Abstract
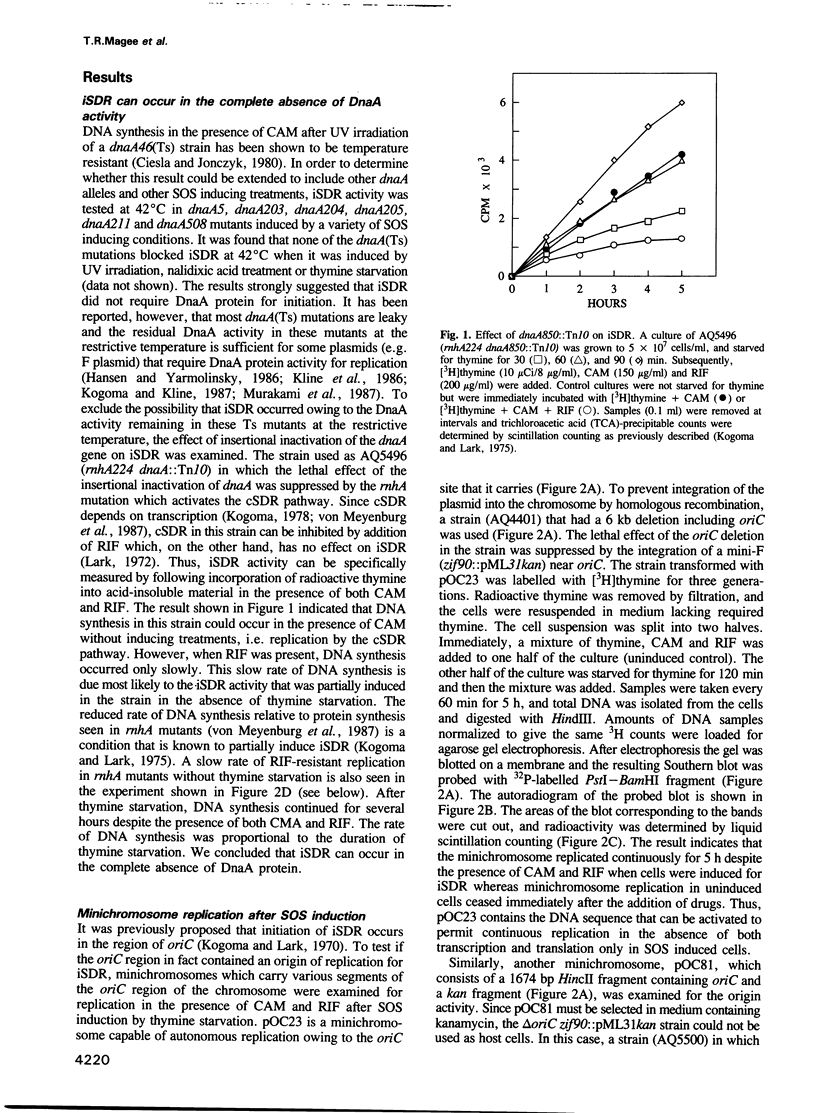
Upon induction of the SOS response in Escherichia coli, the mode of initiation of DNA replication is altered such that it can occur in the absence of normally required protein synthesis. This type of DNA replication has been termed induced stable DNA replication (iSDR). We examined the origin usage during iSDR and found that the initiation of iSDR occurs primarily in the oriC and terC regions of the chromosome in a manner completely independent of transcription, translation and DnaA protein. Minichromosomes (oriC plasmids) pOC23 and pOC81 were induced to replicate in the absence of DnaA protein and transcription after SOS induction. The results localized one of the iSDR origin activities in a 596 bp region which includes the minimal oriC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D. Replication of the bacterial chromosome: location of new initiation sites after irradiation. J Bacteriol. 1969 Mar;97(3):1169–1175. doi: 10.1128/jb.97.3.1169-1175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binding R., Romansky G., Bitner R., Kuempel P. Isolation and properties of Tn10 insertions in the rac locus of Escherichia coli. Mol Gen Genet. 1981;183(2):333–340. doi: 10.1007/BF00270637. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner C. A., Randall S. K., Rayssiguier C., Radman M., Eritja R., Kaplan B. E., McEntee K., Goodman M. F. Purification and characterization of an inducible Escherichia coli DNA polymerase capable of insertion and bypass at abasic lesions in DNA. J Biol Chem. 1988 Dec 15;263(35):18946–18952. [PubMed] [Google Scholar]
- Cieśla Z., Jonczyk P. The dnaA gene product is not required during stable chromosome replication in Escherichia coli. Mol Gen Genet. 1980;180(3):617–620. doi: 10.1007/BF00268069. [DOI] [PubMed] [Google Scholar]
- Connolly B., Parsons C. A., Benson F. E., Dunderdale H. J., Sharples G. J., Lloyd R. G., West S. C. Resolution of Holliday junctions in vitro requires the Escherichia coli ruvC gene product. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6063–6067. doi: 10.1073/pnas.88.14.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Barnsley P., Pritchard R. H. Location and characterisation of a new replication origin in the E. coli K12 chromosome. Mol Gen Genet. 1979 Sep;175(2):151–157. doi: 10.1007/BF00425531. [DOI] [PubMed] [Google Scholar]
- Diaz R., Pritchard R. H. Cloning of replication origins from the E. coli K12 chromosome. Nature. 1978 Oct 12;275(5680):561–564. doi: 10.1038/275561a0. [DOI] [PubMed] [Google Scholar]
- Espion D., Kaiser K., Dambly-Chaudiere C. A third defective lambdoid prophage of Escherichia coli K12 defined by the lambda derivative, lambdaqin111. J Mol Biol. 1983 Nov 5;170(3):611–633. doi: 10.1016/s0022-2836(83)80124-7. [DOI] [PubMed] [Google Scholar]
- Farr S. B., Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991 Dec;55(4):561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman E. C., Jimenez-Sanchez A., Orr E., Pritchard R. H. Heat stress in the presence of low RNA polymerase activity increases chromosome copy number of Escherichia coli. Mol Gen Genet. 1988 May;212(2):203–206. doi: 10.1007/BF00334685. [DOI] [PubMed] [Google Scholar]
- Hansen E. B., Yarmolinsky M. B. Host participation in plasmid maintenance: dependence upon dnaA of replicons derived from P1 and F. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4423–4427. doi: 10.1073/pnas.83.12.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. M., Kuempel P. L. Deletion of the terminus region (340 kilobase pairs of DNA) from the chromosome of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3766–3770. doi: 10.1073/pnas.82.11.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
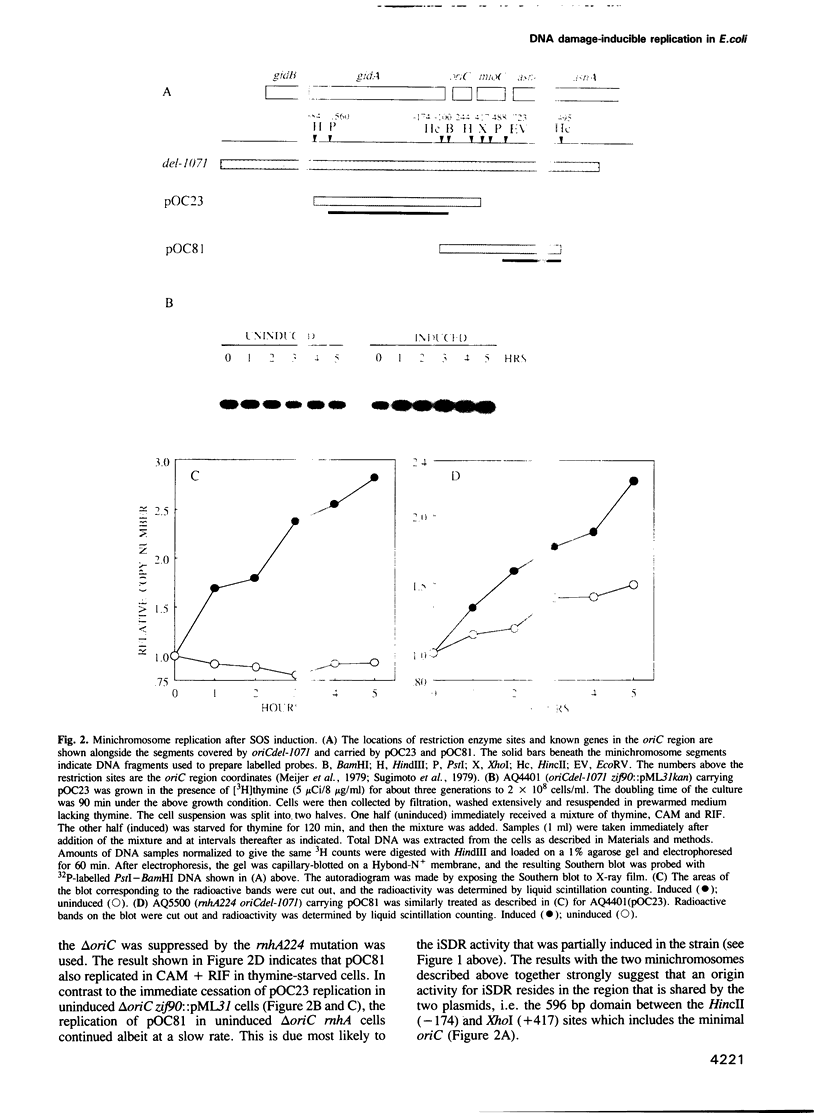
- Hill T. M., Henson J. M., Kuempel P. L. The terminus region of the Escherichia coli chromosome contains two separate loci that exhibit polar inhibition of replication. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1754–1758. doi: 10.1073/pnas.84.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. Novel F prime factors able to replicate in Escherichia coli Hfr strains. Proc Natl Acad Sci U S A. 1976 Jan;73(1):198–202. doi: 10.1073/pnas.73.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khidhir M. A., Casaregola S., Holland I. B. Mechanism of transient inhibition of DNA synthesis in ultraviolet-irradiated E. coli: inhibition is independent of recA whilst recovery requires RecA protein itself and an additional, inducible SOS function. Mol Gen Genet. 1985;199(1):133–140. doi: 10.1007/BF00327522. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Kogoma T., Tam J. E., Shields M. S. Requirement of the Escherichia coli dnaA gene product for plasmid F maintenance. J Bacteriol. 1986 Oct;168(1):440–443. doi: 10.1128/jb.168.1.440-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T. A novel Escherichia coli mutant capable of DNA replication in the absence of protein synthesis. J Mol Biol. 1978 May 5;121(1):55–69. doi: 10.1016/0022-2836(78)90262-0. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Kline B. C. Integrative suppression of dnaA(Ts) mutations mediated by plasmid F in Escherichia coli is a DnaA-dependent process. Mol Gen Genet. 1987 Dec;210(2):262–269. doi: 10.1007/BF00325692. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Lark K. G. Characterization of the replication of Escherichia coli DNA in the absence of protein synthesis: stable DNA replication. J Mol Biol. 1975 May 15;94(2):243–256. doi: 10.1016/0022-2836(75)90081-9. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Lark K. G. DNA replication in Escherihia coli: replication in absence of protein synthesis after replication inhibition. J Mol Biol. 1970 Sep 14;52(2):143–164. doi: 10.1016/0022-2836(70)90022-7. [DOI] [PubMed] [Google Scholar]
- Kogoma T. RNase H-defective mutants of Escherichia coli. J Bacteriol. 1986 May;166(2):361–363. doi: 10.1128/jb.166.2.361-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., Subia N. L., von Meyenburg K. Function of ribonuclease H in initiation of DNA replication in Escherichia coli K-12. Mol Gen Genet. 1985;200(1):103–109. doi: 10.1007/BF00383320. [DOI] [PubMed] [Google Scholar]
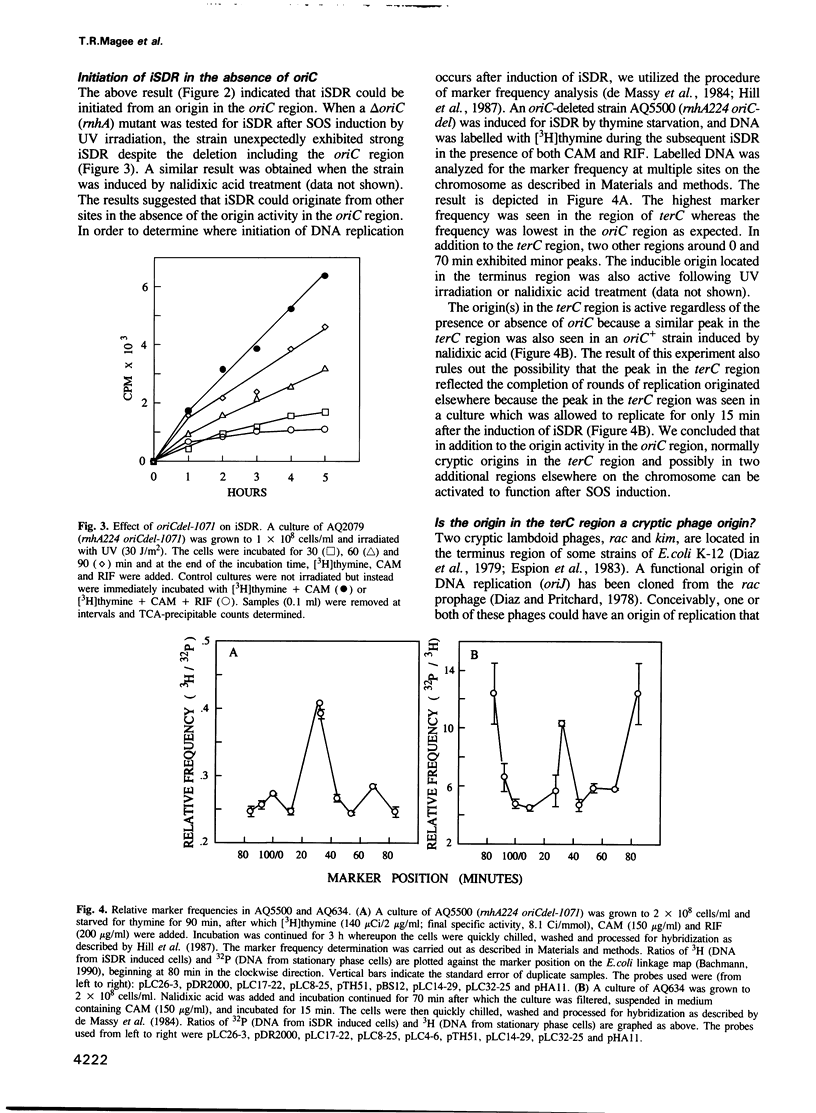
- Kogoma T., Torrey T. A., Connaughton M. J. Induction of UV-resistant DNA replication in Escherichia coli: induced stable DNA replication as an SOS function. Mol Gen Genet. 1979 Oct 2;176(1):1–9. doi: 10.1007/BF00334288. [DOI] [PubMed] [Google Scholar]
- Kogoma T., von Meyenburg K. The origin of replication, oriC, and the dnaA protein are dispensable in stable DNA replication (sdrA) mutants of Escherichia coli K-12. EMBO J. 1983;2(3):463–468. doi: 10.1002/j.1460-2075.1983.tb01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Yap W. Y., Menkens A. E., Engman H. W. Recombination-dependent replication of plasmids during bacteriophage T4 infection. J Biol Chem. 1988 Aug 15;263(23):11366–11373. [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. A. recA-dependent DNA replication in the absence of protein synthesis: characteristics of a dominant lethal replication mutation, dnaT, and requirement for recA+ function. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):537–549. doi: 10.1101/sqb.1979.043.01.059. [DOI] [PubMed] [Google Scholar]
- Luder A., Mosig G. Two alternative mechanisms for initiation of DNA replication forks in bacteriophage T4: priming by RNA polymerase and by recombination. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1101–1105. doi: 10.1073/pnas.79.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Magee T. R., Kogoma T. Rifampin-resistant replication of pBR322 derivatives in Escherichia coli cells induced for the SOS response. J Bacteriol. 1991 Aug;173(15):4736–4741. doi: 10.1128/jb.173.15.4736-4741.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W., Meijer M., Bergmans H. E., Hansen F. G., von Meyenburg K., Beck E., Schaller H. Origin of replication, oriC, of the Escherichia coli K12 chromosome: nucleotide sequence. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):139–145. doi: 10.1101/sqb.1979.043.01.020. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Ohmori H., Yura T., Nagata T. Requirement of the Escherichia coli dnaA gene function for ori-2-dependent mini-F plasmid replication. J Bacteriol. 1987 Apr;169(4):1724–1730. doi: 10.1128/jb.169.4.1724-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Subia N. L., Kogoma T. Concatemer formation of ColE1-type plasmids in mutants of Escherichia coli lacking RNase H activity. J Mol Biol. 1986 Jun 5;189(3):389–399. doi: 10.1016/0022-2836(86)90311-6. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Massy B., Fayet O., Kogoma T. Multiple origin usage for DNA replication in sdrA(rnh) mutants of Escherichia coli K-12. Initiation in the absence of oriC. J Mol Biol. 1984 Sep 15;178(2):227–236. doi: 10.1016/0022-2836(84)90141-4. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Boye E., Skarstad K., Koppes L., Kogoma T. Mode of initiation of constitutive stable DNA replication in RNase H-defective mutants of Escherichia coli K-12. J Bacteriol. 1987 Jun;169(6):2650–2658. doi: 10.1128/jb.169.6.2650-2658.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]