Abstract
Purpose
To study the effect of posterior blepharitis on meibomian glands using infrared meibography and to correlate the results with tear film parameters.
Methods
This is a prospective cohort study. The study included eyes from two groups: 86 eyes of healthy volunteers’ eyes and 72 eyes with posterior blepharitis. Participants were examined, and diagnosis of posterior blepharitis was achieved clinically based on signs of posterior blepharitis. Clinical assessment of dryness was performed including slit lamp examination looking for signs of posterior blepharitis, tear breakup time (TBUT), superficial punctate keratopathy (SPK), Schirmer II test (with anesthesia) and meibum score. Non-contact meibography was performed for both upper and lower eyelids using the meibo-grade system which involved distortion of meibomian gland, shortening and dropout.
Results
Lid margin abnormalities (Telangiectasia, lid margin swelling and hyperemia) were all significantly higher in the posterior blepharitis group. SPK, meibum score, meibography dropout, distortion, shortening, and total meibography were all significantly higher in the posterior blepharitis group as well as meibum score (P value < 0.001). TBUT was significantly shorter in the posterior blepharitis group (P value < 0.001). There was no significant difference between the two groups in Schirmer’s II test.
Conclusion
Meibography can be a helpful non-invasive tool for the clinical evaluation of the extent of the anatomical damage in patients having meibomian glands loss due to posterior blepharitis. Knowing the extent of damage in meibomian glands may help in selecting the appropriate treatment modality and expect the response to treatment in patients with posterior blepharitis.
Keywords: Meibography, Blepharitis, Meibomian glands dysfunction
Introduction
Blepharitis is a term that describes a wide group of disorders that cause an inflammation of eyelid margin and adjacent ocular surface. Eyelid margin anomalies consist of varied clinical entities which many of them have been identified as significant contributing factors to the dry eye syndrome.1, 2 Blepharitis is a common chronic disease whose etiology is poorly understood. It is a common eye condition and affects both children and adults.2 Lindsley et al. categorized blepharitis in several different ways. The first group is based on the length of disease process: acute or chronic blepharitis while the second group is based on the anatomical location of the disease: anterior (e.g. staphylococcal and seborrheic blepharitis), and posterior blepharitis.3
McCann LC et al. showed significant differences in his study when he compared tear physiology and meibomian gland function in patients with blepharitis and normal patients without blepharitis using different clinical parameters such as tear evaporation rate which was higher in blepharitis group.4 His study also demonstrated by meibography a significant meibomian gland dropout in blepharitis group with thicker and more opaque meibum.4
With the use of meibography, the structure of the meibomian glands, including the ducts and acini can be observed and assessed.5 The non- contact meibography was introduced in 2008 by Arita et al.6 In the non-contact meibography technique, the light and dark contrast of the meibomian glands is opposite that of contact technique in that they appear light instead of dark.
Our aim was to study the effect of posterior blepharitis on the function of meibomian glands and to assess and compare between meibomian glands in posterior blepharitis patients and normal population using infrared meibography and to correlate the results with tear film parameters.
Methods
After obtaining the approval of the Institution Review Board and obtaining a written informed consent from each participant, a prospective cohort study was performed on patients visiting King Abdulaziz University Hospital (KAUH) in Riyadh from April 2015 to April 2016. The study was conducted according to the principles contained in the Declaration of Helsinki.
The study included eyes from two groups: healthy volunteers’ eyes and eyes with posterior blepharitis.
Exclusion criteria included contact lens wearers, systemic or ocular diseases that would interfere with tear film production or function, continuous eye drop use, eyes with sequelae of trachoma, facial nerve weakness and any eyelid surgery including cryotherapy or electrocautery treatment for trichiasis. The participants were examined, and the diagnosis of blepharitis was achieved clinically based on signs of blepharitis.
The clinical examinations of dryness and ocular surface were performed including slit lamp examination of the lids, conjunctiva and cornea were performed before and after fluorescein staining. Lid margin telangiectasia, lid margin swelling and lid margin hyperemia were noted. Tear breakup time (TBUT) was measured after fluorescein instillation and was represented by the time elapsed from the last complete eyelid blink until appearance of the first dry spot on the cornea. It was measured 3 times consecutively and the mean value was taken for analysis. Superficial punctate keratopathy (SPK) in the cornea was classified into four grades: no SPK anywhere on the cornea (grade 0); no SPK at the central cornea (grade 1), mild SPK at the central cornea (grade 2), and severe SPK at the central cornea (grade 3).7
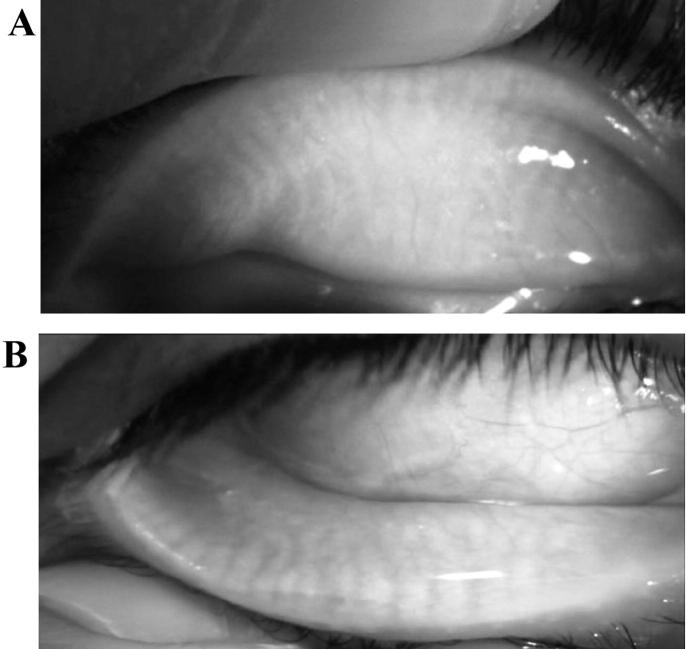
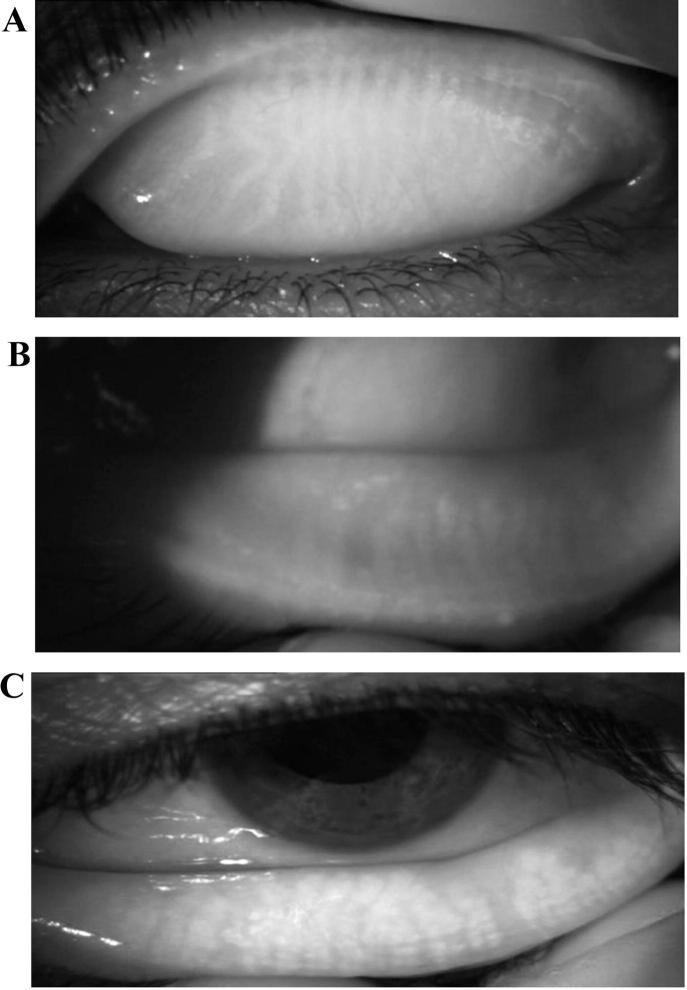
Non-contact meibography was performed on both upper and lower eyelids separately, using the meibo-grade system which was developed and validated by Call et al.8. Normal morphology of the meibomian glands of the upper eyelids appears to be thinner and longer as compared with the lower eyelids which are broader and shorter. (Fig. 1 A and B). The meibo-grade system involves distortion of meibomian gland, shortening and dropout. Gland distortion is abnormal gland to tarsus ratio and/or tortuous glands and/or discordant pattering of glands (Fig.2A). Gland shortening is gland not extending from the eyelid margin to the opposite edge of the tarsal plate (Fig.2B). Gland dropout is zones of meibomian gland dropout (Fig.2C). Each category was graded from 0 to 3 based on the extent of eyelid involvement: grade 0, no significant eyelid involvement; grade 1, less than 33% involved; grade 2, 33%–66% involved; grade 3, more than 66% involved. Then a maximal score of 9 represented complete gland dropout in the lid. Recorded video meibography image of each individual was used to evaluate the appearance of the meibomian glands.
Fig. 1.
(A) Meibography image of normal upper eyelid; meibomian glands are the dark thin and long vertical lines. (B) Meibography image of normal lower eyelid; meibomian glands appear to be broader and shorter compared to upper lid.
Fig. 2.
(A) Meibography image of showing meibomian glands distortion and tortuosity (B) Meibography image showing meibomian glands shortening (C) Meibography image of showing meibomian glands dropout.
Schirmer II test (with anesthesia) was performed to evaluate aqueous production. Meibum score was then performed by applying digital pressure on the tarsus and the degree of ease with which meibomian secretion was evaluated as follows: grade 0, clear meibum easily expressed; grade 1, cloudy meibum expressed with mild pressure; grade 2, cloudy meibum expressed with more than moderate pressure; grade 3, meibum not expressed even with hard pressure.9
Data analysis
Data were collected and stored in a spreadsheet using Microsoft Excel 2010® software. Data management and coding were then done in Excel. Data were analyzed using SPSS® version 22.0 (IBM Inc., Chicago, Illinois, USA).
Results
The study included a total of 86 eyes of 43 healthy volunteers’ eyes (25 male and 18 female; mean age ± SD, 58.6 ± 6.3) and 72 eyes of 36 blepharitis patients (21 male and 15 female; mean age ± SD, 63.5 ± 9.0).
Table 1 shows the lid margin abnormalities between the two groups. Telangiectasia, lid margin swelling and hyperemia were all significantly higher in the blepharitis group. Comparison between the two groups in tear film parameter showed significant abnormality in the posterior blepharitis group (Table 2). TBUT was significantly shorter in the posterior blepharitis group. SPK and meibum score were significantly higher in the blepharitis group. Meibography dropout, distortion, shortening and total meibography were all significantly higher in the blepharitis group. There was no significant difference between the two groups in Schirmer’s II test.
Table 1.
Comparing lid margin abnormalities between posterior blepharitis and Normal eyes (n = 158 eyes).
| Characteristic | Posterior blepharitis group (n = 72 eyes) N (%) | Normal group (n = 86 eyes) N (%) | P value |
|---|---|---|---|
| Telangiectasia | |||
| Present (n = 87) | 66 (91.7) | 21 (24.4) | <0.001* |
| Absent (n = 71) | 6 (8.3) | 65 (75.6) | |
| Lid margin swelling | |||
| Present (n = 58) | 52 (72.2) | 6 (7.0) | <0.001* |
| Absent (n = 100) | 20 (27.8) | 80 (93.0) | |
| Lid margin hyperemia | |||
| Present (n = 27) | 27 (37.5) | 0 (0.0) | <0.001* |
| Absent (n = 131) | 45 (62.5) | 86 (100.0) | |
Statistically significant at 5% level of significance.
Table 2.
Comparing tear film & meibomian glands examination characteristics between posterior blepharitis and normal eyes (n = 158 eyes).
| Characteristic | Posterior blepharitis group (n = 72 eyes) N (%) | Normal group (n = 86 eyes) N (%) | P value |
|---|---|---|---|
| Tear Breakup Time Mean (SD) [Range] | 4.39 (1.76) [1–10] | 10.34 (6.90) [1–40] | <0.001* |
| SPK Mean (SD) [Range] | 1.32 (0.71) [0–3] | 0.88 (0.69) [0–3] | <0.001* |
| Dropout_UL Mean (SD) [Range] | 0.79 (1.01) [0–3] | 0.22 (0.54) [0–3] | <0.001* |
| Dropout_LL Mean (SD) [Range] | 0.43 (0.71) [0–3] | 0.14 (0.35) [0–1] | 0.003* |
| Distortion_UL Mean (SD) [Range] | 1.11 (0.96) [0–3] | 0.65 (0.92) [0–3] | 0.001* |
| Distortion_LL Mean (SD) [Range] | 0.46 (0.71) [0–3] | 0.17 (0.38) [0–1] | 0.005* |
| Shortening_UL Mean (SD) [Range] | 0.94 (0.96) [0–3] | 0.44 (0.75) [0–1] | <0.001* |
| Shortening_LL Mean (SD) [Range] | 0.92 (0.78) [0–3] | 0.62 (0.75) [0–3] | 0.007* |
| Total_UL Mean (SD) [Range] | 2.85 (2.79) [0–9] | 1.24 (1.74) [0–9] | <0.001* |
| Total_LL Mean (SD) [Range] | 1.65 (1.86) [0–9] | 0.92 (1.16) [0–4] | 0.004* |
| Schirmers II Mean (SD) [Range] | 15.15 (7.96) [2–35] | 13.76 (7.56) [2–35] | 0.312 |
| Meibum Score Mean (SD) [Range] | 2.08 (0.75) [1–3] | 0.16 (0.37) [0–1] | <0.001* |
TBUT tear film breakup time, SPK superficial punctate keratopathy, UL upper lid, LL lower lid and SD standard deviation.
Statistically significant at 5% level of significance.
We have conducted several correlation studies between meibography of both eyelids and TBUT, and meibum score. We found that TBUT was inversely correlated to dropout in meibography, which means the higher the glands dropout in meibography the lower the TBUT in patients with posterior blepharitis (P = 0.035). SPK and Meibum score were also correlated to glands dropout in meibography of the posterior blepharitis group. We found the higher the glands dropout in meibography the higher the SPK and Meibum score (P < 0.003) and (P < 0.001), respectively.
Discussion
Blepharitis is a common chronic disease with a significant impact on the ocular surface.1, 2 Mathers et al. reported that most patients with blepharitis have some degree of meibomian gland dysfunction, and they used meibography to show glands dropout and ductal occlusion.10
Lid margin abnormalities that can be seen in posterior blepharitis include telangiectasia, swelling and hyperemia which were significantly higher in the blepharitis group in our study, Jester et al. found that this finding is related to abnormalities of keratinization of the ductal epithelium which ultimately result in plugging of the meibomian gland orifice and increased tear film evaporation.11 This reflects the importance meibomian glands in the production of the lipid layer in tear film.12
Meibography was first described in 1977 by Tapie who used woods light to fluoresce meibomian ducts on biomicroscopy and infrared light to illuminate the meibomian gland on IR photography.13 Non-contact meibography uses slit lamp biomicroscope with infrared filter digitally image an everted eyelid with no light probe needed which makes this technique easier for patients’ cooperation and easier to use than other techniques.
Arita et al.6 used a scoring method based on area of gland dropout that they termed the “meiboscore.” While evaluating gland dropout is critical, Call et al.8 believed other visible meibography changes are also important to consider. These changes are gland distortion and gland shortening; they also added in their study some additional change to the meiboscore described by Arita et al.6 and termed the result meibograde.8
Meibography can be of value in the evaluation of meibomian glands morphology in normal and diseased eyelids.14 Age was found to be a causative factor of progressive reduced quality, meibomian gland secretion and meibomian glands dropout.15, 16
Meibography dropout, distortion, shortening and total meibography were all significantly higher in the posterior blepharitis group in our study with upper eyelids more affected than the lower eyelids. McCann et al. who also showed a significant gland dropout in blepharitis group compared to controls. However, the dropout score was the same or greater for the lower lids than for the upper lids in their study.4 The reason behind the difference of the eyelid predominantly involve in the two study is not clear. The different racial and environmental settings may play a role.
There was no statistically significant difference in Schirmer’s II test between the two groups. This indicate that the aqueous tear production by lacrimal gland may not be affected in patients with posterior blepharitis.
Management of posterior blepharitis is often a source of frustration due to several factors such as lack of correlation between signs and symptoms, complex etiology, poorly understood pathophysiology, and historically limited range of diagnostic tests.17 As a result of these factors, different treatment options with variable response are available. Meibography can be a helpful non-invasive tool for the clinical evaluation of the extent of the anatomical damage in patients having meibomian glands loss due to posterior blepharitis. Knowing the extent of damage in meibomian glands may help in selecting the appropriate treatment modality and expect the response to treatment in patients with posterior blepharitis.
In conclusion, meibography is a clinical as well as diagnostic tool to observe the meibomian gland morphology and evaluates patients with MGD. Upper eyelids were much more affected than the lower eyelids when we compared the total meibography (0–9) of the blepharitis upper eyelids to the blepharitis lower eyelids. TBUT, SPK and meibum score are correlated to the status of meibomian glands and meibography which were significantly different in the blepharitis group.
Financial disclosure
None.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Nichols K.K., Foulks G.N., Bron A.J. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mculley J.P., Dougherty J.M., Deneau D.G. Classification of chronic blepharitis. Ophthalmology. 1982;89:1173–1180. doi: 10.1016/s0161-6420(82)34669-2. [DOI] [PubMed] [Google Scholar]
- 3.Lindsley K., Matsumura S., Hatef E., Akpek E.K. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012;(5) doi: 10.1002/14651858.CD005556.pub2. Art. No.: CD005556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCann L.C., Tomlinson A., Pearce E.l., Diaper C. Tear and meibomian gland function in blepharitis and normal. Eye Contact Lens. 2009;35:203–208. doi: 10.1097/ICL.0b013e3181a9d79d. [DOI] [PubMed] [Google Scholar]
- 5.Pult H., Nicholas J.J. A review of meibography. Opto Vis Sci. 2012;89:E760–E769. doi: 10.1097/OPX.0b013e3182512ac1. [DOI] [PubMed] [Google Scholar]
- 6.Arita R., Itoh K., Inoue K., Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Yokoi N., Kinoshita S. Clinical evaluation of corneal epithelial barrier function with the slit-lamp fluorophotometer. Cornea. 1995;14:485–489. [PubMed] [Google Scholar]
- 8.Call C.B., Wise R.F., Hansen M.R., Carter K.D., Allen R.C. In vivo examination of meibomian gland morphology in patients with facial nerve palsy using infrared meibography. Ophthal Plast Reconstr Surg. 2012;28:396–400. doi: 10.1097/IOP.0b013e3182611641. [DOI] [PubMed] [Google Scholar]
- 9.Shimazaki J., Goto E., Ono M., Shimmura S., Tsubota K. Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology. 1998;105:1485–1488. doi: 10.1016/S0161-6420(98)98033-2. [DOI] [PubMed] [Google Scholar]
- 10.Mathers W.D., Shields W.J., Sachdev M.S. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10:277–285. doi: 10.1097/00003226-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Jester J.V., Rife L., Nii D. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982;22:660–667. [PubMed] [Google Scholar]
- 12.Bron A.J. Contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–164. doi: 10.1016/s1542-0124(12)70150-7. [DOI] [PubMed] [Google Scholar]
- 13.Tapie R. Etude biomicroscopique des glandes de meibomius. Ann Oculistique. 1977;210:637–648. [Google Scholar]
- 14.Alsuhaibani A.H., Carter K.D., Abramoff M.D., Nerad J.A. Utility of meibography in the evaluation of meibomian glands morphology in normal and diseased eyelids. Saudi J Ophthalmol. 2011;25:61–66. doi: 10.1016/j.sjopt.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeotikar N.S., Zhu H., Markoulli M., Nichols K.K., Naduvilath T., Papas E.B. Functional and morphologic changes of meibomian glands in an asymptomatic adult population. Invest Ophthalmol Vis Sci. 2016;57:3996–4007. doi: 10.1167/iovs.15-18467. [DOI] [PubMed] [Google Scholar]
- 16.Arita R. Validity of noninvasive meibography systems: noncontact meibography equipped with a slit-lamp and a mobile pen-shaped meibograph. Cornea. 2013;32(Suppl. 1):S65–S70. doi: 10.1097/ICO.0b013e3182a2c7c6. [DOI] [PubMed] [Google Scholar]
- 17.Milner M.S., Beckman K.A., Luch J.L. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27(Suppl. 1):3–47. doi: 10.1097/01.icu.0000512373.81749.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]