ruiz P, Tzakis AG. The effect of immunosuppression on peritoneal adhesions formation after small bowel transplantation in rats. J Surg Res. 2007;141:294–298. doi: 10.1016/j.jss.2006.12.541. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann NE, Siddiqui SA, Agarwal S, McKellar SH, Kurtz HJ, Gettman MT, Ereth MH. Choice of hemostatic agent influences adhesion formation in a rat cecal adhesion model. J Surg Res. 2009;155:77–81. doi: 10.1016/j.jss.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Kanellos I, Blouhos K, Demetriades H, Pramateftakis MG, Mantzoros I, Zacharakis E, Betsis D. The failed intraperitoneal colon anastomosis after colon resection. Tech Coloproctol. 2004;8 Suppl 1:s53–s55. doi: 10.1007/s10151-004-0111-3. [DOI] [PubMed] [Google Scholar]
- 43.Steffensen B, Häkkinen L, Larjava H. Proteolytic events of wound-healing--coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med. 2001;12:373–398. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- 44.Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 45.Stumpf M, Cao W, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V. Collagen distribution and expression of matrix metalloproteinases 1 and 13 in patients with anastomotic leakage after large-bowel surgery. Langenbecks Arch Surg. 2002;386:502–506. doi: 10.1007/s00423-001-0255-9. [DOI] [PubMed] [Google Scholar]
- 46.Ozel SK, Kazez A, Akpolat N. Does a fibrin-collagen patch support early anastomotic healing in the colon? An experimental study. Tech Coloproctol. 2006;10:233–236. doi: 10.1007/s10151-006-0285-y. [DOI] [PubMed] [Google Scholar]
- 47.Kanellos I, Mantzoros I, Goulimaris I, Zacharakis E, Zavitsanakis A, Betsis D. Effects of the use of fibrin glue around the colonic anastomosis of the rat. Tech Coloproctol. 2003;7:82–84. doi: 10.1007/s10151-003-0014-8. [DOI] [PubMed] [Google Scholar]
- 48.Haukipuro KA, Hulkko OA, Alavaikko MJ, Laitinen ST. Sutureless colon anastomosis with fibrin glue in the rat. Dis Colon Rectum. 1988;31:601–604. doi: 10.1007/BF02556795. [DOI] [PubMed] [Google Scholar]
- 49.Detweiler MB, Kobos JW, Fenton J. Gastrointestinal sutureless anastomosis in pigs using absorbable intraluminal stents, stent placement devices, and fibrin glue - a summary. Langenbecks Arch Surg. 1999;384:445–452. doi: 10.1007/s004230050229. [DOI] [PubMed] [Google Scholar]
- 50.Moran B, Heald R. Anastomotic leakage after colorectal anastomosis. Semin Surg Oncol. 2000;18:244–248. doi: 10.1002/(sici)1098-2388(200004/05)18:3<244::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Gainant A. [Prevention of anastomotic dehiscence in colorectal surgery] J Chir (Paris) 2000;137:45–50. [PubMed] [Google Scholar]
- 52.Ekmektzoglou KA, Zografos GC, Kourkoulis SK, Dontas IA, Giannopoulos PK, Marinou KA, Poulakou MV, Perrea DN. Mechanical behavior of colonic anastomosis in experimental settings as a measure of wound repair and tissue integrity. World J Gastroenterol. 2006;12:5668–5673. doi: 10.3748/wjg.v12.i35.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hjortrup A, Nordkild P, Kiaergaard J, Sjøntoft E, Olesen HP. Fibrin adhesive versus sutured anastomosis: a comparative intraindividual study in the small intestine of pigs. Br J Surg. 1986;73:760–761. doi: 10.1002/bjs.1800730928. [DOI] [PubMed] [Google Scholar]
- 54.Capitán Morales LC, Rodríguez Nuñez E, Morales Conde S, Sanchez Ganfornina F, Del Rio Lafuente FD, Cabot Ostos E, Ortega Beviá JM, Loscertales Abril J, Cantillana Martínez J. Experimental study of sutureless colorectal anastomosis. Hepatogastroenterology. 2000;47:1284–1290. [PubMed] [Google Scholar]
- 55.Akgün A, Kuru S, Uraldi C, Tekin O, Karip B, Tug T, Ongören AU. Early effects of fibrin sealant on colonic anastomosis in rats: an experimental and case-control study. Tech Coloproctol. 2006;10:208–214. doi: 10.1007/s10151-006-0281-2. [DOI] [PubMed] [Google Scholar]
- 56.Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240:255–259. doi: 10.1097/01.sla.0000133186.81222.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izbicki JR, Kreusser T, Meier M, Prenzel KL, Knoefel WT, Passlick B, Kuntz G, Schiele U, Thetter O. Fibrin-glue-coated collagen fleece in lung surgery--experimental comparison with infrared coagulation and clinical experience. Thorac Cardiovasc Surg. 1994;42:306–309. doi: 10.1055/s-2007-1016510. [DOI] [PubMed] [Google Scholar]
- 58.Yol S, Tekin A, Yilmaz H, Küçükkartallar T, Esen H, Caglayan O, Tatkan Y. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008;146:190–194. doi: 10.1016/j.jss.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 59.Cicha I, Garlichs CD, Daniel WG, Goppelt-Struebe M. Activated human platelets release connective tissue growth factor. Thromb Haemost. 2004;91:755–760. doi: 10.1160/TH03-09-0602. [DOI] [PubMed] [Google Scholar]
- 60.Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- 61.Softova EB. Examining cellular communication and role of growth factors during wound healing. J Wound Cage. 2005;14:172. [Google Scholar]
- 62.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 63.Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 64.Wilson DA. Principles of early wound management. Vet Clin North Am Equine Pract. 2005;21:45–62, vi. doi: 10.1016/j.cveq.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 66.Salmela MT, Pender SL, Karjalainen-Lindsberg ML, Puolakkainen P, Macdonald TT, Saarialho-Kere U. Collagenase-1 (MMP-1), matrilysin-1 (MMP-7), and stromelysin-2 (MMP-10) are expressed by migrating enterocytes during intestinal wound healing. Scand J Gastroenterol. 2004;39:1095–1104. doi: 10.1080/00365520410003470. [DOI] [PubMed] [Google Scholar]
- 67.Toy LW. Matrix metalloproteinases: their function in tissue repair. J Wound Care. 2005;14:20–22. doi: 10.12968/jowc.2005.14.1.26720. [DOI] [PubMed] [Google Scholar]
- 68.Tettamanti G, Grimaldi A, Congiu T, Perletti G, Raspanti M, Valvassori R, de Eguileor M. Collagen reorganization in leech wound healing. Biol Cell. 2005;97:557–568. doi: 10.1042/BC20040085. [DOI] [PubMed] [Google Scholar]
- 69.Clark RA, An JQ, Greiling D, Khan A, Schwarzbauer JE. Fibroblast migration on fibronectin requires three distinct functional domains. J Invest Dermatol. 2003;121:695–705. doi: 10.1046/j.1523-1747.2003.12484.x. [DOI] [PubMed] [Google Scholar]
- 70.Ottani V, Martini D, Franchi M, Ruggeri A, Raspanti M. Hierarchical structures in fibrillar collagens. Micron. 2002;33:587–596. doi: 10.1016/s0968-4328(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 71.Steffensen B, Xu X, Martin PA, Zardeneta G. Human fibronectin and MMP-2 collagen binding domains compete for collagen binding sites and modify cellular activation of MMP-2. Matrix Biol. 2002;21:399–414. doi: 10.1016/s0945-053x(02)00032-x. [DOI] [PubMed] [Google Scholar]
- 72.Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis. 2007;39:212–220. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Murray B, Wilson DJ. A study of metabolites as intermediate effectors in angiogenesis. Angiogenesis. 2001;4:71–77. doi: 10.1023/a:1016792319207. [DOI] [PubMed] [Google Scholar]
- 74.Srivastava K, Dash D. Changes in membrane microenvironment and signal transduction in platelets from NIDDM patients-a pilot study. Clin Chim Acta. 2002;317:213–220. doi: 10.1016/s0009-8981(01)00794-x. [DOI] [PubMed] [Google Scholar]
- 75.Rabinovsky ED, Draghia-Akli R. Insulin-like growth factor I plasmid therapy promotes in vivo angiogenesis. Mol Ther. 2004;9:46–55. doi: 10.1016/j.ymthe.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Lygoe KA, Norman JT, Marshall JF, Lewis MP. AlphaV integrins play an important role in myofibroblast differentiation. Wound Repair Regen. 2004;12:461–470. doi: 10.1111/j.1067-1927.2004.12402.x. [DOI] [PubMed] [Google Scholar]
- 77.Oxlund H, Christensen H, Seyer-Hansen M, Andreassen TT. Collagen deposition and mechanical strength of colon anastomoses and skin incisional wounds of rats. J Surg Res. 1996;66:25–30. doi: 10.1006/jsre.1996.0367. [DOI] [PubMed] [Google Scholar]
- 78.Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 2004;12:134–147. doi: 10.1111/j.1067-1927.2004.012208.x. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi T, Inoue T, Okada H, Kikuta T, Kanno Y, Nishida T, Takigawa M, Sugaya T, Suzuki H. Connective tissue growth factor mediates the profibrotic effects of transforming growth factor-beta produced by tubular epithelial cells in response to high glucose. Clin Exp Nephrol. 2005;9:114–121. doi: 10.1007/s10157-005-0347-x. [DOI] [PubMed] [Google Scholar]
- 80.Syk I, Agren MS, Adawi D, Jeppsson B. Inhibition of matrix metalloproteinases enhances breaking strength of colonic anastomoses in an experimental model. Br J Surg. 2001;88:228–234. doi: 10.1046/j.1365-2168.2001.01649.x. [DOI] [PubMed] [Google Scholar]
- 81.Hendriks T, Hesp WL, Klompmakers AA, Lubbers EJ, de Boer HH. Solubility of tissue hydroxyproline in experimental intestinal anastomoses. Exp Mol Pathol. 1985;43:253–259. doi: 10.1016/0014-4800(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 82.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hall MC, Young DA, Waters JG, Rowan AD, Chantry A, Edwards DR, Clark IM. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-beta 1. J Biol Chem. 2003;278:10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- 84.Dabareiner RM, Sullins KE, White NA, Snyder JR. Serosal injury in the equine jejunum and ascending colon after ischemia-reperfusion or intraluminal distention and decompression. Vet Surg. 2001;30:114–125. doi: 10.1053/jvet.2001.21393. [DOI] [PubMed] [Google Scholar]
- 85.Madl C, Druml W. Gastrointestinal disorders of the critically ill. Systemic consequences of ileus. Best Pract Res Clin Gastroenterol. 2003;17:445–456. doi: 10.1016/s1521-6918(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 86.Agren MS, Andersen TL, Mirastschijski U, Syk I, Schiødt CB, Surve V, Lindebjerg J, Delaissé JM. Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery. 2006;140:72–82. doi: 10.1016/j.surg.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 87.Krarup PM, Eld M, Heinemeier K, Jorgensen LN, Hansen MB, Ågren MS. Expression and inhibition of matrix metalloproteinase (MMP)-8, MMP-9 and MMP-12 in early colonic anastomotic repair. Int J Colorectal Dis. 2013;28:1151–1159. doi: 10.1007/s00384-013-1697-6. [DOI] [PubMed] [Google Scholar]
- 88.Nandakumar G, Richards BG, Trencheva K, Dakin G. Surgical adhesive increases burst pressure and seals leaks in stapled gastrojejunostomy. Surg Obes Relat Dis. 2010;6:498–501. doi: 10.1016/j.soard.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 89.Slieker JC, Vakalopoulos KA, Komen NA, Jeekel J, Lange JF. Prevention of leakage by sealing colon anastomosis: experimental study in a mouse model. J Surg Res. 2013;184:819–824. doi: 10.1016/j.jss.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 90.Vakalopoulos KA, Wu Z, Kroese LF, Jeekel J, Kleinrensink GJ, Dodou D, Lam KH, Lange JF. Sutureless closure of colonic defects with tissue adhesives: an in vivo study in the rat. Am J Surg. 2017;213:151–158. doi: 10.1016/j.amjsurg.2016.05.009. [DOI] [PubMed] [Google Scholar]


