Abstract
Background
Transdermal alcohol monitoring technology allows for new research on alcohol use disorders. This study assessed feasibility, acceptability, and adherence with this technology in the context of two clinical research trials.
Methods
Participants were the first 100 community-based alcohol treatment outpatients enrolled in randomized studies that monitored drinking with the secure continuous remote alcohol monitor (SCRAMx®) for 12 weeks. Study 1 participants were randomized to usual care (n=36) or usual care with contingency management incentives for treatment attendance (CM-Att; n=30). Study 2 participants were randomized to usual care (n=17) or usual care with CM for each day of no drinking per SCRAMx (CM-Abst; n=17). After 12 weeks, participants completed a survey about the bracelet.
Results
Nine percent of individuals screened (54 of 595) declined participation because of the bracelet. Of participants, 84% provided 12 weeks of data, and 96% of bracelets were returned fully intact. Ninety-four equipment tampers occurred, affecting 2% of monitoring days; 56% (67) of tampers coincided with detected drinking. Common concerns reported by participants were skin marks (58%), irritation (54%), and interfered with clothing choices (51%), but severity ratings were generally mild (60%-94%). Eighty-one percent of participants reported that the bracelet helped them reduce drinking, and 75% indicated that they would wear it for longer. A common suggestion for improvement was to reduce the size of the bracelet.
Conclusions
Results support the viability of transdermal monitoring in voluntary substance abuse treatment participants for an extended duration. Issues to consider for future applications of this technology are discussed.
Keywords: transdermal alcohol monitoring, alcohol use disorder, outpatient treatment
1. Introduction
Research on alcohol use disorders has been limited by the available objective measures of drinking. The most common biochemical tests (breath, blood) only detect drinking within several hours of consumption given the rapid metabolism of alcohol (ethanol). Consequently, research often relies on self-report, which can be reliable and valid, but can also be biased by contextual factors such as response demands (Babor et al., 2000; Del Boca and Darkes, 2007, 2003; Leigh, 2000; Neal et al., 2006; Sobell and Sobell, 1992, 1990).
The ability to test for drinking using transdermal alcohol detection technology addresses some of these limitations. One device is the Secure Continuous Remote Alcohol Monitor (SCRAMx®) (Alcohol Monitoring Systems, Inc., (AMS), Littleton, CO), an ankle bracelet with a transdermal electrochemical sensor that samples alcohol vapors near the skin. SCRAMx has two circumvention detection sensors (skin temperature and contact with the skin) that make thwarting discovery of drinking unlikely, and a retainer clip that prevents removal. The device collects samples approximately every 30 minutes (more often when drinking or a potential tamper occurs), and it can be worn continuously for months. The date- and time-stamped data are uploaded to a secure central server at AMS via a USB connection or modem. Transdermal alcohol concentration is a valid and reliable measure of drinking that is correlated with and lagged in time relative to blood alcohol concentration (Sakai et al., 2006; Swift, 2003, 1993; Swift et al., 1992) and breath alcohol concentration (Dougherty et al., 2012). Transdermal readings provide a continuous estimate of blood alcohol level and thus improved information on the timing and quantity of drinking. Accuracy increases with at least two standard drinks (Sakai et al., 2006), and may be higher in women compared to men when an episode involves 4 or fewer drinks, and higher at normal BMIs compared to obese BMIs (Barnett et al., 2014). The application of SCRAMx within the justice system had exceeded 65,000 offenders in 46 states by 2008 (Kilmer, 2008). Usage now exceeds 483,000 persons in the United States, United Kingdom and Canada (Alcohol Monitoring Systems Inc., 2016).
This technology presents important opportunities for new research on alcohol use disorder and treatment. Contingency management (CM) is an efficacious intervention that systematically reinforces objective evidence of abstinence with tangible incentives (vouchers, prizes) (Higgins and Petry, 1999; Petry, 2000). With CM, the frequency of objective testing ideally maximizes detection of any use and reinforcement of abstinence. CM improves substance use treatment outcomes across a wide range of treatment settings and populations (Davis et al., 2016; Dutra et al., 2008; Lussier et al., 2006; Prendergast et al., 2006), but few studies have examined effects of CM on reducing drinking due to the alcohol measurement issues discussed above. Thus, research has relied predominately on reinforcing infrequent negative breath tests in the clinic (Bickel et al., 1988; Hagedorn et al., 2013; Helmus et al., 2003; Litt et al., 2007; Miller, 1975; Petry et al., 2000), more frequent negative breath tests in the natural environment (Alessi and Petry, 2013), or clinic urine tests negative for ethyl glucuronide, an alcohol metabolite with about a 2-day detection window (McDonell et al., 2012).
In the first CM study that used transdermal alcohol technology, heavy drinkers not seeking treatment and without other substance use except marijuana (N = 13) wore SCRAMx for a 1-week baseline followed by 2 weeks of CM for each day of no drinking defined by a composite of SCRAMx data and self-report (Barnett et al., 2011). Self-reported percent of days abstinent and drinks per week decreased with CM compared to baseline, as did SCRAMx average and peak transdermal alcohol concentration and area under the curve. In other work, following a pilot (Dougherty et al., 2014), heavy drinkers not seeking treatment (N = 82) wore SCRAMx for a 4-week baseline followed by 12 weeks of CM during which they received $50 each week that transdermal readings did not exceed low levels of drinking (> .3 g/dL) (Dougherty et al., 2015b). Self-reported drinking frequency and intensity decreased during CM compared to baseline and throughout the 3-month follow-up period (Dougherty et al., 2015b); SCRAMx-based heavy drinking also decreased, and no- and low-levels of drinking increased, during CM compared to baseline (Dougherty et al., 2015a). Finally, in a between-groups trial Barnett et al. (2017) assigned 30 participants to either daily contingent reinforcement for no drinking or yoked reinforcement; the intervention was three weeks following one week of baseline. During the intervention weeks, the CM group had a higher proportion of days with no drinking detected and a longer period of consecutive days with no detected drinking; this group difference was not retained at the one-month follow up.
Results of those initial studies are promising, but uptake of this technology by the CM and wider alcohol research community has been slower than in the justice system, perhaps in part due to relatively limited knowledge about feasibility, acceptability and adherence in the context of voluntary research. Therefore, the current study examined the experiences of the first 100 alcohol treatment outpatients who participated in one of two randomized clinical trials with SCRAMx monitoring and CM.
2. Materials and methods
2.1 Participants and Setting
Participants were 100 consecutive alcohol treatment outpatients recruited from four community-based clinics that provide comprehensive substance abuse treatment services, including an intensive day program and long-term aftercare using similar treatment approaches. Eligible individuals for the two studies were at least 18 years old, had past year alcohol use disorder (American Psychiatric Association, 2013), passed an informed consent quiz, agreed to wear SCRAMx for 12 weeks and return the equipment, and agreed to return regularly to the clinic for SCRAMx data uploads. The alcohol abstinence study also required hazardous drinking in the past 3 months (at least 15 drinks/week for men, at least 8 drinks/week for women) (Centers for Disease Control and Prevention, n.d.). Exclusion criteria for both studies were uncontrolled serious psychiatric illness (e.g., suicide risk), being in recovery from pathological gambling (although no evidence exists that prize CM increases gambling) (Petry et al., 2006, 2005; Petry and Alessi, 2010), having an unstable address, intending to participate in activities incompatible with SCRAMx in the next 3 months (e.g., airplane travel, swimming), already wearing a transdermal monitor for legal purposes, or having a medical condition that could interfere with SCRAMx readings (e.g., pregnancy, Type 1 diabetes). The alcohol abstinence study also excluded for clinically significant alcohol withdrawal symptoms (scoring at least 10 on the Clinical Institute Withdrawal Assessment for Alcohol revised scale (Sullivan et al., 1989)). The first five enrollees were excluded because the post-SCRAM survey was not administered.
The Institutional Review Board approved study procedures. Participants provided written informed consent, and research assistants with a Bachelor’s Degree in Psychology or a related field completed assessments. Recruitment occurred between September 2013 and October 2015; end-of-treatment surveys were completed by January 2016. Baseline and demographic characteristics are in Table 1.
Table 1.
Participants’ Demographic and Baseline Characteristics (N = 100)
| Variables | ||
|---|---|---|
| Mean age | 42 | SD = 10 |
| % Female | 48% | |
| % Not Hispanic ethnicity | 81% | |
| Race | ||
| % White | 59% | |
| % African American | 29% | |
| % More than 1 | 5% | |
| % Not reported | 5% | |
| % American Indian or Alaskan | 2% | |
| Mean # of days consumed alcohol in past 90 days | 33 | SD = 26 |
| Mean # of drinks on drinking daysa | 14 | SD = 9 |
| Substance use disorderb | ||
| % Alcohol | 100% | |
| % Cocaine | 50% | |
| % Opiates | 40% | |
| % Marijuana | 25% | |
| % Methamphetamine | 0% | |
| % Benzodiazepine | 14% | |
| % Breath alcohol .02 | 0% | |
| Any positive illicit drug test %c | 19% | |
| Mean SIPd score (45 max.) | 28 | SD = 12 |
| Mean ASI composite scorese (1 max.) | ||
| Medical | .34 | SD =.38 |
| Employment | .80 | SD =.22 |
| Alcohol | .34 | SD =.25 |
| Drug | .07 | SD =.09 |
| Legal | .10 | SD =.20 |
| Family | .14 | SD =.18 |
| Psychiatric | .29 | SD =.22 |
Excluding one outlier a participant who reported drinking a gallon of liquor each day.
Substance use disorder was defined per Diagnostic and Statistical Manual of Mental Disorders-5 criteria (American Psychiatric Association, 2013).
All urine tests were conducted using the 6-panel iCUP (Alere Toxicology Services, Portsmouth, VA).
Short Inventory of Problems (Feinn, Tennen, & Kranzler, 2003).
Addiction Severity Index (McLellan et al., 1992).
2.2 Assessments
At intake, demographic characteristics were recorded. The timeline follow-back calendar procedure captured frequency and intensity of drinking in the three months prior to treatment (Sobell and Sobell, 1992). A breath sample was tested for alcohol using Alcosensor IV (Alcometer, Intoximeters, St. Louis, MO), and a urine sample was tested for cocaine, opiates, marijuana, methamphetamine, benzodiazepine, and amphetamine using the 6-panel iCUP (Alere Toxicology Services, Portsmouth, VA). Substance use disorder was assessed using symptom checklists (American Psychiatric Association, 2013), and problems related to drinking were evaluated using the Short Index of Problems (SIP) (Miller et al., 1995), a shorter version of the Drinker Inventory of Consequences (Miller et al., 1995) that assesses lifetime consequences of alcohol use, with good reliability and validity (Forcehimes et al., 2007). The Addiction Severity Index (McLellan et al., 1992) was used to assess psychosocial functioning across seven domains affected by substance use (alcohol use, drug use, employment, medical, legal, psychiatric, and social functioning). The post-wear survey asked about physical and social comfort while wearing the bracelet, side effects and other consequences, and potential barriers to wearing the bracelet in the future (Barnett et al., 2011); see supplemental Table 11.
Research Electronic Data Capture is a secure web-based application (Harris et al., 2009) that was used to record self-reported data. SCRAMx data were uploaded to the secure AMS website, and AMS provided reports of transdermal alcohol concentration levels and notifications of potential and confirmed tampers and bracelet recalls. Tamper alerts are generated by AMS based on the logic that the IR sensor has detected a deviation that is 12% above or 17% below the established baseline readings. Once an alert is generated, AMS analysts look at the data over a period of no less than two weeks and determine if the movement in the IR supports an obstruction (i.e., tamper confirmed) or if it is standard movement of the bracelet due to, for example, fit issues. To confirm (or dismiss) a potential tamper, the AMS report is closely inspected. At the top of the report produced by AMS, there are three graphs of data over time - TAC readings, IR readings, and temperature readings. The remainder of the report is a reading-by-reading breakdown of these data, as well as daily summaries of number of readings, and all TAC readings indicating drinking, tamper alerts, equipment alerts, and instances in which the bracelet “communicates” (e.g., initialization process completed). Staff inspected these data to determine if a tamper occurred, and coded the case accordingly. The research assistant logged when bracelet fit adjustments were made, reasons for temporary removal of bracelets (e.g., day surgery), and explanations when fewer than 84 days (12 full weeks) of bracelet data were available. In this report, data are not provided on drinking outcomes because the trials are ongoing and will be presented when the studies are completed.
2.3 Procedures
Following consent and establishing eligibility, research staff explained how SCRAMx collects data and proper procedures for use, and answered any questions. After confirming an alcohol breath test of zero, research staff placed the bracelet on the participant’s lower leg and started the bracelet initialization process (this takes approximately 1 h). The project protocol was to install the bracelet after consent but prior to other assessments to allow sufficient time to confirm successful bracelet initialization before the participant left for the day. All participants received usual care outpatient substance use disorder treatment at two community-based clinics. Usual care consists of group therapy sessions, including daily planning, 12-Step treatment, relapse prevention, coping and life skills training, and AIDS education. Intensity starts at 3–4 groups/day up to 3–4 days/week, and reduces over time to a minimum of 1 aftercare group per week. Groups are led by recovering individuals, nurses, and masters level counselors.
In the treatment attendance study, in addition to usual care, brief research visits occurred once every other week for 12 weeks for all participants, to upload bracelet data and track treatment attendance. In the alcohol abstinence study, these brief research visits occurred twice weekly for 12 weeks. For participants in both studies, compensation was $1 for each day of SCRAMx data, a $3 bonus for each full week of data, $20 bonus per month of data, $20 study completion bonus, and $50 for returning study equipment. In addition, in the treatment attendance study, CM-Att participants also earned chances for prizes for each day that they attended all scheduled treatment sessions since the last research visit, with expected earnings of about $440 in prizes. In the alcohol abstinence study, CM-Abst participants additionally earned chances for prizes for each day (6 a.m. to 5:59 a.m.) with no drinking detected, with about $400 in prizes expected on average. Drinking events were determined by processing SCRAMx data through an algorithm developed for this purpose (Barnett et al., 2015). At month 3, all participants completed the post-wear survey.
2.4 Data analysis
Baseline and demographic variables and all outcomes were examined for any differences by study or study condition using Chi-square for nominal variables and analysis of variance for continuous variables. Differences were nonsignificant (p > .05). Therefore, results are presented aggregated across study and study condition.
To explore feasibility, adherence, and acceptability of SCRAMx, we evaluated: (1) recruitment data; (2) the proportion of participants who completed the 12-week monitoring period and reasons for incomplete data; (3) the frequency of confirmed tampers, fit adjustments, bracelet replacements, and temporary removal of the bracelet; (4) the proportion of devices returned and returned intact; and (5) post-wear survey data. Throughout, percentages are presented in whole numbers, and some sets do not add up to 100% due to rounding (i.e., not all 100 participants contributed a data point). Analyses were conducted using IBM® SPSS® Statistics version 21.
3. Results
3.1 Recruitment
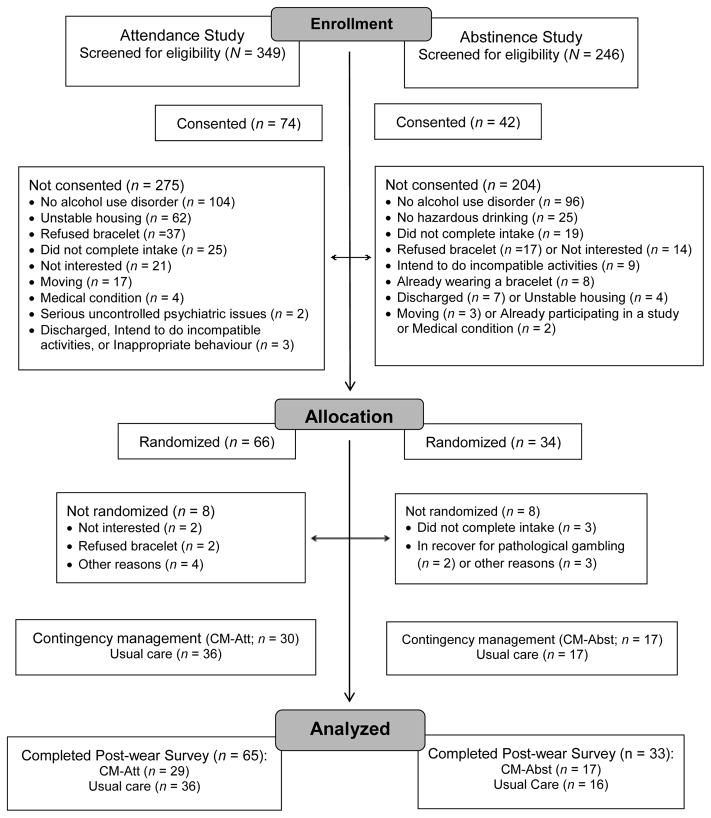
In total, 595 individuals initiated treatment at these clinics and completed screening procedures for the studies. During screening, research staff showed prospective participants the bracelet. Of those screened, 116 (19%) subsequently completed the informed consent process. Details of participant flow through the study are in Figure 1. The most common reason for not being consented was no alcohol use disorder (n = 200), as research staff screened patients initiating treatment at these clinics regardless of their substance use diagnosis. Relatively few persons screened (9%, n = 56) declined participation explicitly because of the bracelet. Reasons for not pursuing participation possibly due to the bracelet were “not interested” (6%, n = 37) and not completing the intake (7%, n = 44). Of the 116 consented, 100 (86%) were fully eligible for the study. The most common reason for consent but not randomized was not completing the intake. Participants were randomized using an urn program (Stout et al., 1994) and balanced on clinic, severity of alcohol problems (scores of 2–5 vs. scores of 6 or more (on symptom checklists)), and submission of a sample positive for alcohol or any illicit drug at baseline to a CM condition (n = 47) or Monitoring only (n = 53).
Figure 1.
CONSORT flow diagram of participants through the study.
3.2 Adherence
Overall, 84% of the 100 randomized participants provided 84 days of data. Reasons for fewer days of data in the remaining 16 participants and associated descriptive statistics are in Table 2. Reasons included relocation, events incompatible with continuing (court appearance for DUI, turning oneself in to law enforcement, day surgery), personal discomfort (no longer wanted to wear it, too uncomfortable, concern about employability, not compatible with compression socks), the last study day fell on a holiday, bracelet malfunction, and the participant cut the strap.
Table 2.
Rates of adherence and days of SCRAMx data
| Participants with: | n | Days of data collected | Days of data misseda |
|---|---|---|---|
| At least 84 days of data | 84 | 7,089 | 0 |
| Fewer than 84 days of data | 16 | 711 | 801 |
| Relocation | 4 | 248 | 88 |
| Incompatible events | 3 | 168 | 84 |
| Personal discomfort | 7 | 111 | 477 |
| Last study day was a holiday | 1 | 83 | 1 |
| Bracelet malfunction | 1 | 72 | 12 |
| Client cut strap | 1 | 29 | 139 |
| Other events | |||
| Confirmed tamper | 34 | 5,169 | 0 |
| Fit adjustments | 39 | 3,166 | 0 |
| Bracelet replacement | 13 | 3,205 | 0 |
| Temporary removal of bracelet | 3 | 259 | 0 |
For non-zero values, the number of days missed was computed assuming that all participants affected would have submitted 84 days of data.
Other occurrences with the potential to affect feasibility, acceptability and/or adherence but that did not result in lost days of data were confirmed tampers, fit adjustments, bracelet replacements, and the need to temporarily remove the bracelet for other reasons (Table 2). There were 94 confirmed tampers across 34 participants, ranging from 1 to 11 tampers per person (excluding the one instance a participant cut the strap noted above, and three instances of law enforcement, not the participant, cutting the strap). These tampers affected 139 days (2%) of bracelet data (but with no lost days of data), and 56% (67 confirmed tampers) coincided with the detection of drinking. For the remaining 44% of confirmed tampers, TAC readings did not indicate drinking and were most likely resultant from participants blocking the sensor inadvertently (e.g., placing a sock between the skin and device for comfort). Fit adjustments were needed on 62 occasions across 39 participants. Of these, participants requested 90% (56 requests) (e.g., too loose, too tight), AMS requested 5% (3 requests for issues such as unusual connection patterns that suggest poor fit), and both the client and AMS requested 5% (3 requests). There were 16 bracelet replacements across 13 participants. Eleven of these were due to AMS recall notifications: approaching the end of the bracelet’s expected life cycle (8 events), replacement of faceplate required (1 event), pump not running at full capacity (1 event), and reinitialization required (1 event). Research staff initiated the remaining replacements due to known or suspected malfunction: running too loudly (n = 3), and unexplained gap in readings (n = 2). Bracelets were also temporarily removed on four occasions: preemptively prior to a medical procedure (1 occasion) and because law enforcement cut the strap (3 occasions).
Staff distributed 120 bracelets to participants over the course of the study (including bracelet replacements when needed); 98% (118) were returned and 97% (114) returned intact and in full working order. Not intact were the 3 devices with the strap cut by law enforcement and the one with the strap cut by the participant, noted above.
3.3 Post-wear survey on side effects, other consequences and barriers
The post-wear survey was completed by 98% of participants. Items rated on a similar scale are presented together, in Table 3 and Table 4. Remaining data are described below. When asked how comfortable the bracelet was, most participants indicated neutrality (rated 5 or 6 on a scale of 1 to 10) (Table 3). Most participants noticed the bracelet only “once or twice” during the day (45%) and/or while sleeping (56%). On how often participants noticed the bracelet during the day, most endorsed “every few hours” (32%) followed by “every hour” (8%). On noticing it while sleeping, most endorsed “never or almost never” (20%) followed by “every few hours” (12%). Most participants also indicated that the bracelet did not cause them to lose sleep (no 79%;yes 21%), and relatively few indicated that it interfered at all with aspects of life other than sleep (Table 3). Of physical side effects, 58% of participants endorsed experiencing some marks on their skin, with most indicating that this occurred “sometimes” (33%) or “rarely” (16%). Of other physical side effects queried, most participants experienced none (46% to 63% depending on the side effect), and when endorsed, most were of mild severity (Table 3).
Table 3.
Side Effects and Other Consequences of Wearing the Bracelet (n = 98).
| Percent of participants who endorsed each response | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survey Item | 1 Not at all | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 Completely |
| How physically comfortable was the bracelet? | 1% | 2% | 4% | 10% | 21% | 22% | 11% | 15% | 4% | 8% |
| How socially comfortable was the bracelet? | 2% | 5% | 6% | 7% | 16% | 21% | 11% | 9% | 3% | 18% |
| Circle the number that describes how | ||||||||||
| Wearing the bracelet interfered with your: | 1 Not at all | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 Completely |
| Ability to concentrate | 86% | 4% | 4% | 0% | 3% | 2% | 0% | 0% | 0% | 1% |
| Mood | 80% | 3% | 4% | 5% | 2% | 3% | 0% | 0% | 0% | 3% |
| Normal work | 80% | 4% | 5% | 2% | 3% | 3% | 0% | 0% | 0% | 3% |
| Social life | 79% | 3% | 4% | 3% | 4% | 3% | 1% | 2% | 0% | 1% |
| Exercise | 78% | 6% | 5% | 1% | 3% | 1% | 3% | 0% | 1% | 2% |
| Enjoyment of life | 71% | 5% | 6% | 4% | 5% | 0% | 4% | 1% | 0% | 3% |
| General activity | 70% | 10% | 5% | 4% | 3% | 2% | 3% | 0% | 0% | 2% |
| Sleep | 55% | 6% | 11% | 3% | 11% | 7% | 4% | 0% | 0% | 2% |
| Choice of clothing | 50% | 5% | 5% | 5% | 11% | 3% | 6% | 4% | 2% | 8% |
| What other side effects (than skin marks) | ||||||||||
| did you have from the bracelet? | 1 Not noticeable | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 Unbearable |
| Itching | 50% | 18% | 11% | 8% | 5% | 1% | 1% | 2% | 1% | 2% |
| Sweating | 57% | 10% | 10% | 6% | 8% | 2% | 1% | 3% | 1% | 1% |
| Soreness | 57% | 16% | 11% | 2% | 4% | 5% | 1% | 2% | 1% | 0% |
| Itching | 50% | 18% | 11% | 8% | 5% | 1% | 1% | 2% | 1% | 2% |
| Irritation | 47% | 17% | 10% | 3% | 8% | 6% | 3% | 2% | 2% | 1% |
Table 4.
Potential Barriers to Wearing the Bracelet for a Longer Duration (n = 98).
| Percent of participants who endorsed each response | |||||
|---|---|---|---|---|---|
| Survey item: Please indicate how true you think the following statements are: | 0 Not at all true | 1 | 2 | 3 | 4 Very true |
| The bracelet is too uncomfortable to wear any longer. | 72% | 6% | 11% | 5% | 5% |
| I want to swim/bathe. | 36% | 12% | 13% | 6% | 33% |
| I am tired of explaining the bracelet to people. | 51% | 11% | 12% | 11% | 14% |
| I wouldn’t want to wear it any longer because I am embarrassed. | 74% | 12% | 7% | 2% | 4% |
| I want to wear shorts or skirts but won’t while wearing the bracelet. | 63% | 6% | 6% | 8% | 16% |
| I am ready to stop wearing the bracelet because I am just ready to be done. | 54% | 13% | 15% | 5% | 12% |
| I would not continue wearing the bracelet because I do not like doing the downloads. | 84% | 11% | 3% | 2% | 0% |
| The financial payment for wearing the bracelet any longer would not be worth it. | 69% | 13% | 8% | 6% | 3% |
| I had difficulty coming to my study appointments. | 81% | 8% | 8% | 2% | 1% |
Three quarters of participants indicated that they would wear the bracelet for longer (yes versus no). Ratings of potential barriers to wearing it for longer are in Table 4. Endorsing wanting to swim or bathe in a tub was most common (e.g., 33% rated this “very true”), and endorsing difficulty coming to study appointments was least common (e.g., 1% rated this “very true”).
Despite some consequences and barriers, most participants endorsed believing that wearing the bracelet helped them reduce their drinking. Specifically, participants indicated that they were “able to completely reduce” (49%), “able to reduce quite a bit” (21%), “able to reduce somewhat” (11%), “tried but didn’t really reduce” (10%), and “did not reduce nor tried” (7%). Life circumstances that may have affected efforts to reduce drinking while wearing the bracelet included moved (n = 15), new, lost or changed jobs (n = 10), moved into or out of sober living (n = 8), medical issues (n = 6), went inpatient (n = 5), changed treatment programs (n = 2), and breakup (n = 2), and n = 1 each for started school, traveled out of state, incarcerated, completed intensive outpatient treatment, went to detox, and became homeless. When asked if they would use an online option for redeeming money earned for wearing the bracelet if available, 38% indicated yes; 62% indicated that they would prefer to wait for a check in person.
Lastly, 39% of participants provided feedback on an open-ended question asking for any other comments. Most (44%, n = 17) suggested changes to the device (e.g., making it smaller, lighter), noted that the bracelet was a good reminder not to drink (21%, n = 8), or expressed satisfaction in general (18%, n = 7). Other comments (each endorsed once) included satisfaction with the research visits and prize draws in the CM conditions, it helped with recovery (not linked explicitly to drinking), helped with understanding addiction, higher compensation would be preferable, payment options would be preferable, suggesting we should open it up to more people, and expressing a desire to stay in the study.
4. Discussion
Results of the current study support the feasibility and acceptability of SCRAMx and high rates of adherence in the context of 12-week randomized clinical trials that incorporated SCRAMx monitoring. These results are consistent with and extend prior studies on CM and SCRAMx monitoring with non-treatment samples, most of which used within-subject nonrandomized designs, and shorter durations of monitoring. Results are also consistent with two prior studies involving SCRAMx but not CM that also observed good adherence and supported the feasibility of transdermal monitoring in individuals’ natural environment (Barnett et al., 2014; Sakai et al., 2006). Although questions about feasibility, acceptability, and adherence may have limited alcohol treatment research using this technology to date, on balance, the current results should attenuate concerns and inform efforts moving forward.
The transdermal monitoring aspect of the current study was not a substantial barrier to recruitment. Relatively few individuals explicitly cited the bracelet as their reason for declining to participate or for withdrawing. Other explicit (“not interested”) and ambiguous (not showing up to complete the intake) reasons for attrition may have been in part due to the bracelet component, which was described briefly when screening participants (when most attrition occurred) and in detail during consent. However, the rate of attrition for such reasons in this study was lower than when recruiting for a study not involving this technology at these clinics (30.2%) (Petry et al., 2012). Going forward, it may be useful to question prospective participants who decline to pursue participation about specific areas of concern.
Adherence with twelve weeks of monitoring was good, with 84% of participants providing the full 84 days of bracelet data. AMS encourages at least 90 days of monitoring for offenders, but it was not clear at the start of the current study if that duration of monitoring would be acceptable in the context of voluntary research. Notably, adherence in this study was remarkably similar to that observed in a prior study with 12 weeks of SCRAMx in a non-treatment sample of heavy drinkers, in which 81% (n = 66) of participants completed the monitoring period (Dougherty et al., 2015b). For those who did not fulfill 12 weeks of monitoring in the current study, there were a variety of reasonable explanations, and no one explanation occurred disproportionately. Further, there was the potential for high incurred costs from lost or damaged inventory, but only 2 devices were not returned.
Confirmed tampers occurred in about one third of participants. CM-Abst participants may have been motivated to tamper over concern about losing incentives should alcohol be detected. What motivated these events in the CM-Att condition and in usual care participants is unclear. Detection of drinking had no intended consequences for those in a usual care condition. For CM-Att participants, the specific nature of the contingency (linked to attendance, not alcohol abstinence) was reviewed in detail during consent and reinforced at each CM session (Petry, 2013; Petry et al., 2010). For all participants, detection of drinking via SCRAMs had no legal consequences. Only research staff (not clinicians at the treatment clinics) had access to participants’ SCRAMx (and other) data, and the strict separation of research and clinic files, and confidentiality and security protections, were discussed at length during the consent process and reviewed as needed. Nevertheless, we cannot rule out the possibility that such considerations motivated some attempts to tamper with the device.
Bracelet fit adjustments were common, and any associated time and effort demands may have incurred some costs in terms of acceptability, even while improving participants’ comfort level. Likewise, the need for bracelet replacements placed some demands on participants, but also avoided inadvertent disruption of compensation for days of lost bracelet data due to, for example, a device malfunctioning.. Overall, most participants did not express distress about how comfortable they were physically and socially while wearing the bracelet, but suggestions to reduce the size/weight/noise level were common. We also provided all participants with an official document stating that they were voluntarily wearing the device as part of a research study, in case anyone (employers, etc.) questioned them.
Although three out of four participants indicated that they would hypothetically wear the bracelet for longer, wanting to swim or bathe was a common perceived potential barrier to doing so. Technological advances that assuage these concerns could improve usability and acceptability in the future. The most common side effects and personal consequences of wearing the bracelet related to placement of the bracelet (marks on the skin, irritation, interfered with choice of clothing). Participants generally rated these and all side effects and consequences as mild in severity, and these effects did not contribute substantially to lost days of bracelet data. Interestingly, a large majority (81%), even those in a usual care condition, perceived the bracelet to be clinically useful in helping them reduce drinking. In response to an open invitation for additional comments, responses from 7 participants (5 in usual care) may provide some insight. Five referred to the bracelet as a tool or that it generally helped with not drinking; one replied it “helped me at times to think different and stay out of trouble”, and one noted “it keeps you mindful”.
One limitation of this study is that individuals who declined at the point of screening due to the bracelet component were not further assessed on eligibility criteria. Future efforts may consider structuring recruitment procedures to distinguish between the rate at which prospective participants decline but are otherwise eligible and the rate of declining and also not otherwise eligible, which may provide a better understanding of acceptability. Notably, the other sources of data firmly indicated high acceptability, and the compensation for wearing the device likely contributed. In this study, we assessed for days of lost data (which rarely occurred); analysis of drinking outcomes will require more fine-grained within-day analysis. Finally, this study does not address the time and effort required of research staff to manage the amount of information resulting from continuous monitoring, fitting and re-fitting devices, and uploading data.
5. Conclusions
This is the largest study to report on feasibility, acceptability and adherence with continuous transdermal alcohol monitoring in detail, and the first randomized clinical trials using this technology in alcohol treatment patients. Recruitment was not unduly affected by the monitoring component. There were high rates of adherence with monitoring for 12 weeks, a duration that coincides with typical lengths of outpatient treatment. Side effects and consequences of wearing the bracelet were generally minimal and resulted in few instances of fewer than 84 days of data, although tolerance for these factors may vary with circumstances. Further, most participants agreed that wearing the bracelet helped them reduce drinking, and most reported no barriers to potentially wearing the bracelet for a longer duration. Together, these results support the use of this technology in research on alcohol use disorder and treatment.
Supplementary Material
Highlights.
Continuous alcohol monitoring technology presents new opportunities for research
High rates of adherence with 12 weeks of monitoring yielded 7,800 days of data
Side effects and other consequences were generally minimal
Most participants agreed that monitoring helped them reduce drinking
Results support the feasibility, acceptability and adherence with monitoring
Acknowledgments
Role of funding source
This work was supported by National Institutes of Health grants P60 AA03510 and R01 AA021446. Preparation of this report was additionally supported by R01 HD075630, R01 AA023502, P50 DA092410, R01 DA013444, and R21 AA020943. Funders did not have any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or, in the decision to submit the paper for publication.
We extend our gratitude to the research participants, and to Matthew Brennan, Trisha Lausier, Steven MacKinnon, and Meghan Rossini for their dedication and expertise in data collection and oversight of the clinical trials that form the basis of this report. Thank you to Tim Souza for his contributions processing SCRAM data, and to AMS. We also thank the patients and staff at Alcohol and Drug Rehabilitation Center, Inc., Behavioral Health Network, Inc., InterCommunity, Inc., The Hospital of Central Connecticut, and Recovery Network of Programs, Inc. Recovery Counseling Services. Ellen Ciesielski and Ruth Fetter managed IRB and other regulatory communications, Sean Sierra assisted with oversight, and Wendy Soneson provided grant-related administrative support.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
Nancy Petry proposed the initial design of the study. Sheila Alessi undertook data analysis and wrote the initial draft of the manuscript. All authors contributed to and approved the final manuscript.
Conflicts of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcohol Monitoring Systems Inc. 2 billion alcohol tests later, famous alcohol anklet has changed the face of community corrections. 2016 [WWW Document]. URL https://www.scramsystems.com/media-room/2-billion-alcohol-tests-later-famous-alcohol-anklet-has-changed-the-face-of/
- Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction. 2013;108:900–909. doi: 10.1111/add.12093. http://dx.doi.org/doi:10.1111/add.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Babor TF, Steinberg K, Anton R, Del Boca F. Talk is cheap: Measuring drinking outcomes in clinical trials. J Stud Alcohol. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. http://dx.doi.org/doi:10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- Barnett NP, Celio MA, Tidey JW, Murphy JG, Colby SM, Swift RM. A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction. 2017:1025–1035. doi: 10.1111/add.13767. http://dx.doi.org/doi:10.1111/add.13767. [DOI] [PMC free article] [PubMed]
- Barnett NP, Meade EB, Glynn TR. Predictors of detection of alcohol use episodes using a transdermal alcohol sensor. Exp Clin Psychopharmacol. 2014;22:86–96. doi: 10.1037/a0034821. http://dx.doi.org/doi:10.1037/a0034821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R, Colby SM. Contingency management for alcohol use reduction: A pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011;118:391–399. doi: 10.1016/j.drugalcdep.2011.04.023. http://dx.doi.org/doi:10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Rizzuto P, Zielony RD, Klobas J, Pangiosonlis P, Mernit R, Knight WF. Combined behavioral and pharmacological treatment of alcoholic methadone patients. J Subst Abuse. 1988;1:161–171. doi: 10.1016/s0899-3289(88)80019-1. http://dx.doi.org/doi:10.1016/S0899-328988)80019-1. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Frequently asked questions about alcohol. n.d [WWW Document]. URL http://www.drugfreeworld.org/drugfacts/alcohol.htm.
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Prev Med (Baltim) 2016;92:36–46. doi: 10.1016/j.ypmed.2016.08.008. http://dx.doi.org/doi:10.1016/j.ypmed.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. Enhancing the validity and utility of randomized clinical trials in addictions treatment research: II. Participant samples and assessment. Addiction. 2007;102:1194–1203. doi: 10.1111/j.1360-0443.2007.01863.x. http://dx.doi.org/doi:10.1111/j.1360-0443.2007.01863.x. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction. 2003;98:1–12. doi: 10.1046/j.1359-6357.2003.00586.x. http://dx.doi.org/doi:10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Charles NE, Acheson A, John S, Furr RM, Hill-Kapturczak N. Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Exp Clin Psychopharmacol. 2012;20:373–381. doi: 10.1037/a0029021. http://dx.doi.org/doi:10.1037/a0029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, Karns TE, Cates SE, Lake SL, Mullen J, Roache JD. Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend. 2014;142:301–306. doi: 10.1016/j.drugalcdep.2014.06.039. http://dx.doi.org/doi:10.1016/j.drugalcdep.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Karns TE, Mullen J, Liang Y, Lake SL, Roache JD, Hill-Kapturczak N. Transdermal alcohol concentration data collected during a contingency management program to reduce at-risk drinking. Drug Alcohol Depend. 2015a;148:77–84. doi: 10.1016/j.drugalcdep.2014.12.021. http://dx.doi.org/doi:10.1016/j.drugalcdep.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Hill-Kapturczak N, Liang Y, Karns TE, Mullen J, Roache JD. Using contingency management procedures to reduce at-risk drinking in heavy drinkers. Alcohol Clin Exp Res. 2015b;39:743–751. doi: 10.1111/acer.12687. http://dx.doi.org/doi:10.1111/acer.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. http://dx.doi.org/doi:10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Forcehimes AA, Tonigan JS, Miller WR, Kenna GA, Baer JS. Psychometrics of the Drinker Inventory of Consequences (DrInC) Addict Behav. 2007;32:1699–1704. doi: 10.1016/j.addbeh.2006.11.009. http://dx.doi.org/doi:10.1016/j.addbeh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Hagedorn HJ, Noorbaloochi S, Simon AB, Bangerter A, Stitzer ML, Stetler CB, Kivlahan D. Rewarding early abstinence in Veterans Health Administration addiction clinics. J Subst Abuse Treat. 2013;45:109–117. doi: 10.1016/j.jsat.2013.01.006. http://dx.doi.org/doi:10.1016/j.jsat.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. http://dx.doi.org/doi:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmus TC, Saules KK, Schoener EP, Roll JM. Reinforcement of counseling attendance and alcohol abstinence in a community-based dual-diagnosis treatment program: A feasibility study. Psychol Addict Behav. 2003;17:249–251. doi: 10.1037/0893-164X.17.3.249. http://dx.doi.org/doi:10.1037/0893-164X.17.3.249. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Petry NM. Contingency management. Alcohol Res Heal. 1999;23:122–127. [PMC free article] [PubMed] [Google Scholar]
- Kilmer B. The Future of DIRECT Surveillance: Drug and alcohol use Information from REmote and Continuous Testing. J Drug Policy Anal. 2008;1 http://dx.doi.org/doi:10.2202/1941-2851.1004. [Google Scholar]
- Leigh BC. Using daily reports to measure drinking and drinking patterns. J Subst Abuse. 2000;12:51–65. doi: 10.1016/s0899-3289(00)00040-7. http://dx.doi.org/doi:10.1016/S0899-3289(00)00040-7. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Kabela-Cormier E, Petry NM. Changing network support for drinking: Initial findings from the Network Support Project. J Consult Clin Psychol. 2007;75:542–555. doi: 10.1037/0022-006X.75.4.542. http://dx.doi.org/doi:10.1037/0022-006X.75.4.542. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. http://dx.doi.org/doi:10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McDonell MG, Howell DN, McPherson S, Cameron JM, Srebnik D, Roll JM, Ries RK. Voucher-based reinforcement for alcohol abstiennce using the ethyl-glucoronide alcohol biomarker. J Appl Behav Anal. 2012;45:161–165. doi: 10.1901/jaba.2012.45-161. http://dx.doi.org/doi:10.1901/jaba.2012.45-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller PM. A behavioral intervention program for chronic public drunkenness offenders. Arch Gen Psychiatry. 1975;32:915. doi: 10.1001/archpsyc.1975.01760250107012. http://dx.doi.org/doi:10.1001/archpsyc.1975.01760250107012. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R. An Instrument for Assessing Adverse Consequences of Alcohol Abuse. 1995. The Drinker Inventory of Consequences (DrInC) Test Manual. … MATCH Monogr. Ser. 4. [Google Scholar]
- Neal DJ, Corbin WR, Fromme K. Measurement of alcohol-related consequences among high school and college students: Application of item response models to the Rutgers Alcohol Problem Index. Psychol Assess. 2006;18:402–414. doi: 10.1037/1040-3590.18.4.402. http://dx.doi.org/doi:10.1037/1040-3590.18.4.402. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency management for substance abuse treatment: A guide to implementing this evidence-based practice. Routledge: 2013. [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. http://dx.doi.org/doi:10.1016/S0376-8716(99)00071-X. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. J Subst Abuse Treat. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. http://dx.doi.org/doi:10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the Contingency Management Competence Scale. Drug Alcohol Depend. 2010;109:167–174. doi: 10.1016/j.drugalcdep.2009.12.027. http://dx.doi.org/doi:10.1016/j.drugalcdep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012;80:276–285. doi: 10.1037/a0026883. http://dx.doi.org/doi:10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML, Hamilton JA. Prize-based contingency management does not increase gambling. Drug Alcohol Depend. 2006;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. http://dx.doi.org/doi:10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: Contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. http://dx.doi.org/doi:10.1037/0022-006X.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic Francis J. Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. J Consult Clin Psychol. 2005;73:354–359. doi: 10.1037/0022-006X.73.2.354. http://dx.doi.org/doi:10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. http://dx.doi.org/doi:10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Sakai JT, Mikulich-Gilbertson SK, Long RJ, Crowley TJ. Validity of transdermal alcohol monitoring: Fixed and self-regulated dosing. Alcohol Clin Exp Res. 2006;30:26–33. doi: 10.1111/j.1530-0277.2006.00004.x. http://dx.doi.org/doi:10.1111/j.1530-0277.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back. In: Allen RZLJP, editor. Measuring Alcohol Consumption. Humana Press; Totowa, NJ: 1992. pp. 41–72. http://dx.doi.org/doi:10.1007/978-1-4612-0357-5_3. [Google Scholar]
- Sobell LC, Sobell MB. Self-report issues in alcohol abuse: state of the art and future directions. Behav Assess. 1990;12:77–90. [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol, Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. http://dx.doi.org/doi:10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. http://dx.doi.org/doi:10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Swift RM. Direct measurement of alcohol and its metabolites. Addiction. 2003;98:73–80. doi: 10.1046/j.1359-6357.2003.00605.x. http://dx.doi.org/doi:10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- Swift RM. Transdermal measurement of alcohol consumption. Addiction. 1993;88:1037–1039. doi: 10.1111/j.1360-0443.1993.tb02122.x. http://dx.doi.org/doi:10.1111/j.1360-0443.1993.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Swift RM, Martin CS, Swette L, LaConti A, Kackley N. Studies on a Wearable, Electronic, Transdermal Alcohol Sensor. Alcohol Clin Exp Res. 1992;16:721–725. doi: 10.1111/j.1530-0277.1992.tb00668.x. http://dx.doi.org/doi:10.1111/j.1530-0277.1992.tb00668.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.