Abstract
AIM
To assess the diagnostic value of FIB-4, aspartate aminotransferase-to-platelet ratio index (APRI), and liver stiffness measurement (LSM) in patients with hepatitis B virus infection who have persistently normal alanine transaminase (PNALT).
METHODS
We enrolled 245 patients with chronic hepatitis B: 95 in PNALT group, 86 in intermittently elevated alanine transaminase (PIALT1) group [alanine transaminase (ALT) within 1-2 × upper limit of normal value (ULN)], and 64 in PIALT2 group (ALT > 2 × ULN). All the patients received a percutaneous liver biopsy guided by ultrasonography. LSM, biochemical tests, and complete blood cell counts were performed.
RESULTS
The pathological examination revealed moderate inflammatory necrosis ratios of 16.81% (16/95), 32.56% (28/86), and 45.31% (28/64), and moderate liver fibrosis of 24.2% (23/95), 33.72% (29/86), and 43.75% (28/64) in the PNALT, PIALT1, and PIALT2 groups, respectively. The degrees of inflammation and liver fibrosis were significantly higher in the PIALT groups than in the PNALT group (P < 0.05). No significant difference was found in the areas under the curve (AUCs) between APRI and FIB-4 in the PNALT group; however, significant differences were found between APRI and LSM, and between FIB-4 and LSM in the PNALT group (P < 0.05 for both). In the PIALT1 and PIALT2 groups, no significant difference (P > 0.05) was found in AUCs for all comparisons (P > 0.05 for all). In the overall patients, a significant difference in the AUCs was found only between LSM and APRI (P < 0.05).
CONCLUSION
APRI and FIB-4 are not the ideal noninvasive hepatic fibrosis markers for PNALT patients. LSM is superior to APRI and FIB-4 in PNALT patients because of the influence of liver inflammation and necrosis.
Keywords: Liver stiffness measurement, Hepatitis B virus, FIB-4, Aspartate aminotransferase-to-platelet ratio index, Normal, Alanine aminotransferase
Core tip: To assess the diagnostic value of FIB-4, aspartate aminotransferase-to-platelet ratio index (APRI), and liver stiffness measurement (LSM) in patients with hepatitis B virus infection who have persistently normal alanine transaminase (PNALT), we enrolled 245 patients with chronic hepatitis B: 95 in PNALT group, 86 in intermittently elevated alanine transaminase (PIALT1) group [alanine transaminase (ALT) within 1-2 × upper limit of normal value (ULN)], and 64 in PIALT2 group (ALT > 2 × ULN). The results showed that APRI and FIB-4 are not the ideal noninvasive hepatic fibrosis markers for PNALT patients. LSM is superior to APRI and FIB-4 in PNALT patients because of the influence of liver inflammation and necrosis.
INTRODUCTION
Approximately a third of the world’s population have serological evidence of past or present hepatitis B virus (HBV) infection, and 350-400 million people are known to be chronic HBV surface antigen (HBsAg) carriers. The disease spectrum and natural history of chronic HBV infection are diverse and varied, ranging from an inactive carrier state to progressive chronic hepatitis B (CHB), which may progress to cirrhosis and hepatocellular carcinoma (HCC)[1,2]. Chronic HBV infection is a dynamic process, and its natural history was schematically divided into five phases by the European Association for the Study of the Liver Clinical Practice Guidelines (2012) as follows[1]: (1) the “immune tolerant” phase; (2) the “immune reactive HBeAg-positive phase”; (3) the “inactive HBV carrier phase”; (4) “HBeAg-negative CHB” phase; and (5) the “HBeAg-negative CHB” or “HBsAg-negative” phase.
Although serum levels of alanine transaminase (ALT), an enzyme released from hepatocytes during liver injury, should reflect the degree of liver damage[3], not all patients with chronic HBV infection have persistently elevated ALT levels. Patients in the immune-tolerant phase and inactive carriers have persistently normal ALT (PNALT) levels[4,5], while a proportion of patients with HBeAg-negative CHB may have intermittently normal ALT levels. Histological injury in patients with normal ALT levels has also been reported[5-10]. Furthermore, some large cohort studies have shown that patients with CHB who have normal serum ALT levels were also at risk for the development of cirrhosis and HCC[11,12]. Liver biopsy (LB) is the current gold standard for assessing hepatic inflammation and fibrosis in patients with chronic HBV infection who have PNALT[7]. The invasiveness of liver puncture, the limitation of the specimen, and the poor patient compliance have restricted the application of LB, which has led to the development of noninvasive methods such as FIB-4[13] and aspartate aminotransferase (AST)-to-platelet ratio index (APRI)[14] for evaluating fibrosis in patients with chronic HBV infection. Liver stiffness measurement (LSM) using transient elastography (FibroScan) has been widely used in the diagnosis of chronic liver fibrosis[15,16]. However, the diagnostic value of FIB-4, APRI, and LSM in patients with HBV infection with PNALT is not clear.
In this study, we comprehensively evaluated the characteristics of histological abnormalities in a large population of Chinese CHB patients with PNALT, with an aim to analyze the diagnostic value of FIB-4, APRI, and LSM in patients with HBV who have PNALT.
MATERIALS AND METHODS
Ethics statement
The study was approved by the Medical Ethics Committee of The Third Hospital of Zhenjiang Affiliated Jiangsu University (No. 2013011), and written informed consent was obtained from each patient prior to participation. The study was conducted in accordance with the Declaration of Helsinki.
Patients
This was a retrospective cohort study of patients with CHB diagnosed between January 2011 and June 2016 at the Department of Hepatology, The Third Hospital of Zhenjiang Affiliated Jiangsu University. The patients were examined every 3 to 6 mo, or more often if clinically indicated. At each visit, liver biochemistry and HBV serology, including HBsAg, HBeAg, anti-HBe, and HBV DNA levels, and HBV genotype, were evaluated. The inclusion criteria were as follows[17]: (1) being HBsAg positive for at least 6 mo; (2) HBV DNA level > 1000 copies/mL; and (3) patients with PNALT levels who had at least three ALT values taken in the year prior to baseline LB, with all values > 40 IU/L and remaining so until the start of treatment or the last follow-up if not treated. Patients were categorized as having PIALT levels if they had at least three ALT values taken, and at least one measurement of > 40 IU/L in the year prior to the baseline LB, or any time until the start of treatment or the last follow-up if not treated (intermittently elevated)[6-8,10,18,19]. The exclusion criteria were as follows: (1) hepatitis A, C, or D, or human immunodeficiency virus coinfection; (2) evidence of liver disease with another etiology; (3) use of hepatotoxic drugs or regular consumption of alcohol; (4) previous antiviral (HBV) therapy or any liver functional protection therapy to alleviate hepatic inflammation; and (5) less than three normal ALT values taken prior to the biopsy. The clinical data from these participants were given new numbers and anonymized before analysis. All data were provided separately as Supporting Information.
Biochemical and serologic tests
Biochemical tests and complete blood cell counts were performed using routine automated analyzers. The upper limit of normal value (ULN) of ALT level was 40 IU/L. HBsAg, HBeAg, and anti-HBe levels were assayed with commercially available enzyme-linked immunosorbent assay (ELISA) kits. HBV DNA level was measured using real-time polymerase chain reaction (PCR), with a lower detection limit of 1000 copies/mL (DaAn Gene Co, China).
Genotyping by multiplex PCR
Genotyping was performed using multiplex PCR with specific primers for each genotype (A-F) of HBV[20].
LB and histological assessment
Liver biopsies were obtained using a 16-G core aspiration needle, a biopsy length of at least 1.5 cm, and six portal tracts or more. Biopsies were fixed, paraffin-embedded, and stained with hematoxylin and eosin for morphological evaluation and Masson’s trichrome stain for the assessment of fibrosis. The pathologist who reviewed all biopsy specimens was blinded to the biochemical and virological results of the patients, the amount of necrosis and inflammation, and the degree of fibrosis according to the Knodell scoring system[21]. Knodell necroinflammatory scores were classified into four categories as follows: Minimal (0-3), mild (4-6), moderate (7-9), and severe (10-14)[22]. Minimal and mild necroinflammatory scores were considered insignificant, while moderate and severe scores were considered significant. Knodell fibrosis scores were also classified into four categories as follows: Minimal (0), mild (1), moderate (2), and severe (3). Minimal and mild fibrosis scores were considered insignificant, while moderate and severe scores were considered significant.
LSM
LSM was assessed using transient elastography (FibroScan502, Echosens, Paris, France) with the 3.5-MHz standard probe by the same operator (experience, > 10000 measurements) who was blinded to the other parameters of the patients, as previously described. The examination was performed with the patient lying in the dorsal decubitus position, with the right arm in maximal abduction. The tip of the probe transducer was placed on the skin, between the ribs at the level of the right lobe of the liver. The results are expressed in kPa, and each LSM corresponded to the median of 10 validated measurements.
Statistical analysis
Results are presented as median (range) or mean ± SD as appropriate. Data on demographic and clinical features of the CHB patients were analyzed using Statistical Package for the Social Sciences (SPSS) version 21.0 (SPSS Inc, Chicago, IL, United States). Statistical analyses were performed using χ2 and Fisher exact tests for categorical variables. The Student’s t-test or one-way analysis of variance was used for group comparisons of parametric quantitative data. The equations for the two noninvasive markers analyzed were as follows: FIB-4 = (Age × AST)/(PLT × ALT1/2) and APRI = (AST/ULN) × 100/PLT. The receiver-operating characteristic (ROC) curves were used to calculate the cutoff values of FIB-4, APRI, and LSM. The ROC analysis was performed using MedCalc software version 10.4.7.0 (MedCalc, Mariakerke, Belgium). All P-values were two-sided.
RESULTS
Clinical and pathological characteristics of CHB patients with different levels of ALT
Table 1 shows that among 245 cases of CHB, 95 were in the PNALT group, 86 in the PIALT1 group (ALT within 1-2 × ULN), and 64 in the PIALT2 group (ALT > 2 × ULN). Body mass index (BMI), platelet count (PLT), prothrombin activity (PTA), ALT and AST (aspiration aminotransferase), serum albumin, E antigen status (positive or negative), HBsAg level, and HBV DNA expression level (≥ 3, < 5 and ≥ 5) were analyzed. We found that the differences in age, ALT, AST, PLT, and other factors were statistically significant (P < 0.05) between the PNALT and PIALT groups. No significant differences were found in E antigen status, HBsAg level, and HBV DNA. The pathological examination revealed moderate inflammatory necrosis ratios of 16.81% (16/95), 32.56% (28/86), and 45.31% (28/64), and moderate liver fibrosis of 24.2% (23/95), 33.72% (29/86), and 43.75% (28/64) in the PNALT, PIALT1, and PIALT2 groups, respectively. The degrees of inflammation and liver fibrosis in the PIALT groups were significantly higher than those in the PNALT group (P < 0.05).
Table 1.
Demographic and clinical characteristics of chronic hepatitis B patients n (%)
| Characteristic | PNALT (n = 95) | PIALT1 (n = 86) | PIALT2 (n = 64) | Statistic | P value |
| Age (yr) | 34.5 ± 11.2 | 34.2 ± 12.5 | 36.5 ± 13.5 | 5.423 | 0.0211 |
| Sex | |||||
| Male | 70 (73.7) | 58 (67.4) | 46 (71.9) | 0.885 | 0.6422 |
| Female | 25 (26.3) | 28 (32.6) | 18 (28.1) | ||
| BMI | 23.4 ± 2.65 | 24.0 ± 3.6 | 24.25 ± 3.37 | 1.231 | 0.5431 |
| PLT (× 109/L) | 200.1 ± 60.3 | 196.8 ± 65.4 | 186.5 ± 74.5 | 6.364 | 0.0181 |
| PTA (%) | 99.6 ± 6.7 | 99.6 ± 8.7 | 102.3 ± 10.3 | 0.674 | 0.7741 |
| ALB (g/L) | 41.3 ± 3.4 | 41.6 ± 3.7 | 42.4 ± 4.1 | 1.536 | 0.5431 |
| ALT (U/L) | 21.4 ± 4.3 | 56.2 ± 19.4 | 113.6 ± 55.3 | 25.754 | < 0.0011 |
| AST (U/L) | 22.6 ±6.8 | 55.4 ± 16.6 | 124.5 ± 57.6 | 31.644 | < 0.0011 |
| APRI | 0.32 ± 0.14 | 0.61 ± 0.44 | 1.25 ± 0.62 | 284.92 | < 0.0011 |
| FIB-4 | 0.72 ± 0.36 | 1.12 ± 0.53 | 1.67 ± 0.84 | 56.37 | < 0.0011 |
| FibroScan (kPa) | 5.33± 2.45 | 7.36 ± 3.14 | 10.22 ± 5.53 | 46.34 | < 0.0011 |
| HBsAg [lg (IU/L)] | 4.41 ± 0.73 | 4.53 ± 0.88 | 4.38 ± 0.64 | 0.743 | 0.437 |
| HBV DNA [lg (IU/mL)] | 6.78 ± 2.13 | 6.53 ± 2.43 | 6.42 ± 2.54 | 0.864 | 0.2541 |
| ≥ 3, < 5 | 15 (15.8) | 11 (12.8) | 10 (15.6) | 0.384 | 0.8252 |
| ≥ 5 | 80 (84.2) | 75 (87.2) | 54 (84.4) | ||
| E antigen | |||||
| Positive | 42 (44.2) | 31 (36) | 29 (45.3) | 1.78 | 0.4112 |
| Negative | 53 (55.8) | 55 (64) | 35 (54.7) | ||
| Necroinflammatory score | 2.21 ± 2.14 | 3.47 ± 3.64 | 4.74 ± 3.65 | 23.43 | 0.001 |
| Minimal | 35 (32.4) | 20 (23.3) | 8 (12.5) | 18.69 | 0.005 |
| Mild | 44 (38) | 38 (44.2) | 27 (42.4) | ||
| Moderate | 15 (15.8) | 26 (30.2) | 22 (34.4) | ||
| Severe | 1 (0.9) | 2 (2.3) | 7 (10.9) | ||
| Fibrosis score | 1.53 ± 0.46 | 2.38 ± 1.27 | 2.85 ± 1.75 | 15.237 | 0.0051 |
| Minimal | 32 (33.7) | 18 (20.9) | 13 (20.3) | 13.275 | 0.0352 |
| Mild | 40 (42.1) | 40 (51.2) | 23 (35.9) | ||
| Moderate | 21 (22.1) | 24 (27.9) | 21 (32.8) | ||
| Severe | 2 (2.1) | 5 (5.8) | 7 (10.9) |
One-way analysis of variance;
Pearson χ2. APRI = [AST (U/L)/ULN × 100/PLT (109/L)]; FIB-4 = [age (yr) × AST (U/L)]/[PLT (109/L) × (ALT U/L)1/2].
Diagnostic value of APRI in the three groups of CHB
We considered hepatic fibrosis (insignificant/significant) as a categorical variable and APRI as a variable to test the AUC of APRI in the PNALT, PIALT1, and PIALT2 groups and in all the patients. The AUC of APRI was 0.518 in the PNALT group (95%CI: 0.414-0.622; specificity, 43.1%; sensitivity, 69.6%; cutoff value, 0.202; P = 0.7852), 0.659 in the PNALT1 group (95%CI: 0.548-0.757; specificity, 34.5%; sensitivity, 82.4%; cutoff value, 0.524; P = 0.011), 0.735 in the PNALT2 group (95%CI: 0.609-0.837; specificity, 83.7%; sensitivity, 85.7%; cutoff value, 1.26; P < 0.001), and 0.65 for all the patients (95%CI: 0.587-0.710; specificity, 88.5%; sensitivity, 54.4%; cutoff value, 1.15; P < 0.001). APRI showed a high diagnostic value for hepatic fibrosis in CHB patients with abnormal ALT levels in comparison with those with normal ALT levels (Figure 1).
Figure 1.
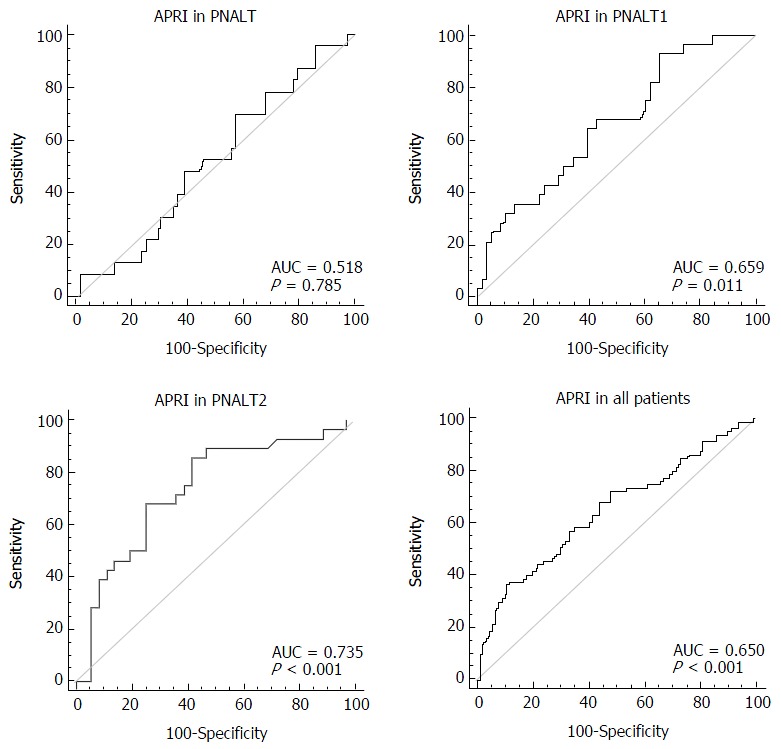
Diagnostic value of aspartate aminotransferase-to-platelet ratio index in different groups of chronic hepatitis B patients. PNALT: Persistently normal alanine transaminase; APRI: Aspartate aminotransferase-to-platelet ratio index; AUC: Areas under the curve.
Diagnostic value of FIB-4 in the three groups of CHB
We considered hepatic fibrosis (insignificant/significant) as a categorical variable and FIB-4 as a variable to test the AUC of FIB-4 in the PNALT, PIALT1, and PIALT2 groups and in all the patients. The AUC of FIB-4 was 0.597 in the PNALT group (95%CI: 0.492-0.697; specificity, 68.1%; sensitivity, 52.2%; cutoff value, 0.698; P = 0.152), 0.642 in the PNALT1 group (95%CI: 0.531-0.742; specificity, 67.9%; sensitivity, 56.9%; cutoff value, 1.174; P = 0.021), 0.667 in the PNALT2 group (95%CI: 0.538-0.780; specificity, 52.8%; sensitivity, 78.7%; cutoff value, 1.46; P = 0.015), and 0.659 for all the patients (95%CI: 0.596-0.718; specificity, 66.8%; sensitivity, 74.2%; cutoff value, 0.96; P < 0.001). FIB-4 showed a high diagnostic value for hepatic fibrosis in CHB patients with abnormal ALT levels in comparison with those with normal ALT levels (Figure 2).
Figure 2.
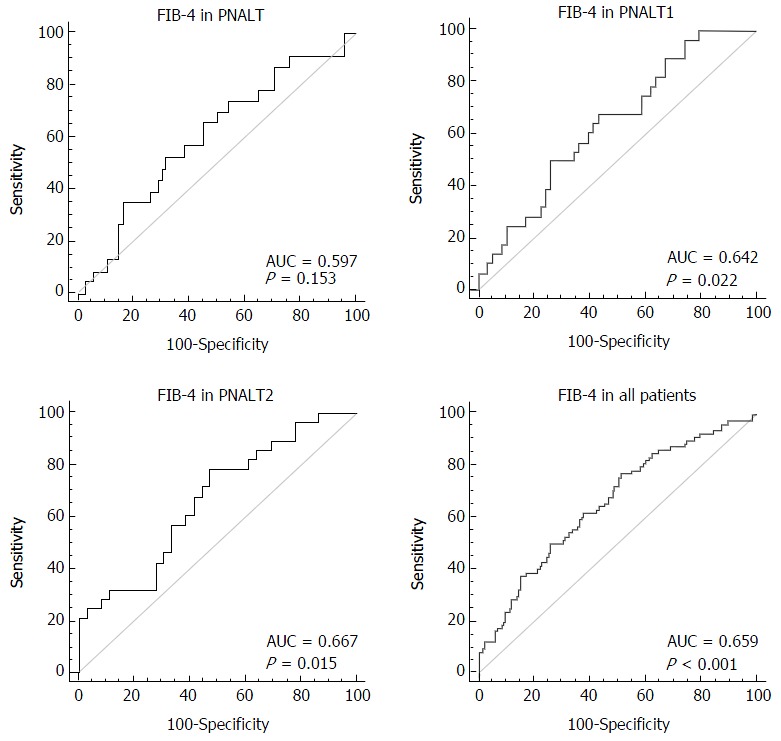
Diagnostic value of FIB-4 in different groups of chronic hepatitis B patients. PNALT: Persistently normal alanine transaminase; AUC: Areas under the curve.
Diagnostic value of LSM in the three groups of CHB
We considered hepatic fibrosis (insignificant/significant) as a categorical variable and LSM as a variable to test the AUC of LSM in the PNALT, PIALT1, and PIALT2 groups and in all the patients. The AUC of LSM was 0.769 in the PNALT group (95%CI: 0.009-0.879; 95%CI: 0.709-0.850; specificity, 79.2%; sensitivity, 70.1%; cutoff value, 7.3; P < 0.001), 0.800 in the PNALT1 group (95%CI: 0.700-0.879; specificity, 72.4%; sensitivity, 82.7%; cutoff value, 7.5; P < 0.001), 0.708 in the PNALT2 group (95%CI: 0.581-0.815; specificity, 72.7%; sensitivity, 67.9%; cutoff value, 8.5; P = 0.017), and 0.763 for all the patients (95%CI: 0.596-0.718; specificity, 78.2%; sensitivity, 70.1%; cutoff value, 7.5; P < 0.001). LSM showed a high diagnostic value for the three groups of CHB, although the sensitivity and specificity in the PNALT2 showed a downward trend (Figure 3).
Figure 3.
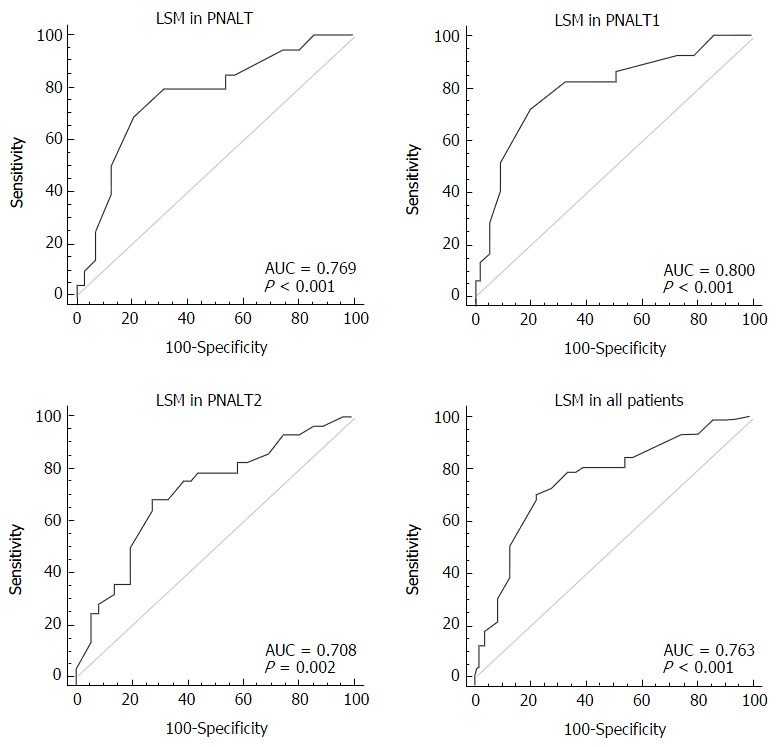
Diagnostic value of liver stiffness measurement in different groups of chronic hepatitis B patients. PNALT: Persistently normal alanine transaminase; LSM: Liver stiffness measurement; AUC: Areas under the curve.
Comparison of the diagnostic value of the three noninvasive liver fibrosis markers in CHB
As shown in Table 2 and Figure 4, there was no significant difference in the AUCs between APRI and FIB-4 in the PNALT group; however, significant differences were found between APRI and LSM, and between FIB-4 and LSM in the PNALT group (P < 0.05 for both). In the PIALT1 and PIALT2 groups, no significant difference (P > 0.05) was found in AUCs for all comparisons (P > 0.05 for all). In the overall patients, a significant difference in the AUCs was found only between LSM and APRI (P < 0.05).
Table 2.
Pairwise comparisons of receiver-operating characteristic curves
| PNALT | PIALT1 | PIALT2 | All patients | |
| APRI vs FIB-4 | ||||
| Difference between areas | 0.0628 | 0.0169 | 0.068 | 0.00904 |
| Standard error | 0.0557 | 0.0699 | 0.0738 | 0.0245 |
| 95%CI | -0.046 to 0.172 | -0.120 to 0.154 | -0.077 to 0.213 | -0.039 to 0.057 |
| z statistic | 1.129 | 0.242 | 0.921 | 0.369 |
| P value | 0.2588 | 0.8087 | 0.3569 | 0.712 |
| APRI vs LSM | ||||
| Difference between areas | 0.256 | 0.124 | 0.0263 | 0.111 |
| Standard error | 0.104 | 0.0896 | 0.0882 | 0.0518 |
| 95%CI | 0.0525-0.459 | -0.0511 to 0.30 | -0.147 to 0.19 | 0.00909-0.212 |
| z statistic | 2.465 | 1.389 | 0.298 | 2.135 |
| P value | 0.0137 | 0.1649 | 0.7655 | 0.0327 |
| FIB 4 vs LSM | ||||
| Difference between areas | 0.193 | 0.141 | 0.0417 | 0.102 |
| Standard error | 0.106 | 0.0938 | 0.0957 | 0.0531 |
| 95%CI | -0.014 to 0.400 | -0.0425 to 0.325 | -0.146 to 0.229 | -0.00246 to 0.206 |
| z statistic | 1.829 | 1.507 | 0.435 | 1.914 |
| P value | 0.0374 | 0.1318 | 0.663 | 0.0557 |
Figure 4.
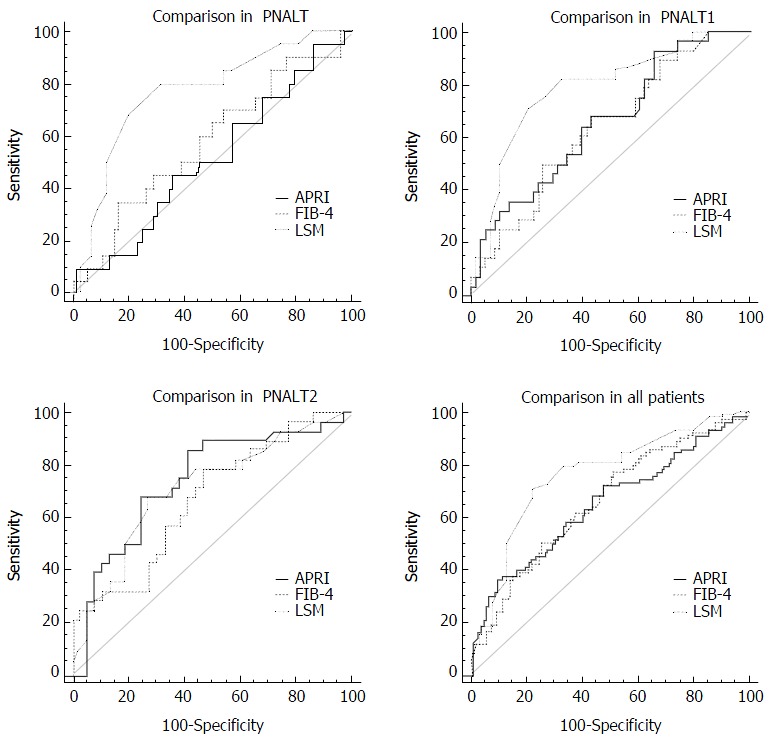
Comparison of the diagnostic value of the three noninvasive liver fibrosis markers in different groups of chronic hepatitis B patients. APRI: Aspartate aminotransferase-to-platelet ratio index; FIB-4: Fibrosis-4; LSM: Liver stiffness measurement.
DISCUSSION
Hepatic fibrosis is a compensatory repair process associated with inflammation and necrosis of the liver. Therefore, 25% to 40% of liver fibrosis cases will eventually progress to cirrhosis and even liver cancer. Early liver fibrosis can be reversed after correct treatment; thus, early diagnosis of liver fibrosis will be beneficial to the treatment of CHB.
LB is still the gold standard for assessment of liver fibrosis in CHB, although it is invasive, expensive, and associated with risk of complications and poor patient compliance, and of subjective differences in pathologists. The accuracy of the pathological diagnosis of liver fibrosis can only be approximately 90% and even reported to be < 80%[23]. Therefore, noninvasive diagnostic markers for liver fibrosis have been developed, such as serum markers and models, imaging, transient liver hardness, and other noninvasive techniques. Although these noninvasive diagnostic methods have their own advantages and disadvantages, they have not completely replaced LB. However, new techniques and methods have become greatly improved. Among the noninvasive tests developed are FIB-4, APRI, and LSM; previous studies have shown these noninvasive markers and techniques to be strong predictors of liver fibrosis.
A multicenter, retrospective study reported that the AUCs of APRI were 0.72, 0.812, and 0.707 in CHB patients with F2, F3, and F4 fibrosis, respectively[24]. The AUCs of APRI were 0.65, 0.659, and 0.735 in our PNALT, PIALT1, and PIALT2 CHB patients, respectively. These results showed that the diagnostic value of APRI in the CHB patients with elevated ALT levels was better than that in the CHB patients with normal ALT. APRI is the ratio of AST to PLT, and elevated AST levels has a higher APRI value and thus is more likely to distinguish patient groups with different AST levels.
In a study of 388 cases of cirrhosis of varied severity assessed using APRI and FIB-4, the AUCs were 0.68 (95%CI: 0.63-0.74) and 0.73 (95%CI: 0.68-0.78)[25], respectively. In our study, the AUC of FIB-4 was 0.597 for PNALT, 0.642 for PNALT1, and 0.667 for PNALT2. The diagnostic value of FIB-4 in the CHB patients with elevated ALT levels was better than that in the CHB patients with normal ALT levels and APRI.
We detected LSM by FibroScan in the CHB patients, with an AUC of 0.769 for PNALT, 0.800 for PIALT1, 0.708 for PIALT2, and 0.763 for all the patients. LSM showed a good diagnostic value for the three groups of CHB patients. Furthermore, the diagnostic value of LSM in the high ALT group was not as good as that in the PNALT group, and the sensitivity and specificity in the PNALT2 group even had a downward trend. In a report on FibroScan in China[26], the AUCs were 0.916 and 0.971 for the diagnosis of ≥ F2 liver fibrosis (F0-1 vs F2-4) and cirrhosis (F = 4, F0-3 vs F4), and the sensitivity and accuracy in the ALT level ≥ 2 × ULN group were significantly lower than those in the other lower-level ALT groups. The reason is that LSM is susceptible to liver inflammation[27,28] and cholestasis[29,30].
We compared the three noninvasive methods for the diagnosis of hepatic fibrosis. The results showed that the AUC differences between LSM and APRI, and between LSM and FIB-4 were statistically significant in the PNALT group (P < 0.05 for both), but not in the PIALT1 and PIALT2 groups (P > 0.05 for all). For all the patients, we found that the AUC difference was statistically significant only between LSM and APRI (P < 0.05).
In a previous report from South Korea, the diagnostic value of LSM for hepatic fibrosis was compared with that of APRI. The results suggested that LSM is superior to APRI in 916 patients with CHB (AUC: 0.774 vs 0.72 for ≥ F2, 0.849 vs 0.812 for ≥ F3, and 0.902 vs 0.707 for F4; all P < 0.05)[24]. Another report revealed that LSM was better than APRI and FIB-4 when the LSM cutoff value was > 13.6 kPa for the diagnosis of portal hypertension in cirrhosis patients[31].
In conclusion, we evaluated three common noninvasive hepatic fibrosis techniques in CHB patients with different ALT levels. The results showed that APRI and FIB-4 are not the ideal noninvasive hepatic fibrosis markers in the PNALT patients and that APRI and FIB-4 established according to the common blood biochemical indicators are more suitable for active CHB. LSM, which determines liver hardness for assessment of the degree of liver fibrosis, is superior to APRI and FIB-4 in patients with PNALT because of the influence of liver inflammation and necrosis.
COMMENTS
Background
Although serum levels of alanine transaminase (ALT), an enzyme released from hepatocytes during liver injury, should reflect the degree of liver damage, not all patients with chronic HBV infection have persistently elevated ALT levels. Furthermore, some large cohort studies have shown that patients with chronic hepatitis B (CHB) who have normal serum ALT levels are also at risk for the development of cirrhosis and hepatocellular carcinoma. Liver biopsy is the current gold standard for assessing hepatic inflammation and fibrosis in patients with chronic hepatitis B virus (HBV) who have persistently normal alanine transaminase (PNALT). The invasiveness of liver puncture, the limitation of the specimen, and the poor patient compliance have restricted the application of liver biopsy.
Research frontiers
The limitation of liver biopsy has led to the development of noninvasive methods such as FIB-4 and aspartate aminotransferase-to-platelet ratio index (APRI) for evaluating fibrosis in patients with chronic HBV infection. Liver stiffness measurement (LSM) using transient elastography (FibroScan) has been widely used in the diagnosis of chronic liver fibrosis. However, the diagnostic value of FIB-4, APRI, and LSM in patients with HBV infection with PNALT is not clear.
Innovations and breakthroughs
The authors evaluated three common noninvasive hepatic fibrosis markers in CHB patients with different ALT levels. The results showed that APRI and FIB-4 are not the ideal noninvasive hepatic fibrosis markers for PNALT patients. LSM is superior to APRI and FIB-4 in PNALT patients because of the influence of liver inflammation and necrosis.
Applications
Noninvasive markers such as FIB-4, APRI and liver stiffness measurement for evaluating fibrosis in patients with chronic HBV infection.
Terminology
PNALT means patients with hepatitis B virus infection who have persistently normal alanine transaminase.
Peer-review
The manuscript is well written and the numerical simulations are well performed.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was approved by the Medical Ethics Committee of the Third Hospital of Zhenjiang Affiliated Jiangsu University (No. 2013011).
Informed consent statement: All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: All the authors have no conflict of interest related to the manuscript.
Data sharing statement: The original anonymous dataset is available on request from the corresponding author at tyw915@sina.com.
Peer-review started: May 4, 2017
First decision: June 6, 2017
Article in press: July 12, 2017
P- Reviewer: Nakao T S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Xu XR
Contributor Information
You-Wen Tan, Department of Hepatology, the Third Hospital of Zhenjiang Affiliated Jiangsu University, Zhenjiang 212001, Jiangsu Province, China. tyw915@sina.com.
Xing-Bei Zhou, Department of Hepatology, the Third Hospital of Zhenjiang Affiliated Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Yun Ye, Department of Hepatology, the Third Hospital of Zhenjiang Affiliated Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Cong He, Department of Hepatology, the Third Hospital of Zhenjiang Affiliated Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Guo-Hong Ge, Department of Hepatology, the Third Hospital of Zhenjiang Affiliated Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
References
- 1.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liver Dis. 2004;24 Suppl 1:17–21. doi: 10.1055/s-2004-828674. [DOI] [PubMed] [Google Scholar]
- 3.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 4.Nunnari G, Pinzone MR, Cacopardo B. Lack of clinical and histological progression of chronic hepatitis C in individuals with true persistently normal ALT: the result of a 17-year follow-up. J Viral Hepat. 2013;20:e131–e137. doi: 10.1111/jvh.12029. [DOI] [PubMed] [Google Scholar]
- 5.Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760–767. doi: 10.1016/j.jhep.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Xue L, Yan R, Zhou Y, Wang MS, Cheng MJ, Hai-Jun Huang. Comparison of histologic characteristics of Chinese chronic hepatitis B patients with persistently normal or mildly elevated ALT. PLoS One. 2013;8:e80585. doi: 10.1371/journal.pone.0080585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol. 2012;57:196–202. doi: 10.1016/j.jhep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, Chauhan R, Bose S. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376–1384. doi: 10.1053/j.gastro.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 9.Lin CL, Liao LY, Liu CJ, Yu MW, Chen PJ, Lai MY, Chen DS, Kao JH. Hepatitis B viral factors in HBeAg-negative carriers with persistently normal serum alanine aminotransferase levels. Hepatology. 2007;45:1193–1198. doi: 10.1002/hep.21585. [DOI] [PubMed] [Google Scholar]
- 10.Dai CY, Chuang WL, Huang JF, Yu ML. Hepatitis B e antigen-negative patients with persistently normal alanine aminotransferase levels and hepatitis B virus DNA > 2000 IU/mL. Hepatology. 2009;49:704–705; author reply 705-706. doi: 10.1002/hep.22723. [DOI] [PubMed] [Google Scholar]
- 11.Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, Chan AO, Wong BC, Lai KC, Lai CL. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610–1614. doi: 10.1136/gut.2005.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH; REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 13.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 14.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 15.Huang R, Jiang N, Yang R, Geng X, Lin J, Xu G, Liu D, Chen J, Zhou G, Wang S, et al. Fibroscan improves the diagnosis sensitivity of liver fibrosis in patients with chronic hepatitis B. Exp Ther Med. 2016;11:1673–1677. doi: 10.3892/etm.2016.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? World J Gastroenterol. 2016;22:7236–7251. doi: 10.3748/wjg.v22.i32.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao B, Wang Z, Lin S, Xu Y, Yi J, Xu M, Huang Z, Zhou Y, Zhang F, Hou J. Significant fibrosis is not rare in Chinese chronic hepatitis B patients with persistent normal ALT. PLoS One. 2013;8:e78672. doi: 10.1371/journal.pone.0078672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Xue L, Yan R, Zhou Y, Wang MS, Cheng MJ, Huang HJ. Comparison of FIB-4 and APRI in Chinese HBV-infected patients with persistently normal ALT and mildly elevated ALT. J Viral Hepat. 2013;20:e3–e10. doi: 10.1111/jvh.12010. [DOI] [PubMed] [Google Scholar]
- 19.Arora S, O'Brien C, Zeuzem S, Shiffman ML, Diago M, Tran A, Pockros PJ, Reindollar RW, Gane E, Patel K, et al. Treatment of chronic hepatitis C patients with persistently normal alanine aminotransferase levels with the combination of peginterferon alpha-2a (40 kDa) plus ribavirin: impact on health-related quality of life. J Gastroenterol Hepatol. 2006;21:406–412. doi: 10.1111/j.1440-1746.2005.04059.x. [DOI] [PubMed] [Google Scholar]
- 20.Kirschberg O, Schüttler C, Repp R, Schaefer S. A multiplex-PCR to identify hepatitis B virus--enotypes A-F. J Clin Virol. 2004;29:39–43. doi: 10.1016/s1386-6532(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 21.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 22.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 23.Jin SY. [Role of liver biopsy in the assessment of hepatic fibrosis--its utility and limitations] Korean J Hepatol. 2007;13:138–145. [PubMed] [Google Scholar]
- 24.Seo YS, Kim MY, Kim SU, Hyun BS, Jang JY, Lee JW, Lee JI, Suh SJ, Park SY, Park H, et al. Accuracy of transient elastography in assessing liver fibrosis in chronic viral hepatitis: A multicentre, retrospective study. Liver Int. 2015;35:2246–2255. doi: 10.1111/liv.12808. [DOI] [PubMed] [Google Scholar]
- 25.Martin J, Khatri G, Gopal P, Singal AG. Accuracy of ultrasound and noninvasive markers of fibrosis to identify patients with cirrhosis. Dig Dis Sci. 2015;60:1841–1847. doi: 10.1007/s10620-015-3531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen XB, Zhu X, Chen LY, Chen EQ, Tang H. [Accuracy of FibroScan for the diagnosis of liver fibrosis influenced by serum alanine aminotransferase levels in patients with chronic hepatitis B] Zhonghua Gan Zang Bing Za Zhi. 2011;19:286–290. doi: 10.3760/cma.j.issn.1007-3418.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Liang XE, Chen YP, Zhang Q, Dai L, Zhu YF, Hou JL. Dynamic evaluation of liver stiffness measurement to improve diagnostic accuracy of liver cirrhosis in patients with chronic hepatitis B acute exacerbation. J Viral Hepat. 2011;18:884–891. doi: 10.1111/j.1365-2893.2010.01389.x. [DOI] [PubMed] [Google Scholar]
- 28.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Chan HL. Increased liver stiffness measurement by transient elastography in severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2009;24:1002–1007. doi: 10.1111/j.1440-1746.2009.05779.x. [DOI] [PubMed] [Google Scholar]
- 29.Harata M, Hashimoto S, Kawabe N, Nitta Y, Murao M, Nakano T, Arima Y, Shimazaki H, Ishikawa T, Okumura A, et al. Liver stiffness in extrahepatic cholestasis correlates positively with bilirubin and negatively with alanine aminotransferase. Hepatol Res. 2011;41:423–429. doi: 10.1111/j.1872-034X.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- 30.Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718–1723. doi: 10.1002/hep.22577. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Wang L, Wang L, Li G, Huang A, Yin P, Yang Z, Ling C, Wang L. Liver stiffness measurement, better than APRI, Fibroindex, Fib-4, and NBI gastroscopy, predicts portal hypertension in patients with cirrhosis. Cell Biochem Biophys. 2015;71:865–873. doi: 10.1007/s12013-014-0275-z. [DOI] [PubMed] [Google Scholar]


