Abstract
Polymorphisms in the Fat Mass and Obesity Associated (FTO) gene are robustly associated with overweight and obesity among children, although the underlying mechanisms are poorly understood. We tested if appetitive traits partially mediated the association between FTO genotype and increased BMI among a sample of US preadolescents. Data were from 178 unrelated 9–10 year olds who participated in an experimental study between 2013 and 2015. Children’s DNA was isolated from buccal swabs, and the rs9939609 SNP in the FTO gene was genotyped. Children’s age- and sex-adjusted BMI z-scores were computed using height and weight measured at the laboratory. Parents completed the Child Eating Behavior Questionnaire that includes three validated scales of habitual appetitive traits related to drive and regulation: satiety responsiveness, enjoyment of food and food responsiveness. Structural equation modeling was used to assess if those traits mediated the relationship between FTO and BMI z-score. The sample of children was 48.9% male and 91.0% non-Hispanic white. FTO distribution was in Hardy Weinberg equilibrium, and 16.3% of participants were homozygous for the high-risk allele. Mean BMI z-score was greatest among those with the high-risk genotype (ANOVA P<0.01). In separate structural equation models adjusted for the child’s sex and maternal education, decreased satiety responsiveness and increased food responsiveness each partially mediated the positive association between the high-risk genotype and increased BMI z-score (P-value for each indirect effect <0.05). Continued research is needed to better understand how other known genetic obesity risk factors may impact appetitive traits among children.
Introduction
Childhood obesity is a global public health issue.1 In the US, about one-third of children under the age of 12 are overweight or obese.2 Overweight or obese children are likely to face a lifetime battle with excess weight,3,4 which increases the risk for numerous health problems.5,6 Appetitive traits develop early in childhood,7–10 and variations in appetitive traits such as enjoyment of food, impulsivity, increased eating rate and decreased satiety responsiveness have been consistently associated with obesity risk among children. 9 Furthermore, strong evidence supports that appetitive traits are heritable,10,11 and may, in part, underlie a genetic predisposition to obesity.10 As summarized in a review of the behavioral susceptibility theory first put forth by Dr. Jane Wardle,10 there is strong observational and experimental evidence to support that eating behaviors are under significant genetic control among infants and children. While several studies have assessed the heritability of appetitive traits among children,10 the few studies that have included genotype data have reported mixed findings,12–18 and all have been outside of the US.
The Fat Mass and Obesity Associated (FTO) gene was the first gene identified as having robust associations with obesity among children and adults.19 The high-risk allele of the FTO gene includes the rs9939609 single-nucleotide polymorphism, and a meta-analysis19 reported a linear, increased risk of overweight and obesity with each additional copy of the FTO high risk allele. Those associations appear specific to increased fat mass versus lean body mass19, 20 and unrelated to differences in metabolic efficiency20 or physical activity levels21 among children. Importantly, FTO may relate to increased weight status via disrupted appetite regulation. For example, animal models have documented that FTO is highly expressed in a region of the hypothalamus that regulates appetite and energy balance and that FTO expression in the hypothalamus affects energy intake.22 Studies among children further support that FTO may impact appetitive traits. In a behavioral eating experiment among 131 3–4 years old,21 children were offered highly palatable snack foods within one hour of eating a regular meal at home and were allowed to consume those snacks ad libitum for 10 minutes; the amount of snacks consumed was operationalized as eating in the absence of hunger. Children with one or two copies of the high-risk FTO allele engaged in more eating in the absence of hunger, supporting that FTO may impact behavioral phenotypes related to appetite.
Parental report of children’s appetitive traits may accurately assess obesogenic eating behaviors. The Children’s Eating Behavior Questionnaire (CEBQ)23 is a parent-reported scale that measures aspects of children’s appetitive behaviors, and three scales measure appetitive drive and regulation: satiety responsiveness, enjoyment of food and food responsiveness. Satiety responsiveness reflects a child’s sensitivity to feelings of fullness (e.g., My child gets full easily); enjoyment of food reflects normal variation in overall appetite and desire to eat (e.g., My child is interested in food); and food responsiveness reflects more obesogenic responses to the presentation of food (e.g., If given the chance, my child would always have food in his/her mouth). Those three measures have been validated against objective measures of obesogenic eating,24 have high 2-week test-retest reliability,23 and have demonstrated stability over time.25 Importantly, those appetitive traits may reflect appetitive phenotypes linked to FTO. In a UK cohort of 3337, 8–11 year old children,12 being homozygous for the high-risk FTO allele was associated with decreased satiety responsiveness and increased enjoyment of food as measured with the CEBQ; food responsiveness was not included in that analysis. Furthermore, decreased satiety responsiveness partially mediated the positive relationship between FTO and increased BMI z-score in that cohort. Similar findings were found in a UK cohort of 2258 10-year olds that assessed a genetic predisposition score of 28 known obesity-related SNPs.13 More studies are needed to understand if such associations generalize to US-based populations.
In this study, we examined how appetitive traits mediated the association between FTO and BMI z-score among a US sample of 9–10 year olds. We included the same two measures of appetitive traits as reported in the previous UK cohort, satiety responsiveness and enjoyment of food,12 and we also included a measure reflective of more obesogenic appetitive traits, food responsiveness. In addition, we were interested in exploring how FTO and appetitive traits were associated with short term food intake, which might suggest a behavioral mechanism for how eating behaviors impact obesity risk.
Methods
Design and Study Participants
Data are from a randomized experimental study that examined the impact of TV food advertising exposure on eating in the absence of hunger (EAH) among preadolescents.26 Children aged 9–10 years old and one parent or guardian (referred to as parent from here on) were recruited from the greater Hanover, NH area between August 2013 and March 2015. Participants were recruited from the community using a contact list of patients who seek care from our primary care pediatric clinic and community fliers, and one dyad at a time visited the laboratory to complete the experiment. All visits were scheduled around noon and children were provided lunch at the study visits. Parents were asked to provide their children a normal breakfast but to not have their children eat for 45 minutes before the appointment. Upon arrival, a buccal swab from the child was collected for genotyping as described below, and the parent completed a study questionnaire while the child completed the EAH experiment. Eligibility criteria included English fluency, absence of food allergies and dietary restrictions in the child, absence of health conditions, medication use that may impact appetite and attention span of the child, and willingness to participate in a 2-hour study. Of the 252 dyads screened, 224 were eligible, and 200 enrolled. There were 21 families that enrolled more than one child into the study, and we limited analyses to unrelated participants (179) by randomly selecting one child in each sibling pair. Analyses further excluded one underweight child. This final analysis therefore included 178 children. Participating children provided assent for study procedures, and parents provided consent for their children and themselves. The Committee for the Protection of Human Subjects at Dartmouth College approved of all study procedures.
FTO rs9939609 Genotyping
Collected buccal cells were stored at room temperature with desiccant capsules (Isohelix, Kent, U.K.). DNA was then isolated from the swabs by the Dartmouth Translational Research Laboratory using DDK-50 isolation kits (Isohelix, Kent, U.K.). Genotyping for the rs9939609 single-nucleotide polymorphism in FTO was implemented with real time PCR and Taqman chemistry using primers and the 7500 Fast Real-time instrument (both from Thermo Fisher Scientific, Waltham, MA, USA). The context sequence for the rs9939609 SNP genotyping Taqman Assay from Thermofisher is: [VIC/FAM] GGTTCCTTGCGACTGCTGTGAATTT[A/T]GTGATGCACTTGGATAGTCTCTGT. The extraction volume for each sample was 80 μl with a mean (SD) DNA concentration of 31.7 (24.6) ng/μl. All 200 samples were successfully genotyped for rs9939609. There was 100% genotyping consistency among the 10% of samples that were blindly replicated.
Child Appetitive Traits
Parents completed the Children’s Eating Behavior Questionnaire (CEBQ),23 a questionnaire to assess habitual eating behaviors among children. Our analysis focused on three CEBQ scales of appetitive traits related to appetite drive and regulation: satiety responsiveness (5 items), enjoyment of food (4 items), and food responsiveness (5 items). Those scales were chosen because they are validated and have high reliabilty.23, 24 As noted above, satiety responsiveness reflects a child’s sensitivity to feelings of fullness; enjoyment of food reflects normal variation in appetitive drive and responsiveness to food; and food responsiveness reflects more obesogenic responses to the presentation food. All items in each scale are scored on a 5-point Likert scale anchored at 1=never and 5=always. Final scores for each scale are computed as the mean score over the individual items. These three scales have demonstrated good internal consistency (Cronbach’s alpha range: 0.74–0.91) and high 2-week test-retest reliability (Pearson’s r range: 0.83–0.87) in initial scale development.23
Objectively Measured Eating Behaviors
Total intake at a meal and eating in the absence of hunger (EAH) are two measures of short-term eating, which may reflect usual eating behaviors indicative of overeating.27 Thus, we compared FTO and appetitive traits to the total caloric intake at lunch and EAH in the experimental study. Full details of the experimental study, including details about the pre-load meals are published elsewhere.26 Briefly, in the experimental study, children were provided with one of three highly palatable lunches (macaroni and cheese, cheese pizza bagel bites, or chicken nuggets with ketchup), along with string cheese, carrots and ranch dressing, apple slices, bread rolls, butter, milk, and water. Each meal provided 1153–1183 kCals total and contained between 552 and 704 kCals of carbohydrates, 315 and 405 kCals of fat, and 152 and 188 kCals of protein. Children self-reported satiety after lunch using a 5-point visual Likert scale (I am very hungry, I am a little hungry, I am neither full nor hungry, I am a little full, I am very full.) All lunch items were weighed pre- and post-lunch, and the total caloric intake at lunch (kCals) was computed using the difference in weights along with the manufacturers’ nutritional information. All participants in this study also completed an EAH assessment as part of a randomized experiment26 where half of the children were exposed to TV food advertisements while viewing a TV program and the other half were exposed to TV toy advertisements while viewing the same program. In each experimental condition, the TV program was 34-minutes, inclusive of 7.7 minutes of advertisements interspersed throughout the program. All children were provided four highly palatable snack foods to consume ad libitum while viewing the TV program, specifically gummy candy (546 kCals), cheese puffs (536 kCals), cookies (692 kCals) and chocolate candies (1000 kCals). Snacks were weighed before and after viewing the TV program, and the kilocalories consumed was computed using the manufacturers’ nutritional information. We computed the associations between the child’s FTO genotype and appetitive traits with total intake at lunch in all participants and with EAH among the children in the control condition of the experiment (i.e., those who were not exposed to TV food advertisements, n=89), excluding those who reported I am hungry or I am a little hungry on the pictorial Likert satiety scale after eating lunch (n=2), for a final sample of 87 participants in the control condition with EAH data.
Additional Measures
Parents reported the time his/her child spent engaging in physical activity (i.e., running around, climbing, biking, dancing, swimming, playing sports, etc.) for an average school day and for an average weekend day or summer day. A weighted response was created to reflect typical minutes of physical activity per day. The parent also reported on his/her child’s age, sex, ethnicity, and race, relationship to the child, marital status, household income, and educational attainment for him/herself and his/her spouse as appropriate. The educational attainment of the mother was used in analyses because most parents enrolled were mothers, and mothers often play a primary role in child feeding.28 The child’s weight (kg) and height (cm) were measured at the study visit using a Seca 763 Medical Scale and Seca 213 Stadiometer (Hamburg, Germany), respectively. All measurements were taken without shoes and in light indoor clothing. Measurements were used to compute age- and sex-adjusted BMI-percentiles using the Center for Disease Control and Prevention 2000 growth charts.29 Overweight or obese was defined as ≥85th percentile. One participant was classified as underweight per the Centers for Disease Control and Prevention criteria (<5th BMI-percentile)30 and was excluded from analyses.
Statistical Analyses
We first present univariate distributions of variables as counts and percentages. Bivariate analyses then compared the distribution of the child’s BMI z-score by child and family characteristics using two sample t tests when comparing means across two groups or one-way ANOVA to compare means across more than two groups. For ANOVAs, post-hoc pairwise comparisons were completed using Tukey’s Honest Significant Difference (HSD) approach. Bivariate analyses using Chi-Square tests also compared child and family characteristics by the child’s FTO to assess the distribution of baseline measures by genotype. The child’s BMI z-score, weight status (healthy weight vs. overweight or obese), and each appetitive trait were compared across FTO using ANOVAs for continuous outcomes or Chi-Square test for categorical outcomes. We computed Pearson’s correlation coefficients (r) to assess the unadjusted relationships between each appetitive trait and child BMI z-score. To address the indirect effects of FTO on BMI z-score via appetitive traits, we used structural equation modeling,31 adjusted for child sex and maternal education because those were the only measures that were associated with child BMI z-score in bivariate analyses at the P<0.10 level. For point estimates in the SEM analyses, we computed bias and acceleration corrected 95% bootstrap confidence intervals using 5000 draws.32 We considered a 95% confidence interval for any indirect effect that excluded zero as statistically significant. To explore how FTO and appetitive traits related to objective measures of short-term eating independently of current weight, total intake at lunch was regressed on FTO and on each appetitive trait using linear regression adjusted for the child’s BMI z-score, the child sex and maternal education. Similar adjusted analyses were completed to compare EAH by FTO and to each appetitive trait among the participants enrolled in the study’s control condition, although these analyses were expected to have low statistical power with the limited sample size. In analyses that fit BMI z-score on FTO genotype, we did not assume a genetic model a priori, and instead fit FTO genotype as nominal variable. Because findings from those bivariate analyses supported a recessive genetic model, the final regression models we present were fit using a recessive genetic model. However, we present regression models using an additive genetic model as a sensitivity check. All analyses were completed using the R language and environment for statistical computing, version 3.0.233 and MPlus, version 6.12.34
Results
Among the sample of 178 unrelated 9–10 year olds, 48.9% were male, 91.8% were non-Hispanic white, and most parents who accompanied the children were mothers (83.1%) (Table 1). Approximately 20% of mothers did not have a college degree and 25% of children came from families with an annual household income of less than $65,000. In bivariate analyses, boys had a greater mean BMI z-score than girls (0.6 vs 0.2; t test P<0.01) and mean BMI z-score was inversely associated with maternal education in a linear manner (ANOVA P-value<0.01). Parental report of children’s physical activity was not associated with BMI z-score (Pearson’s r: −0.07; P=0.385).
Table 1.
Sample characteristics.1
| n (%) | |
|---|---|
| Child characteristics | |
| Age | |
| 9 | 97 (54.5%) |
| 10 | 81 (45.5%) |
| Male | 87 (48.9%) |
| Ethnicity | |
| Non-Hispanic | 172 (96.6%) |
| Hispanic | 6 (3.4%) |
| Race | |
| White race | 166 (93.3%) |
| Non-white | 12 (6.7%) |
| Family characteristics | |
| Relationship to child | |
| Mother | 148 (83.1%) |
| Father | 28 (15.7%) |
| Other | 2 (1.1%) |
| Marital status of parent/guardian | |
| Married | 150 (84.3%) |
| Singe, never-married | 8 (4.5%) |
| Separated or divorced | 17 (9.6%) |
| Other | 3 (1.7%) |
| Maternal education | |
| Associates degree or less | 36 (20.2%) |
| College graduate | 52 (29.2%) |
| Professional or Graduate School | 90 (50.6%) |
| Total annual household income | |
| <$65,000 | 44 (24.7%) |
| $65,000 – $144,999 | 83 (46.6%) |
| $145,000 – $225,000 | 32 (18.0%) |
| >$225,000 | 19 (10.7%) |
| Child FTO allele frequency2 | |
| T | 211 (59.3%) |
| A | 145 (40.7%) |
| Child FTO genotype | |
| TT | 62 (34.8%) |
| AT | 87 (48.9%) |
| AA | 29 (16.3%) |
Among 178 unrelated 9–10 year olds.
Allele frequency among 356 total alleles (178 x 2) in the sample.
FTO and child weight
The frequency of the high-obesity-risk FTO rs9939609 allele (A) was similar to that observed in other studies19 and consistent with Hardy-Weinberg equilibrium ( 2 test P value > 0.99). Table 2 presents the associations between FTO and child’s physical activity, child BMI z-score, and weight status. FTO was statistically associated with BMI z-score, with mean BMI z-score being greatest among those homozygous for the high-risk allele. According to post-hoc pair-wise comparisons, mean BMI z-score was not statistically different between those heterozygous (AT) or homozygous (TT) for the low-risk allele (data not shown, Tukey’s HSD P=0.653). Rates of overweight or obesity were also greatest among those homozygous for the high-risk allele (P=0.034), again with no post-hoc differences between those heterozygous or homozygous for the low-risk allele (data not shown, unadjusted Chi-Square P=0.812).
Table 2.
| Overall (n=178) | By FTO genotype | ||||
|---|---|---|---|---|---|
| TT (n=62) | AT (n=87) | AA (n=29) | P-value3 | ||
| Child’s physical activity, minutes/day, mean (SD) | 135.0 (74.4) | 127.7 (71.0) | 144.4 (73.5) | 122.4 (71.0) | 0.225 |
| BMI z-score, mean (SD) | 0.4 (1.0) | 0.4 (0.8) | 0.2 (1.0) | 0.9 (1.0) | 0.0024 |
| Weight status, n (%) | |||||
| Healthy weight | 137 (77.0%) | 51 (82.2%) | 69 (79.3%) | 17 (58.6%) | 0.034 |
| Overweight or obese | 41 (23.0%) | 11 (17.7%) | 18 (20.7%) | 12 (41.4%) | |
| Child appetitive traits2 | |||||
| Satiety responsiveness | 2.9 (0.6) | 3.0 (0.7) | 2.9 (0.6) | 2.7 (0.6) | 0.0705 |
| Enjoyment of food | 3.7 (0.7) | 3.7 (0.7) | 3.7 (0.7) | 3.9 (0.7) | 0.317 |
| Food responsiveness | 2.2 (0.7) | 2.1 (0.6) | 2.3 (0.7) | 2.4 (0.7) | 0.150 |
Among 178 unrelated 9–10 year olds.
Child appetitive traits as measured with the Child Eating Behavior Questionnaire.
P-value from ANOVA for means and Chi-Square tests for percentages.
Post-hoc test using Tukey’s HSD documented that mean BMI z-score was not statistically different between TT and AT FTO genotypes; P=0.653.
Post-hoc test using Tukey’s HSD documented that mean satiety responsiveness was not statistically different between TT and AT FTO genotypes; P=0.945.
FTO and child appetitive traits
Table 2 also presents the associations between FTO and appetitive traits. Mean satiety responsiveness scores appeared to decrease with increasing copies of the high-risk allele (A), although findings were borderline statistically significant (P=0.070). Mean enjoyment of food (P=0.317) and food responsiveness (P=0.150) were not statistically different by FTO, although means appeared greatest among those homozygous for the high-risk allele (AA). Unadjusted linear associations between each habitual appetitive behavior and BMI z-score were in the expected directions (Table 3), with decreased satiety responsiveness, increased food responsiveness, and increased enjoyment of food each being statistically associated with increased BMI z-score (all P<0.01). Satiety responsiveness and food responsiveness were significantly, inversely associated (Pearson’s r=−0.41; P<0.001).
Table 3.
| Correlation with BMI z-score | ||
|---|---|---|
| Pearson's r | P-value | |
| Child appetitive drive | ||
| Satiety responsiveness | −0.26 | <0.01 |
| Enjoyment of food | 0.22 | <0.01 |
| Food responsiveness | 0.37 | <0.001 |
Among 178 unrelated 9–10 year olds.
Child appetitive traits as measured with the Child Eating Behavior Questionnaire.
Indirect effects of FTO on child weight via child appetitive traits
Table 4 presents separate linear regression models fitting BMI z-score on FTO, adjusted for child sex and maternal education. Because BMI z-score did not differ between those homozygous low-risk and heterozygous for FTO in the bivariate analyses, we completed adjusted analyses assuming a recessive genetic model. Inclusion of satiety responsiveness (model 2), enjoyment of food (model 3), or food responsiveness (model 4) each independently decreased the main effect due to the presence of two copies of the high risk allele (AA genotype) vs. one or no copies of that allele (AT or TT genotype) by 9.3%, 6.7%, and 13.3%, respectively. In an adjusted linear regression model that included all three appetitive traits (model 5), two copies of the high-risk allele (AA genotype) remained related to increased BMI z-score and only food responsiveness was statistically, significantly related to increased BMI z-score.
Table 4.
Adjusted associations between FTO genotype and BMI z-score, without and with adjustment for each measure of child appetitive behavior.1, 2, 3
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (SE) | P-value | (SE) | P-value | (SE) | P-value | (SE) | P-value | (SE) | P-value | |
| Child's FTO genotype | ||||||||||
| TT or AT | Reference | Reference | Reference | Reference | Reference | |||||
| AA | 0.75 (0.18) | <0.001 | 0.68 (0.18) | <0.001 | 0.70 (0.18) | <0.001 | 0.65 (0.17) | <0.001 | 0.64 (0.17) | <0.001 |
| Further adjusted for each measure of child appetitive behavior2 | ||||||||||
| Satiety responsiveness | -- | -- | −0.20 (0.11) | 0.069 | -- | -- | -- | -- | −0.02 (0.12) | 0.781 |
| Enjoyment of food | -- | -- | -- | -- | 0.22 (0.1) | 0.023 | -- | -- | 0.06 (0.11) | 0.606 |
| Food responsiveness | -- | -- | -- | -- | -- | -- | 0.37 (0.09) | <0.001 | 0.33 (0.11) | 0.002 |
|
| ||||||||||
| Model adjusted R2: | 0.18 | 0.19 | 0.20 | 0.24 | 0.24 | |||||
-- Indicates variable not included in the model.
Among 178 unrelated 9–10 year olds.
Child appetitive traits as measured with the Child Eating Behavior Questionnaire.
Series of least-squares linear regression multivariable models presented.
All models also adjusted for child’s sex and maternal education level.
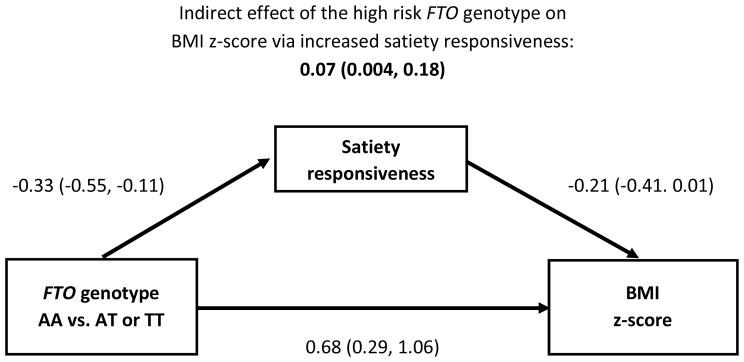
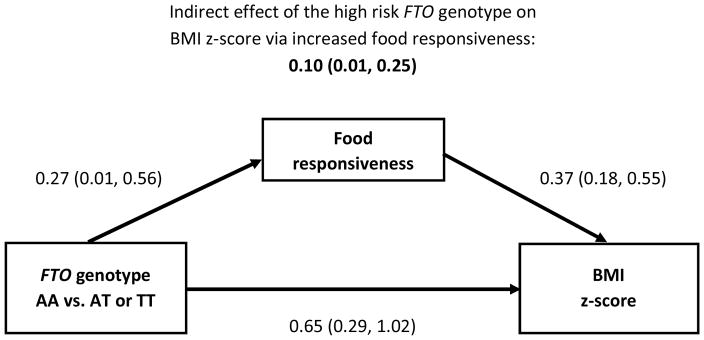
In structural equation models, there was statistical evidence that being homozygous for the high-risk allele (AA) was related to an increased BMI z-score indirectly via decreased satiety responsiveness (Figure 1) and increased food responsiveness (Figure 2) (both P-values<0.05). There was no statistical evidence of an indirect effect via enjoyment of food (model not shown, P>0.05). In all models, the high-risk FTO genotype remained strongly, positively associated with increased BMI z-score even after accounting for the indirect effects via appetitive traits. In the adjusted analysis that considered both satiety responsiveness and food responsiveness as indirect effects in the same model, only the indirect effect via food responsiveness remained statistically significant (0.09, 95% CI: 0.01, 0.24) while the indirect effect via satiety responsiveness was not statistically significant (0.02, 95% CI: −0.04, 0.12); the direct effect of the high-risk FTO genotype remained highly significant (0.63, 95% CI: 0.26, 1.00). In sensitivity analyses that assumed an additive genetic model, the indirect effects of FTO (per 1-unit increase in the number of A alleles) on BMI z-score via satiety responsiveness or via food responsiveness were statistically significant (0.03; 95% CI: 0.002, 0.10 and 0.06; 95% CI: 0.01, 0.14; respectively), while the direct effects of FTO on BMI z-score in those models were not statistically significant (0.18; 95% CI: −0.02, 0.37 and 0.15; 95% CI: −0.04, 0.33; respectively).
Figure 1. Associations between the presence of the high risk FTO genotype and BMI z-score through satiety responsiveness.1,2,3.
Coefficients labeling arrows are the main effects from full models that also account for the indirect effect of FTO to BMI z-score.
1Among 178 unrelated 9–10 year olds. β̂ and 95% confidence intervals are presented.
2Satiety responsiveness as measured with the Child Eating Behavior Questionnaire.
3Structural equation model adjusted for child’s gender and maternal education level (associate’s degree or less, college graduate or professional/graduate school).
Figure 2. Associations between the presence of the high risk FTO genotype and BMI z-score through food responsiveness.1,2,3.
Coefficients labeling arrows are the main effects from full models that also account for the indirect effect of FTO to BMI z-score.
1Among 178 unrelated 9–10 year olds. β̂ and 95% confidence intervals are presented.
2Food responsiveness as measured with the Child Eating Behavior Questionnaire.
3Structural equation model adjusted for child’s gender and maternal education level (associate’s degree or less, college graduate or professional/graduate school).
FTO and measured eating
Finally, we explored how FTO and appetitive traits were associated with short-term caloric consumption (Table 5). In a linear regression model adjusted for child BMI z-score, child sex and maternal education, there was a trend for increasing total caloric intake at lunch among homozygous high-risk children, although differences were not statistically significant (P=0.125). In similarly adjusted linear regression models, total intake at lunch was statistically, inversely associated with satiety responsiveness (P=0.003), while there were no statistical associations with enjoyment of food (P=0.111) or food responsiveness (P=0.536), although findings were in the expected directions. In similar adjusted linear regression models assessing EAH among the children enrolled in the control arm of the experiment who did not report being hungry after the pre-load lunch (n=87), there were no statistically significant associations between FTO or appetitive traits and EAH (Table 5; all P>0.520).
Table 5.
Adjusted associations between and FTO genotype or appetitive traits and caloric intake among children.
| Total caloric intake at lunch kCals (n=178) | Total caloric intake during EAH kCals (n=87)2 | |||
|---|---|---|---|---|
| (SE) | P-value | (SE) | P-value | |
| Model 11 | ||||
| FTO Genotype | ||||
| TT | - | Reference | - | Reference |
| AT | 1.6 (28) | 0.955 | 17.6 (62) | 0.777 |
| AA | 59.7 (38.7) | 0.125 | −49.9 (82.8) | 0.549 |
| BMI z-score, per 1 unit increase | 51.6 (14.8) | 0.001 | 99.6 (29.4) | 0.001 |
| Model 21 | ||||
| Satiety responsiveness, per 1 unit increase | −63.9 (20.9) | 0.003 | −19.3 (49) | 0.695 |
| BMI z-score, per 1 unit increase | 50.7 (13.9) | <0.001 | 90.9 (28.3) | 0.002 |
| Model 31 | ||||
| Enjoyment of food, per 1 unit increase | 30.5 (19) | 0.111 | 7.4 (44.4) | 0.869 |
| BMI z-score, per 1 unit increase | 54.3 (14.2) | <0.001 | 91.9 (28.4) | 0.002 |
| Model 31 | ||||
| Food responsiveness, per 1 unit increase | 12.1 (19.5) | 0.536 | 29.8 (46.5) | 0.524 |
| BMI z-score, per 1 unit increase | 56.0 (14.8) | <0.001 | 87.8 (28.9) | 0.003 |
EAH: Eating in the absence of hunger.
Each model adjusted for child gender and maternal education.
Caloric intake during EAH was among children enrolled in the control arm of the experiment who did not report being hungry after the pre-load lunch (n=87).
Discussion
FTO genotype has been robustly associated with an increased risk of overweight and obesity among children and adults.19 In this study among 178 unrelated 9–10 year old children, two parent-reported measures of appetitive traits, namely satiety responsiveness and food responsiveness, each partially mediated the positive relationship between the presence of the high-risk FTO genotype and increased BMI z-score. While effect sizes were small, our findings are similar to those from a subset of 3337 8–12 year olds enrolled in the UK TEDS cohort12 and with a subset set of 2258 children in the UK Twins Early Development study.13 Also consistent with that previous UK TEDS cohort,12 we found that normal variation in appetitive drive as measured by enjoyment of food did not mediate the association between FTO and BMI z-score. Overall, study findings support that FTO may relate to a behavioral phenotype specific to dysregulation in appetitive traits.
In our sample, the association between FTO and increased BMI z-score or weight status was limited to children who were homozygous for the high-risk allele (AA). This is in contrast to many,12, 19 but not all,35,36 studies among children that demonstrated an additive increase in the odds of overweight or obesity with each copy of the high-risk allele. Our finding of a recessive model linking FTO to BMI z-score may have occurred by chance. However, our sample reflected a population of higher socioeconomic status, and it is possible that family and environmental factors moderated the impact of being heterozygous for the FTO and increased BMI z-score. For example, there are many factors that modify the relationship between FTO and weight status, such as usual dietary composition,37 physical activity levels,38,39 and parental feeding behaviors.40 Additional studies are needed to understand what individual and family-level characteristics might explain the observed differences in FTO relating to weight gain in either an additive, dominant, or recessive genetic model.
We found that food responsiveness demonstrated the strongest and most robust associations indirectly linking FTO to BMI z-score. However, satiety responsiveness and food responsiveness were moderately correlated with each other, and our study was likely underpowered to detect two independent indirect effects. Thus, while study findings suggest that FTO may foremost act on food responsiveness, future research should consider how a genetic predisposition to obesity may affect different aspects of appetitive traits independently. It is also important to consider how children’s environments modify their genetic risk of obesity if those environments trigger obesogenic appetitive traits.10 For example, findings from the full randomized EAH experiment in this same sample of 9–10-year-olds26 documented a statistically significant interaction between TV food advertisement exposure (vs. TV toy advertisement exposure) and FTO on EAH. Specifically, EAH increased linearly in response to TV food advertisement exposure with each copy of the high-risk FTO allele. Future research that is able to operationalize a child’s usual environment may shed insight on the impact of gene-environment interactions on shaping habitual appetitive behaviors.
We also found that satiety responsiveness was substantially and significantly inversely associated with measured lunch consumption in adjusted analyses. This finding agrees with data from the Gemini twin birth cohort, where satiety responsiveness measured using the CEBQ was inversely associated with meal consumption measured via parent-reported 3-day food diaries.41 Though findings did not reach statistical significance, we also observed a greater caloric intake at lunch among children homozygous for the FTO high-risk allele compared to other children, even after adjusting for current BMI. This suggested association is in line with other studies that have observed a relation between FTO genotype and measured caloric intake.20, 21 Taken together, these findings add further support to the hypothesis that FTO may relate to BMI through decreased satiety responsiveness and increased caloric consumption. We did not observe statistically significant associations between other appetitive traits and caloric intake at lunch, though the associations were in the expected direction. We also did not observe significant associations with FTO or appetitive traits with EAH, though our study was likely underpowered to observe such associations in the subsample of 87 children in the control arm of the experiment. Research from other groups provides preliminary support that EAH is related to FTO21 and appetitive traits.24 s
In this study we only examined the associations between FTO and children’s appetitive traits. There are strong and robust associations between FTO and obesity, and variants in the FTO are common.19 However, there are several other genetic loci that have been associated with both increased body mass and protein expression in brain regions involved in energy regulation,42 and FTO may not be the gene that has a primary impact on eating behaviors. For example, data from two independent large cohorts documented that while a genetic predisposition risk score constructed from either 2813 or 9742 known obesity SNPs was associated with decreased satiety responsiveness, findings in either study did not appear driven by FTO genotype. Furthermore, research among a cohort of 258 Chilean 8–14 year olds support that polymorphisms in the melanocortin-4 receptor relate to variations in the CEBQ subscales food responsiveness and satiety responsiveness,17 and findings from a case-control study among another sample of 377 Chilean 6–12 year olds support that melanocortin-4 receptor polymorphisms relate to variations in the CEBQ subscales of satiety responsiveness and enjoyment of food.14 Additionally, our measures of appetitive traits were limited to those assessed with the CEBQ, a valid and reliable assessment of satiety responsiveness, enjoyment of food and food responsiveness. However, there are other appetitive phenotypes such as impulsivity and increased eating rate10,11 that might also partially mediate a genetic predisposition to obesity.
Strengths of this study include the use of a valid psychometric measure for appetitive traits among children. BMI z-scores were based on objective measures, and the methods to assess FTO are valid and reliable. Thus, our study has high internal validity. However, the generalizability of our study is limited because we recruited a convenience sample from a mostly white, non-Hispanic, and highly educated population from a rural area of New England. While our study is the first to assess how appetitive traits mediate the relationship between FTO and weight status among children in the US, more research is necessary to determine if results generalize to more ethnically and racially diverse samples in the US. Also, our study is limited by the use of an un-validated parent-reported measure for the child’s physical activity; such a measure is likely impacted by reporting bias. However, our finding of a null association between FTO and physical activity is consistent with previous studies among children21 and the growing body of evidence supporting that FTO is not related to physical activity levels.44 Finally, we did not observe an unadjusted association between FTO status and appetitive traits. While our structural equation modeling methods do not require such a direct effect in order to accurately estimate indirect effects,45 it must be acknowledged that the indirect effects documented may be an artifact. However, the validity of the findings is strengthened by the consistency with previous research.12
Conclusions
Appetitive phenotypes linked to a genetic predisposition to obesity emerge at a young age. This study documented that the positive relationship between the presence of the high-risk FTO genotype and increased BMI z-score was, in part, indirectly explained by variations in satiety responsiveness (sensitivity to feelings of fullness) and food responsiveness (responsiveness to the presentation of food). Findings support FTO variants may act on obesity risk via appetite regulation. Given that many other genetic loci have been associated with both an increased obesity risk, continued research is needed to consider how additional genetic variants act on appetitive traits. Understanding such phenotypes can better tailor intervention programs to help children and their parents manage children’s eating behaviors and risk for excess weight gain.
Acknowledgments
Participating children and their caregivers enabled this research study to happen. Archana Ramanujam, Horacio Romero and other study staff members contributed significantly to running this study.
Funding/Support: This study was supported by grant R21HD076097 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH) (Dr. Gilbert-Diamond).
Role of the Sponsors: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- CEBQ
Children’s Eating Behavior Questionnaire
- FTO
Fat Mass and Obesity Associated gene
- kCals
kilocalories
Footnotes
Conflict of Interest Declaration: The authors declare they have no actual or potential competing financial interests or other conflicts of interest related to the described work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. [Accessed January 29];Report of the commission on ending childhood obesity. 2016 Jan; Available at http://www.who.int/end-childhood-obesity/final-report/en/
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obesity Rev. 2008;9(5):474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. NEJM. 2014;370(5):403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biro FM, Wien M. Childhood obesity and adult morbidities. AJCN. 2010;91(5):1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375(9727):1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neale BM, Mazzeo SE, Bulik CM. A twin study of dietary restraint, disinhibition and hunger: an examination of the eating inventory (three factor eating questionnaire) Twin Res. 2003;6(6):471–478. doi: 10.1375/136905203322686455. [DOI] [PubMed] [Google Scholar]
- 8.Svensson V, Lundborg L, Cao Y, Nowicka P, Marcus C, Sobko T. Obesity related eating behaviour patterns in Swedish preschool children and association with age, gender, relative weight and parental weight--factorial validation of the Children's Eating Behaviour Questionnaire. Int J Behav Nutr Phy. 2011;8:134. doi: 10.1186/1479-5868-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson EL, Kreichauf S, Wildgruber A, et al. A narrative review of psychological and educational strategies applied to young children's eating behaviours aimed at reducing obesity risk. Obesity Rev. 2012;13(Suppl 1):85–95. doi: 10.1111/j.1467-789X.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- 10.Llewellyn CH, Fildes A. Behavioural Susceptibility Theory: Professor Jane Wardle and the Role of Appetite in Genetic Risk of Obesity. Curr Obes Rep. 2017 doi: 10.1007/s13679-017-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llewellyn CH, van Jaarsveld CH, Boniface D, Carnell S, Wardle J. Eating rate is a heritable phenotype related to weight in children. AJCN. 2008;88(6):1560–1566. doi: 10.3945/ajcn.2008.26175. [DOI] [PubMed] [Google Scholar]
- 12.Wardle J, Carnell S, Haworth CM, Farooqi IS, O'Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocr Metab. 2008;93(9):3640–3643. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 13.Llewellyn CH, Trzaskowski M, van Jaarsveld CH, Plomin R, Wardle J. Satiety mechanisms in genetic risk of obesity. JAMA Peds. 2014;168(4):338–344. doi: 10.1001/jamapediatrics.2013.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho-Urriola J, Guzmán-Guzmán IP, Smalley SV, et al. Melanocortin-4 receptor polymorphism rs17782313: association with obesity and eating in the absence of hunger in Chilean children. Nutrition. 2014;30(2):145–9. doi: 10.1016/j.nut.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Valladares M, Obregón AM, Weisstaub G, et al. Association between feeding behavior, and genetic polymorphism of leptin and its receptor in obese Chilean children. Nutr Hosp. 2014;31(3):1044–51. doi: 10.3305/nh.2015.31.3.8049. [DOI] [PubMed] [Google Scholar]
- 16.Aris IM, Tint MT, Teh AL, et al. MC3R gene polymorphisms are associated with early childhood adiposity gain and infant appetite in an Asian population. Pediatr Obes. 2016;11(6):450–458. doi: 10.1111/ijpo.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obregón AM, Oyarce K, Santos JL, Valladares M, Goldfield G. Association of the melanocortin 4 receptor gene rs17782313 polymorphism with rewarding value of food and eating behavior in Chilean children. J Physiol Biochem. 2017;73(1):29–35. doi: 10.1007/s13105-016-0521-5. [DOI] [PubMed] [Google Scholar]
- 18.Ibba A, Pilia S, Zavattari P, et al. The role of FTO genotype on eating behavior in obese Sardinian children and adolescents. J Pediatr Endocrinol Metab. 2013;26(5–6):539–44. doi: 10.1515/jpem-2012-0417. [DOI] [PubMed] [Google Scholar]
- 19.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. NEJM. 2008;359(24):2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 21.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obesity. 2009;33(1):42–45. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- 22.Tung YC, Ayuso E, Shan X, et al. Hypothalamic-specific manipulation of FTO, the ortholog of the human obesity gene FTO, affects food intake in rats. Plos One. 2010;5(1):e8771. doi: 10.1371/journal.pone.0008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42(7):963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 24.Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: validation of the child eating behaviour questionnaire. Appetite. 2007;48(1):104–113. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 25.Ashcroft J, Semmler C, Carnell S, van Jaarsveld CH, Wardle J. Continuity and stability of eating behaviour traits in children. Eur J CLin Nutr. 2008;62(8):985–990. doi: 10.1038/sj.ejcn.1602855. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert-Diamond D, Emond JA, Lansigan RK, et al. Television food advertisement exposure and FTO rs9939609 genotype in relation to excess consumption in children. Int J Obesity. 2016 doi: 10.1038/ijo.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. AJCN. 2002;76(1):226–231. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101(3 Pt 2):539–549. [PubMed] [Google Scholar]
- 29.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics. Series 11, Data from the national health survey. 2002;(246):1–190. [PubMed] [Google Scholar]
- 30.Meyers A, Joyce K, Coleman SM, et al. Health of children classified as underweight by CDC reference but normal by WHO standard. Pediatrics. 2013;131(6):e1780–1787. doi: 10.1542/peds.2012-2382. [DOI] [PubMed] [Google Scholar]
- 31.Hayes AF. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Communication Monographs. 2009;76(4):408–420. [Google Scholar]
- 32.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- 33.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing V; Austria: 2016. [Google Scholar]
- 34.Muthén LKaMBO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- 35.Duicu C, Marginean CO, Voidazan S, Tripon F, Banescu C. FTO rs 9939609 SNP Is Associated With Adiponectin and Leptin Levels and the Risk of Obesity in a Cohort of Romanian Children Population. Medicine. 2016;95(20):e3709. doi: 10.1097/MD.0000000000003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luczynski W, Zalewski G, Bossowski A. The association of the FTO rs9939609 polymorphism with obesity and metabolic risk factors for cardiovascular diseases in Polish children. J Physiol Pharmacol. 2012;63(3):241–248. [PubMed] [Google Scholar]
- 37.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfalt E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. AJCN. 2009;90(5):1418–1425. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- 38.Rampersaud E, Mitchell BD, Pollin TI, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med. 2008;168(16):1791–1797. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57(1):95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 40.Tovar A, Emond JA, Hennessy E, Gilbert-Diamond D. An FTO Gene Variant Moderates the Association between Parental Restriction and Child BMI. Plos One. 2016;11(5):e0155521. doi: 10.1371/journal.pone.0155521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syrad H, Johnson L, Wardle J, Llewellyn CH. Appetitive traits and food intake patterns in early life. AJCN. 2016;103(1):231–235. doi: 10.3945/ajcn.115.117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monnereau C, Jansen PW, Tiemeier H, Jaddoe VW, Felix JF. Influence of genetic variants associated with body mass index on eating behavior in childhood. Obesity. 2017;25(4):765–772. doi: 10.1002/oby.21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo GS. The role of the FTO (Fat Mass and Obesity Related) locus in regulating body size and composition. Mol Cell Endocrinol. 2014;397(1–2):34–41. doi: 10.1016/j.mce.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]