Abstract
Purpose
To study the antifibrotic effectiveness of mitomycin-C in the tissues of the ostium site after it application for endonasal endoscopic dacryocystorhinostomy.
Material and methods
The study included 45 patients (48 cases) with primary obstruction of the nasolacrimal duct. All patients underwent endoscopic endonasal dacryocystorhinostomy (EEDCR). At the final stage of the operation, a swab with MMC was placed in the region of the formed ostium at a concentration of 0.2 mg/ml for 3 min. An ostium was not intubated. After that, biopsies of the mucous of the nasal cavity and lacrimal sac were performed to study the morphological changes that occur in the tissues overtime, as well as to calculate the concentration of the drug in the tissues.
Results
According to the chemical analysis, the concentration of MMC immediately after application was 0.626 ± 0.176 μg/g; after 30 min the concentration of the drug was reduced to 0.23 ± 0.06 μg/g; a day after the operation the drug was not found in the tissue samples. Morphological study established that the repair processes occurring in the mucosa of the nasal cavity and the lacrimal sac after EEDCR are similar to the reparative processes without the use of MMC. The effectiveness of surgical treatment: “positive results” - 77.1% of cases, “relapses” - 22.9% of cases.
Conclusions
Application of MMC for prevention of excessive scarring after EEDCR is impractical as it is not possible to achieve antifibrotic concentration of the drug at dacryocystorhinostomy ostium site using this method.
Keywords: Dacryocystorhinostomy, Mitomycin-C, Fibrosis, High-performance liquid chromatography-mass spectrometry
Introduction
Mitomycin-C (MMC) is an alkylating antibiotic used in dacryology for prevention of excessive scarring of the area of the formed ostium after dacryocystorhinostomy. In vitro study showed its inhibitory effect on the proliferation of fibroblasts. However, clinical effectiveness of its application has remained controversial until now.
Mitomycin-C (MMC) is used in dacryology since 1998 for the prevention of excessive scarring of the region of the formed ostium after dacryocystorhinostomy.20
The mechanism of action of the drug includes inhibition of the synthesis of DNA, cellular RNA and protein, which leads to suppression of collagen synthesis by fibroblasts.2, 9, 10, 12, 19, 20
In 2013 Ali et al. conducted a fundamental study that determined the optimal concentration suppressing the growth of fibroblasts and not causing their apoptosis (0.2–0.3 mg/ml) and optimal exposure (3 min).2 In their further studies, the authors, using electron microscopy, studied the biopsy material of the nasal mucosa after endonasal dacryocystorhinostomy with application of MMC at concentration of 0.2 mg/ml and 3 min exposure. It was proved that the application of the drug causes ultrastructural changes affecting epithelium, vessels and fibroblasts.1
To date, enough information has been accumulated about the possibility of using MMC as an antifibrotic agent, confirmed by in vitro studies.2, 10 However, its clinical effectiveness remains controversial.
We study the antifibrotic effectiveness of mitomycin-C in the tissues of the ostium site after it application for endonasal endoscopic dacryocystorhinostomy.
Material and methods
The study included 45 patients (48 cases) with primary obstruction of the nasolacrimal duct, at the age of 63 ± 15 years. The study was conducted after the approval of the local ethics committee. Nature of the forthcoming study was explained to the patients and they provided informed consent.
The study did not include patients with traumatic lesions of the lacrimal passages and their secondary changes, previous surgical interventions on the lacrimal pathways, diseases of the nasal cavity and paranasal sinuses that required treatment. A standard ophthalmological and dacryological examination were performed. Complaints for lacrimation were assessed on the Munk scale.15 The patients underwent lacrimal meniscometry with an optical coherent tomograph RTVue-100-2 (Optovue, USA) with the definition of the conditional depth of the lacrimal meniscus according to the procedure described by us earlier.5 Endoscopy of the nasal cavity was performed. In all cases computed tomography with topical contrast enhancement of lacrimal passages was performed with GE Optima CT 660 (General Electric, USA).
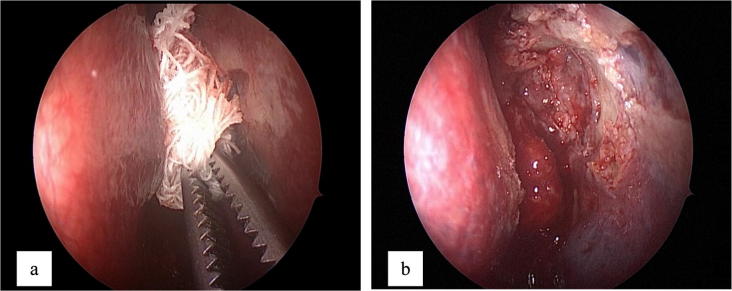
Endoscopic endonasal dacryocystorhinostomy (EEDCR) was performed by forming an ostium at the level of the lacrimal canaliculi orifice, the medial wall of the lacrimal sac was excised along the perimeter of the bone “window”. Fragment of the mucous of the nasal cavity was resected till the edge of the ostium. No intubation was performed. All patients at the final stage of the operation underwent application of MMC with a swab treated with 2 ml of MMC at a concentration of 0.2 mg/ml for 3 min at the ostium site. After the removal of the swab the lacrimal passages were rinsed with physiological saline (Fig. 1).
Figure 1.
The stage of EEDCR. Endoscopic view. (a) Installation of a swab with mitomycin-C in the area of the operation; (b) endoscopic view after removal of a swab.
Fifteen patients (15 cases) had histological examination of the mucous of the nasal cavity and lacrimal sac on the second, fifth, tenth, fourteenth, twenty first, twenty-eighth and sixtieth days (according to the physiological laws of wound healing).
Study protocol: a biopsy of the nasal mucosa from the ostium region was performed endonasally under the control of a 30-degree optic with a diameter of 2.7 mm (Karl Storz, Germany) after anemization of the nasal cavity with lidocaine and epinephrine and local infiltration anesthesia with an articaine solution with epinephrine 1:100,000 - 1.7 ml (Ultracaine D-C Fort, Sanofi Aventis, France), each time from various areas. The resulting samples were placed in a fixing solution (10% neutral formalin) for 24 h, washed in running water, dehydrated in an upward concentration alcohol, clarified in xylene and encased in paraffin. Serial transverse sections with 4–6 μm thickness were prepared on a rotational Historange microtome 2218 (LKB, Sweden), stained with toluidine blue, or hematoxylin and eosin. The resulting histological specimens were examined on a Leica DM-2500 photomicroscope (Leica, Germany). The images were photographed with a digital camera DFC320 (Leica, Germany), followed by image analysis using the ImageScope Color software (Aperio Technologies, USA).
In 14 patients (15 cases) concentration of MMC in the mucosa of the nasal cavity was studied immediately after the administration of the drug, after 30 min, and also on the 1st day after the surgery.
Study protocol: the biopsy was performed in a manner similar to that described above. Before the chemical analysis, the samples were placed in 1 ml of deionized water, and then weighed on a precision scale (Sartorius AG, Germany) within 0.001 g precision and held in an ultrasonic bath WiseClean (Daihan Scientific, Korea) for 15 min. Samples were stored for no more than a day at a temperature of 5° C. The concentration of the drug in the resulting solution was determined, and concentration of MMC was found in the initial tissue sample comparing it to the weight of the corresponding tissue sample.
The determination of MMC was carried out by high-performance liquid chromatography-mass spectrometry. The study was performed on Agilent 1260 liquid chromatograph (Agilent, USA) with a mass spectrometer detector Maxis Impact (Bruker, Germany) with electrospray ionization, quadrupole and time-of-flight mass analyzers (Fig. 2). Zorbax SB-C18 column 150 mm × 2.1 mm × 5 μm (Agilent, USA) was used for separation, elution mode: isocratic, 100% acetonitrile (JT Baker, Netherlands), and mobile phase rate: 0.25 ml/min. Total analysis time: 2.1 min.
Figure 2.

Mass-spectrometric detector Maxis Impact (Bruker, Germany).
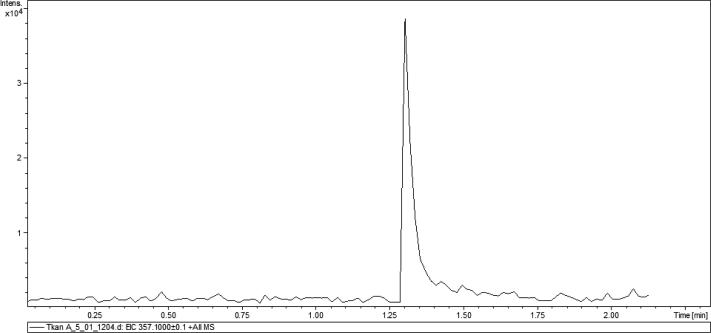
Mitomycin-C Kyowa (Kyowa Hakko Kogyo, Japan) was used as a standard of mitomycin. Standard solution was prepared in deionized water (Millipore, France) at a concentration of 1 μg/ml by accurate weighing, and subsequent standard solutions at concentrations of 500, 200, 100, 50, 20 and 10 ng/ml were prepared by successive dilution. To construct the calibration curve, the area of the peak with a retention time of 1.3 min was used as the analytical signal for detection in the mode of monitoring of isolated ions, m/z = 357.1 ± 0.1 Da (corresponds to MMC adduct with sodium ion) (Fig. 3).
Figure 3.
Chromatogram of a standard solution of MMC, detection in the mode of monitoring of isolated ions, m/z = 357.1 ± 0.1 Da.
Criteria for assessing of postoperative result of patients were as follows:
“Recovery”:
-
1.
severity of epiphora according to Munk scale—0 points;
-
2.
the absence of a purulent discharge;
-
3.
decrease of the depth of the lacrimal meniscus;
-
4.
positive nasal dye disappearing test;
-
5.
free possibility of lacrimal passages during washing;
-
6.
the presence of a formed ostium confirmed by endoscopy of the nasal cavity.
“Improvement”:
-
1.
severity of epiphora according to Munk scale—0–2 points;
-
2.
the absence of a purulent discharge;
-
3.
preservation or reduction of the former depth of the lacrimal meniscus;
-
4.
positive or delayed nasal dye disappearing test;
-
5.
passage of fluid into the nasal cavity after pressure on the plunger of the syringe or passing it in the form of thin jet;
-
6.
the presence of a formed ostium confirmed by endoscopy of the nasal cavity.
“Relapse”:
-
1.
1 severity of epiphora according to Munk scale—3–4 points;
-
2.
purulent discharge from lacrimal passages;
-
3.
increased depth of lacrimal meniscus;
-
4.
negative nasal dye disappearing test;
-
5.
obstruction of lacrimal passages during washing;
-
6.
cicatricial deformation of the formed ostium confirmed with endoscopy of the nasal cavity.
Positive results included “recovery” and “improvement”, negative – “relapse” of the disease.
The follow-up period was 9–24 (14 ± 5) months after the surgery.
Statistical processing was carried out using the Microsoft Office 2013 program suite and SOFA Statistics 1.4.4.
Result
Results of histological examination
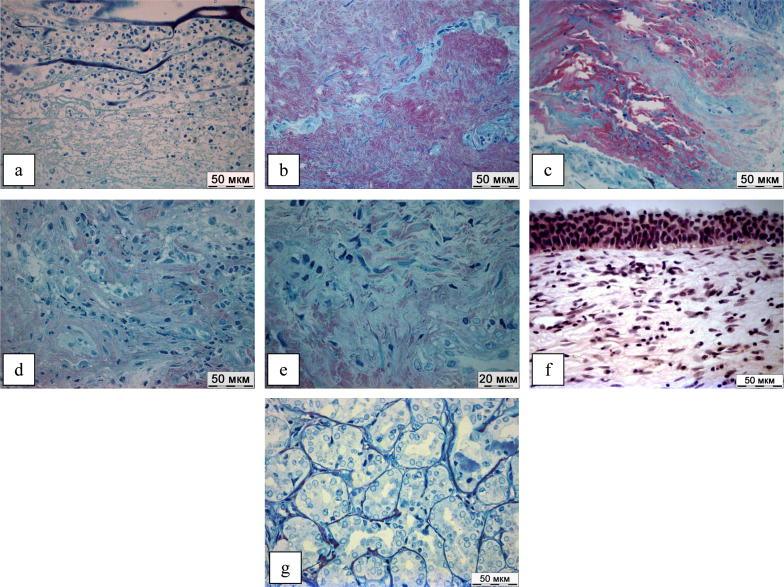
Fig. 4 presents the results of a histological examination of the mucous of the nasal cavity and lacrimal sac in patients after EEDCR with the application of MMC.
Figure 4.
(a–g) Results of histological study.
On the 2nd day after the operation, a pronounced inflammatory reaction was persisted in the mucous membrane of the nasal cavity, and fibrinous exudation and leukocyte infiltration were observed in all samples (Fig.4a). However, by the 5th day the activity of inflammation decreased, histological examination revealed fibroblast proliferation areas which synthesized collagen extracellular matrix (Fig.4b). This trend persisted on the 7th and 14th days after the surgery (Fig.4c and d). On the 21st day after the operation, the formation of loose connective tissue was observed (Fig.4e) with duration of up to 60 days, in two cases, active proliferative inflammation was determined on the 28th day with predominance of activated unidirectional fibroblasts in the cellular infiltrate; small numbers of plasmocytes and lymphocytes were determined (Fig.4f). However, on the 60th day regeneration was completed, and the formation of the epithelial lining and functioning glands was noted (Fig.4g).
Results of the study of the concentration of MMC in tissues
Determination of MMC concentration in the initial samples of tissue found that immediately after application the concentration of the drug was 0.626 ± 0.176 μg/g; after 30 min the concentration of the drug was reduced to 0.23 ± 0.06 μg/g; a day after the operation the drug was not determined in the tissue samples.
Clinical results
Clinical results of the performed surgical treatment are presented in Table 1.
Table 1.
Clinical effect.
| Parameters | Patients (cases) |
||
|---|---|---|---|
| Before operation | After operation | ||
| Munk (scores) | 2–4 | 0–4 | |
| Median 4 | Median 1 | ||
| Purulent | Presence | 48 (100%) | 11 (22.9%) |
| Discharge | Absence | – | 37 (77.1%) |
| Depth of lacrimal meniscus (µm) | 425 ± 180 | 368 ± 135 | |
| Dye disappearing test, cases (%) | Positive | – | 37 (77.1%) |
| Negative | 47 (100%) | 11 (22.9%) | |
| Washing of lacrimal passages, cases (%) | Free | – | 25 (52.1%) |
| Thin jet | – | 12 (25%) | |
| Not passable | 47 (100%) | 11 (22.9%) | |
| Endorinoscopy of EEDCR, cases (%) | Epithelial lining | – | 37 (77.1%) |
| Cicatricial deformity | – | 11 (22.9%) | |
“Recovery” was observed in 25 cases (52.1%), “improvement” – in 12 cases (25%), “relapse” – in 11 cases (22.9%). Thus, positive results after the operation were recorded in 37 cases (77.1%), negative – in 11 cases (22.9%).
Postoperative monitoring revealed the following complications: granulation in ostium area in 10 cases (20.8%), synechiae formation between the front end of the middle turbinate and the lateral wall of the nasal cavity in ostium area – in 8 cases (16.7%).
Discussion
Dacryocystorhinostomy is a common operation for the treatment of patients with obstruction of the nasolacrimal duct. Despite the use of optical technologies, improvements in surgical techniques, the use of modern materials for intubation, and positive results after the surgery, according to various authors, are ranged from 82% to 91%.12, 18 The main cause of relapses is imperforation of artificial anastomosis with scar tissue, granulation tissue development, occluding lumen of the ostium and synechia formation in the nasal cavity impeding the outflow of tears.
Among the cytostatic drugs used in dacryology with an antifibrotic purpose, the most studied is MMC, which is used in the clinics after the publication of the study of Ugurbas et al. in 1998.20 Different researchers used MMC in various concentrations from 0.2 to 0.5 mg/ml.3, 7, 14, 19 The exposure time was also variable from 2 to 10 min.8, 14 A number of authors used MMC for primary interventions,3, 7, 21 others for repeated.8, 17 Some researchers in addition to MMC conducted intubation of the ostium with a silastic implant.7, 19 The period of observation of patients in these studies was also different and lasted from 6 to 18 months.3, 6, 7, 8, 10, 12, 13, 14, 16, 17, 18, 19, 21 Thus, the mentioned studies were conducted on non-standardized clinical material, as a result of which generalization of their results is impossible. In addition, in the significant number of works3, 6, 7, 8, 14, 21 there is no comparison group, and none of the works was performed on a statistically significant representative sample. In this regard, it is not possible to draw reliable conclusions about the clinical effectiveness of MMC application in any mode of application.
Our study has proven that after application of MMC on mucosa of the nasal cavity drug concentration is only 0.626 ± 176 µg/g which is approximately three hundred times lower than proven effective antifibrotic drug concentration (0.2–0.3 mg/ ml) established by Ali et al. in in vitro studies.2 Histological study did not reveal difference of ostium repair after EEDCR without the use of any antifibrotic agents4 and with the application of MMC.
Analysis of clinical efficacy showed that positive results (“recovery” and “improvement”) were achieved in 77.1% of cases which is comparable with the results of operations performed previously in the same manner, but without using the MMC, and the data of other researchers.11, 12, 18
We suppose that the absence of a clinically significant antifibrotic effect after application of MMC is associated with a low concentration of the drug in the tissues of the ostium site. Theoretically, to achieve local cytotoxic action, it is necessary either to increase the exposure time of the drug, or to increase the concentration of the solution applied to the swab which can lead to the development of systemic and local complications. The latter are due to the fact that in addition to the cytostatic effect the direct cytopathic effect of MMC can be realized with increasing concentration leading to alteration and, subsequently, necrosis of surrounding tissues.
Thus, it is advisable to develop alternative methods of MMC administration in the tissue which ensure achievement of local antifibrotic concentration and which do not allow significant systemic absorption of the drug. Such MMC application method was suggested by Kamal et al.11 The authors injected the drug into the mucosa of the nasal cavity at the final stage of dacryocystorhinostomy. Clinical efficacy of the surgery with injection of MMC was 97.3% of cases.
It can be concluded that the application of MMC to prevent the scarring of artificial anastomosis after dacryocystorhinostomy is impractical, as for this method of administration, the cytostatic concentration of the drug is not achieved, there is no histologically confirmed inhibition of collagenogenesis, and, as a consequence, clinically significant effects.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
E.L. Atkova, Email: evg.atkova@mail.ru.
A.A. Fedorov, Email: info@eyeacademy.ru.
A.O. Root, Email: anna.root@mail.ru, a.root@niigb.ru.
S.D. Iartsev, Email: yartsew1@yandex.ru.
N.N. Krakhovetsky, Email: krahovetskiynn@mail.ru.
V.D. Yartsev, Email: yartsew@ya.ru, v.yartsev@niigb.ru.
References
- 1.Ali M.J., Baig F., Lakshman M., Naik M.N. Electron microscopic features of nasal mucosa treated with topical mitomycin C: implications in dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2015;31(2):103–107. doi: 10.1097/IOP.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 2.Ali M.J., Mariappan I., Maddileti S., Ali M.H., Naik M.N. Mitomycin C in dacryocystorhinostomy: the search for the right concentration and duration–a fundamental study on human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg. 2013;29(6):469–474. doi: 10.1097/IOP.0b013e3182a23086. [DOI] [PubMed] [Google Scholar]
- 3.Apuhan T., Yıldırım Y., Eroglu F., Sipahier A. Effect of mitomycin C on endoscopic dacryocystorhinostomy. J Craniofac Surg. 2011;22(6):2057–2059. doi: 10.1097/SCS.0b013e3182319863. [DOI] [PubMed] [Google Scholar]
- 4.At'kova E.L., Fedorov A.A., Root A.O., Krakhovetskiy N.N., Yartsev V.D., Rein D.A. Morphological analysis of reparative process at osteotomy site after endoscopic endonasal dacryocystorhinostomy. Ann Ophthalmol. 2016;132(6):87–92. doi: 10.17116/oftalma2016132687-92. [DOI] [PubMed] [Google Scholar]
- 5.At'kova E.L., Yartsev V.D., Krakhovetskiy N.N., Root A.O., Rein D.A. Use of optical coherence tomography based lacrimal meniscometry in dacryology. Ann Ophthalmol. 2016;132(6):101–107. doi: 10.17116/oftalma20161326101-107. [DOI] [PubMed] [Google Scholar]
- 6.Dolmetsch A. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in nasolacrimal duct obstruction in adults. Ophthalmology. 2010;117:1037–1040. doi: 10.1016/j.ophtha.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S., Roychoudhury A., Roychaudhuri B.K. Use of mitomycin C in endo-DCR. Indian J Otolaryngol Head Neck Surg. 2006;58(4):368–369. doi: 10.1007/BF03049597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Görgülü O., Ozdemir S., Görgülü F.F., Altin A., Selçuk T., Akbaş Y. Adjunctive use of mitomycin C in endoscopic revision dacryocystorhinostomy. B-ENT. 2012;8(2):123–126. [PubMed] [Google Scholar]
- 9.Hata T., Hoshi T., Kanamori K., Matsumae A., Sano Y., Shima T. Mitomycin, a new antibiotic from Streptomyces. I. J Antibiot (Tokyo) 1956;9(4):141–146. [PubMed] [Google Scholar]
- 10.Hu D., Sires B., Tong D., Royack G., Oda D. Effect of brief exposure to mitomycin C on cultured human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg. 2000;16(2):119–125. doi: 10.1097/00002341-200003000-00006. 10.1097/00002341-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Kamal S., Ali M., Naik M. Circumostial injection of mitomycin C (COS-MMC) in external and endoscopic dacryocystorhinostomy: efficacy, safety profile, and outcomes. Ophthal Plast Reconstr Surg. 2014;30(2):187–190. doi: 10.1097/IOP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 12.Leong S., Karkos P., Burgess P., Halliwell M., Hampal S. A comparison of outcomes between nonlaser endoscopic endonasal and external dacryocystorhinostomy: single-center experience and a review of British trends. Am J Otolaryngol. 2010;31(1):32–37. doi: 10.1016/j.amjoto.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Leong S., Macewen C., White P. A systematic review of outcomes after dacryocystorhinostomy in adults. Am J Rhinol Allergy. 2010;24(1):81–90. doi: 10.2500/ajra.2010.24.3393. [DOI] [PubMed] [Google Scholar]
- 14.Mudhol R.R., Zingade N.D., Mudhol R.S., Harugop A.S. Das AT2. Prospective randomized comparison of mitomycin C application in endoscopic and external dacryocystorhinostomy. Indian. J Otolaryngol Head Neck Surg. 2013;65(Suppl 2):255–259. doi: 10.1007/s12070-011-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munk P.L., Lin D.T., Morris D.C. Epiphora: treatment by means of dacryocystoplasty with balloon dilation of the nasolacrimal drainage apparatus. Radiology. 1990;177(3):687–690. doi: 10.1148/radiology.177.3.2243969. [DOI] [PubMed] [Google Scholar]
- 16.Nair A.G., Ali M.J. Mitomycin-C in dacryocystorhinostomy: from experimentation to implementation and the road ahead: a review. Indian J Ophthalmol. 2015;63(4):335–339. doi: 10.4103/0301-4738.158082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penttilä E., Smirnov G., Seppä J., Kaarniranta K., Tuomilehto H. Mitomycin C in revision endoscopic dacryocystorhinostomy: a prospective randomized study. Am J Rhinol Allergy. 2011;25(6):425–428. doi: 10.2500/ajra.2011.25.3676. [DOI] [PubMed] [Google Scholar]
- 18.Prasannaraj T., Kumar P., Narasimhan I., Shivaprakash K. Significance of adjunctive mitomycin C in endoscopic dacryocystorhinostomy. Am J Otolaryngol-Head Neck Med Surg. 2010;33:47–50. doi: 10.1016/j.amjoto.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Tirakunwichcha S., Aeumjaturapat S., Sinprajakphon S. Efficacy of mitomycin C in endonasal endoscopic dacryocystorhinostomy. Laryngoscope. 2011;121(2):433–436. doi: 10.1002/lary.21292. Epub 2011 Jan 4. [DOI] [PubMed] [Google Scholar]
- 20.Ugurbas S.H., Zilelioglu G., Sargon M.F., Anadolu Y., Akiner M., Aktürk T. Histopathologic effects of mitomycin-C on endoscopic transnasal dacryocystorhinostomy. Ophthalmic Surg Lasers. 1997;28:300–304. [PubMed] [Google Scholar]
- 21.Zilelioğlu G., Uğurbaş S.H., Anadolu Y., Akiner M., Aktürk T. Adjunctive use of mitomycin C on endoscopic lacrimal surgery. Br J Ophthalmol. 1998;82(1):63–66. doi: 10.1136/bjo.82.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]