Summary
This article presents a novel formulation for preparation of Lactobacillus casei 01 encapsulated in soy protein isolate and alginate microparticles using spray drying method. A response surface methodology was used to optimise the formulation and the central composite face-centered design was applied to study the effects of critical material attributes and process parameters on viability of the probiotic after microencapsulation and in simulated gastrointestinal conditions. Spherical microparticles were produced in high yield (64%), narrow size distribution (d50=9.7 µm, span=0.47) and favourable mucoadhesive properties, with viability of the probiotic of 11.67, 10.05, 9.47 and 9.20 log CFU/g after microencapsulation, 3 h in simulated gastric and intestinal conditions and four-month cold storage, respectively. Fourier-transform infrared spectroscopy confirmed the probiotic stability after microencapsulation, while differential scanning calorimetry and thermogravimetry pointed to high thermal stability of the soy protein isolate-alginate microparticles with encapsulated probiotic. These favourable properties of the probiotic microparticles make them suitable for incorporation into functional food or pharmaceutical products.
Key words: Lactobacillus casei 01, alginate, soy protein isolate, microparticles, spray drying, formulation optimisation
Introduction
The modern lifestyle and consequences of unhealthy eating habits on the one hand, and the strong evidence of health benefits gained by probiotic consumption on the other have stimulated the food and pharmaceutical industry to produce novel probiotic ingredients and products, with estimated market share of approx. 36.7 billion dollars by 2018 (1). The probiotics have been found to be effective for a large number of gut disorders and for improving/maintaining the oral and urogenital health (2–4). Other studies have indicated protective cardiovascular effects and promising effects in cancer, brain, obesity, allergy and atopic diseases, and in combatting diseases where dysbiosis has been observed (5–9). However, providing and maintaining the required minimum of viable probiotic cells to exert health effects (107 CFU/g at a point of delivery) is a great challenge, considering their sensitivity to processing, storage and administration. Microencapsulation could be applied as an effective method for achieving these goals (10). Among many microencapsulation techniques, spray drying is one of the most challenging due to low costs, industrial application, high probiotic stability in the product and preserved cellular integrity during drying, when both process and formulation parameters are optimised correctly. So far, alginate was mostly used as a protective polymeric matrix for probiotic microencapsulation, producing a particularly strong molecular framework and thermoirreversible and freeze-thaw stable microparticles in the presence of Ca2+ (11). However, the Ca-alginate beads alone have shown low ability to protect the cells at a low pH due to the sensitivity of Ca2+/COO– linkage to Ca2+-chelating agents in the gastrointestinal tract (GIT) (12). For this reason, complexation of alginate with other polymers has been commonly used to control the disintegration of alginate beads and to target the probiotic release within the GIT, although with variable success (11, 13).
In search for novel microparticulated probiotic formulation that can provide high viability of Lactobacillus casei 01 and that can dictate its release in the small intestine (pH≥6.8), we have prepared several formulations in which alginate was combined with other polymers such as whey protein (14, 15) and chitosan (16). Lactobacillus casei 01 was chosen based on its previously confirmed effects in colon inflammation (15) and increased blood pressure (17). In addition, in vivo and in vitro evidence confirms the L. casei role in the improvement of lactose tolerance (18) and suppression of tumour growth by modulating non-specific immune responses (19). However, as other probiotics, L. casei 01 is easily degraded during production and storage of the final products, or when orally administered, as was previously confirmed (14, 16). In order to overcome the disadvantages of the previously used methods for microencapsulation, such as difficulties in scale-up and high costs of the emulsion method (14), and significant probiotic viability loss when spray drying method was combined with freeze drying (16), in this study, an attempt was made to achieve high viability of L. casei 01 during processing, storage and digestion by its encapsulation in alginate and soy protein isolate (SPI) microparticles using spray drying only. The SPI was chosen due to its confirmed nutritional and health benefits, availability, biodegradability, low immunogenicity and similarity with the components of the tissue extracellular matrix (20, 21). It can be easily processed and linked to other biocompatible polymers, giving materials with enhanced mechanical properties. It was expected of the cell cross-linked SPI-alginate microparticles to protect the probiotic from the harsh processing and in vivo conditions. The additional aim of the study was to find out whether this protection is sufficient to provide the required probiotic viability or whether enhancement in the mechanical stability of SPI-alginate microparticles by ionic cross-linking is required. For this reason, L. casei 01 was encapsulated both in Ca2+ cross-linked and non- -cross-linked SPI-alginate microparticles. The critical formulation and process factors affecting significantly the viability of L. casei 01 after spray drying and in simulated GIT conditions were identified and, based on the obtained results and established correlations between the variables of interest, a subsequent optimisation of the formulation and microencapsulation process was performed. In addition, the physicochemical and biological properties of the optimal formulation of probiotic microparticles were determined. To our knowledge, this is the first time the probiotic cells have been encapsulated in microparticles prepared with SPI-alginate and for that aim, spray drying technique was used.
Materials and Methods
Materials
FD-DVS nu-trish® Lactobacillus casei 01, a freeze-dried probiotic culture from Chr. Hansen, Copenhagen, Denmark, was used as a bacterial strain. Commercial soy protein isolate (SPI) was purchased from Sojaprotein a.d. Bečej (Bečej, Serbia), whereas alginate (Protanal® LF 10/60 LS sodium alginate, guluronic acid content 35–45%) was kindly donated by FMC Biopolymer UK Ltd. (Girvan, UK). Calcium chloride, MRS (de Man, Rogosa, Sharpe) agar and broth, and peptone water were purchased from Merck KGaA (Darmstadt, Germany). Bile salts (ox gall) were supplied from Sigma-Aldrich (Poole, UK), while pepsin from porcine gastric mucosa (800–2500 U/mg) and pancreatin (>100 U/mg) from porcine pancreas were from Sigma-Aldrich (St. Louis, MO, USA). All reagents and laboratory equipment were autoclaved before usage.
Preparation of L. casei 01 encapsulated in SPI-alginate microparticles
Preparation of the probiotic for microencapsulation
For microencapsulation, the probiotic cells were activated in 5 mL of MRS broth at 37 °C for 24 h under aerobic conditions, as previously described (14, 16). Afterwards, the cells were harvested by centrifugation at 1500×g for 10 min and washed twice with sterile 0.1% (by mass per volume) peptone water.
Preparation of microparticles
The SPI-alginate microparticles with encapsulated L. casei 01 were prepared by spray drying method. In detail, aqueous mixture of alginate and SPI (pH=7.0) was inoculated with 1% (by mass per volume) bacterial suspension (cell load approx. 12 log CFU/g) and homogenised by stirring on a magnetic stirrer (600 rpm, model 4803-02; Cole- -Palmer, Vernon Hills, IL, USA) at room temperature for 30 min. The resultant mixture kept under low speed agitation on a magnetic stirrer (200 rpm) was infused into a spray drying nozzle (model Mini Spray Dryer B-290; Büchi Labortechnik AG, Flawil, Switzerland) and continuously sprayed using an automatic infusion/withdrawal pump (model 6530R096 3.1 A/ph 9407; Sonceboz SA, Sonceboz-Sombeval, Switzerland). The conditions of the spray drying process were: nozzle diameter 0.7 mm, aspirator pressure 90%, atomizer pressure 600 nL/h and flow rate 5 mL/min. As stipulated by the experimental design, series of cross-linked SPI-alginate microparticles with encapsulated probiotic were prepared by introduction of the probiotic microparticles collected from the cyclone into aqueous solution of CaCl2, followed by continuous stirring (800 rpm) at room temperature for 1 h. Thus obtained microparticles were removed from the solution of CaCl2 by centrifugation (1000×g, 5 min, Centric 322B centrifuge; Tehtnica, Železniki, Slovenia), washed three times with sterile water, frozen at –20 °C and freeze dried at 7 Pa and –50 °C for 24 h (FreeZone 2.5 Liter Benchtop Freeze Dry System; Labconco Corp., Kansas City, MO, USA).
Experimental design study – process and formulation optimisation
The critical material attributes (type and concentration of alginate, SPI and CaCl2) and process parameters (inlet temperature (IT)) were identified varying one parameter at a time. In order to characterise the influence of the determined critical variables and, subsequently, to find their optimal values for the production of SPI-alginate microparticles with encapsulated L. casei 01, response surface methodology based on central composite face- -centered design of experiments was used. The concentrations of alginate (A), SPI (B) and CaCl2 (C), and IT (D) applied for preparation of the probiotic microparticles were systematically varied in given ranges and the probiotic viability after microencapsulation and testing in simulated gastrointestinal (GI) conditions (pH=1.5 after 3 h and pH= 6.8 after 3 h) was determined as the response of interest. Probiotic viability in both CFU per g of microparticles and in percentage of viable cells from the initial cell count was determined in order to evaluate the number of viable cells after microencapsulation and in simulated GI conditions, as well as the efficacy of the encapsulation process. The process and formulation parameters were represented by their coded and actual values.
Physicochemical characterisation of L. casei 01 encapsulated in SPI-alginate microparticles
Encapsulation efficacy
The encapsulation efficacy (EE/%), i.e. the percentage of bacterial cells that survived the process and was encapsulated inside the microparticles was calculated as follows:
where N0 is the number of viable bacteria in CFU per g of culture and N is the number of viable bacteria in CFU per g of microparticles.
Particle size analysis
The size distribution of the optimised microparticles was determined using laser diffraction particle size analyser (Mastersizer 2000, equipped with Hydro 2000S/G; Malvern Instruments Ltd., Worcestershire, UK) in the wet dispersions. The wet dispersions were prepared by dispersing the particles (15–20 mg) in 2 mL of 2% (by mass) solution of Polysorbate® 80 (Sigma-Aldrich, Poole, UK), in an ultrasonic bath (Ultrawave Limited, Cardiff, UK) for 10 min. A small aliquot of the resulting suspension was transferred to the measurement cell containing the blank dispersing medium (distilled water). The measurements were performed under stirring (2520 rpm) and ultrasound (50% duty cycle), previously applied for 5 min. The obscuration was set between 10 and 12%. At least six measurements were done. The particle diameter was determined, and particle size distribution was expressed in terms of span factor:
where d90, d10 and d50 are the diameter sizes and the numbers in subscript indicate the percentage of particles smaller than that size (span<2 indicates a narrow size distribution).
Morphological analysis
The morphology of the microparticles in the different phases of preparation was observed using a scanning electron microscope (SEM JSM-5300; JEOL Ltd., Tokyo, Japan). Microparticle samples were attached to an adhesive tape and coated with gold by sputter coater (SEM JFC-1100E ion sputter; JEOL Ltd.).
Moisture content
The moisture content of the optimised probiotic microparticles was calculated by two methods: infrared moisture measurement method, where 2 g of sample were placed in an aluminium pan and dried at 105 °C for 24 h (Mettler Toledo AG, Greifensee, Switzerland), and Karl Fischer method, with one-component system using HydranalTM-Composite 5 (model V20S Compact Volumetric KF Titrator; Mettler-Toledo, Columbus, OH, USA) as a titrating solution (22). The experiments were carried out in triplicates and the average value was taken as a result.
Humidity
The humidity of the optimised probiotic microparticles was determined by drying to constant mass of 5 g of powder for 3 h at 105 °C. The heated sample was cooled in a desiccator and weighed. The procedure was repeated until a constant mass was obtained. The humidity was calculated by the following equation (23):
Solubility
The solubility of the optimal formulation was determined by dissolving 1 g of sample in 100 mL of distilled water, homogenised at room temperature, then collected by centrifugation (1500×g, 15 min, Centric 322B centrifuge; Tehtnica) and dried in an oven (model 7802; Sutjeska Beograd, Belgrade, Serbia) at 75 °C for 5 h. The solubility percentage was calculated from the difference between the initial mass of microparticles and the mass obtained after solubilisation (23).
Wettability
To evaluate the ability of the solid surface to absorb moisture when in contact with water, 2 g of microparticles (optimal formulation) were placed in 400 mL of water. The time required for all the particles to become moist was evaluated visually (23).
Storage test
The optimised microparticles of L. casei 01 were stored in penicillin tubes that were hermetically sealed and placed in a desiccator. Probiotic survival was evaluated by counting the viable cells after 1 and 4 months of storage at 4 °C. The experiments were performed in triplicates.
Fourier-transform infrared study
Fourier-transform infrared (FTIR) spectroscopy (PerkinElmer 2000 FTIR; Waltham, MA, USA) was used to determine the interactions between the probiotic and polymers, and the stability of L. casei 01 during microencapsulation, as previously described (14, 16). The FTIR-attenuated total reflection (FTIR-ATR) spectra were recorded at room temperature by Specac®Golden Gate ATR Mk II with ZnSe lenses (Specac Ltd., Orpington, UK). The characteristic absorption bands of the polymers and the probiotic were recorded in a frequency range of 4000–400 cm–1 for the following samples (2 mg of homogenised sample or 100 µL of dispersion): pure alginate and SPI, their physical mixture, L. casei 01, physical mixture of alginate, SPI and L. casei 01, and blank and microparticles with encapsulated L. casei 01. The molecular changes of the probiotic structure during the microencapsulation process were examined by comparing the spectra of non-encapsulated probiotic and L. casei 01 released from the microparticles.
Thermal property study
The thermal properties of the samples were studied by thermogravimetry (TG) and differential scanning calorimetry (DSC). The TG thermograms of pure alginate and SPI, their physical mixture, physical mixture of alginate, SPI and L. casei 01, and blank and loaded microparticles (7–10 mg per sample) were recorded by a thermogravimetric analyser (model Pyris 1 TGA; PerkinElmer, Shelton, CT, USA), in a temperature range of 30–900 °C, at a heating rate of 10 °C/min. DSC study was carried out in a differential scanning calorimeter (model DSC 204 F1 Phoenix®, Netzsch-Gerätebau GmbH, Selb, Germany), at a heating rate of 10 °C/min up to 300 °C. The investigations were done under nitrogen atmosphere.
Biological characterisation of L. casei 01 encapsulated in SPI-alginate microparticles
Swelling studies
The swelling behaviour of the optimal formulation of microparticles was determined in the simulated GI fluids by exchanging the intestinal juices (24). Namely, after each treatment in the adequate medium, the microparticles were collected by centrifugation, washed once with distilled water and placed in the next medium. Certain amount of microparticles was consequently suspended in 5 mL of simulated gastric juice (SGJ; 0.08 mol/L of HCl, pH=1.5) and simulated intestinal juice (SIJ; 0.05 mol/L of KH2PO4, pH=6.8) for 3 h. Samples of microparticles were taken from the gastric fluid after 0.5 and 3 h, and from the intestinal fluid, with pH=6.8, after 0.5 and 3 h. The adsorbed water on the surface of the microparticles was immediately removed with filter paper and the particle size distribution was measured by laser diffraction particle size analyser (Mastersizer 2000, equipped with Hydro 2000S/G; Malvern Instruments Ltd.). The percentage of swelling was calculated according to:
where dt and d0 are the mean volume diameters of the microparticles at time t and in the dry state, respectively.
Enumeration of Lactobacillus casei 01
The viability of L. casei 01 alone and encapsulated in the SPI-alginate microparticles was assessed after dissolution of 1 g of probiotic cells and 1 g of microparticles in 9 g of phosphate buffer saline (1 mol/L, pH=7.0), using the plate count method as previously described (14, 16). The cell counts were determined by sequential dilutions using 0.1% (by mass per volume) peptone water to achieve countable cell numbers. Quantitative determination of viable cells was performed in triplicate on selective MRS agar after incubation for 72 h at 37 °C under aerobic conditions, counting the plates with 30–250 colonies. The average results were expressed in CFU per g of sample.
Viability of free and encapsulated L. casei 01 in simulated gastrointestinal conditions
The viability of L. casei 01 encapsulated in the SPI-alginate microparticles was determined in the simulated GI fluids by exchanging the intestinal juices (24). Additionally, the viability of L. casei 01 in the optimised microparticles was compared with the viability of non-encapsulated probiotic under the same conditions. In detail, the probiotic cells alone (1:10 by volume, cell load approx. 12 log CFU/g) or microencapsulated (1:10 by mass per volume) were incubated in the SGJ (0.08 mol/L of HCl with 0.2%, by mass per volume, NaCl and 0.3%, by mass per volume, pepsin, pH=1.5) at 37 °C for 3 h in a horizontal shaker (75 rpm, model SWB 20; Haake, Waltham, MA, USA). Consequently, the separated and washed probiotic cells and microparticles were transferred (1:9 by mass per volume) into the SIJ with pH=6.8 (0.05 mol/L of KH2PO4 with 1%, by mass per volume, bile salts (ox gall) and 1%, by mass per volume, pancreatin) and pH=7.4 (0.05 mol/L of KH2PO4), respectively, and incubated for additional 3 and 4 h, respectively, under the same conditions. Samples were taken after 0.5 h and at the end of the incubation period from the SGJ (pH=1.5) and SIJ (pH=6.8) and after 2 and 4 h from the SIJ (pH=7.4), and the viability of L. casei 01 was determined with the already described enumeration method.
Adhesive properties of L. casei 01 encapsulated in SPI-alginate microparticles
The adherence capacity of L. casei 01 alone and encapsulated in the optimised SPI-alginate microparticles was tested in vitro. Pig mucin was mixed with cells (1:4, by volume) or microparticles (1:4, by mass per volume) and incubated in different buffer solutions (pH=2.0, 4.5, 6.8 and 7.4) (25) at 37 °C. After 1, 3, 5 and 24 h of incubation, the samples were centrifuged (1500×g, 10 min, model Centric 322B; Tehtnica) and the remaining free pig mucin in the supernatants was measured by UV/Vis spectrophotometer (model Lambda 16; PerkinElmer) at 251 nm. The binding efficiency of cells or microparticles was calculated using the following equation:
where c0 is the initial concentration of pig mucine used for incubation, and cs is the concentration of free pig mucin determined in the supernatant.
Statistical analysis
Design-Expert® trial v. 8 software was used for the design of experiments (Stat-Ease, Inc., Minneapolis, MN, USA). One-way ANOVA for the fitted model for all responses of interest was applied. Values of p<0.05 indicated that the model terms were significant. The predicted and adjusted R2 within 0.2 of each other and adequate precision values greater than 4 suggested that the models can be used for navigation in the designed space. The paired t-test was used for comparison of viability and adhesive properties of L. casei 01 and probiotic microparticles (Microsoft Excel®, Microsoft Corp. Redmond, WA, USA). Differences were considered statistically significant at p<0.05.
Results and Discussion
Preparation and optimisation of probiotic encapsulated in microparticles
A total of 30 experiments were designed and carried out at random to optimise the process and formulation for the microencapsulation of the probiotic. The equations describing the mathematical correlation between the followed responses and the studied factors were established based on the obtained results for the responses of interest (Table 1). The negative sign in front of the factors indicates inverse relation between the response of interest and the studied factors, while vice versa applies for the positive sign.
Table 1. Coded values of studied variables and obtained responses for designed formulations of soy protein isolate-alginate microparticles with encapsulated Lactobacillus casei 01.
| After preparation | t=3 h | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH=1.5 | pH=6.8 | ||||||||||||
| Std | Run | A | B | C | D |
N(viable cell) log CFU/g |
N(viable cell) log CFU/g |
N(viable cell) log CFU/g |
|||||
| 20 | 1 | 0 | 1 | 0 | 0 | 9.60 | 80.00 | 4.32 | 45.00 | 4.07 | 42.40 | ||
| 24 | 2 | 0 | 0 | 0 | 1 | 10.07 | 83.92 | 4.37 | 43.40 | 3.30 | 32.78 | ||
| 13 | 3 | –1 | –1 | 1 | 1 | 10.37 | 86.42 | 5.00 | 48.22 | 3.00 | 28.93 | ||
| 23 | 4 | 0 | 0 | 0 | –1 | 11.39 | 94.92 | 3.00 | 26.34 | 4.00 | 35.12 | ||
| 25 | 5 | 0 | 0 | 0 | 0 | 11.00 | 91.67 | 9.10 | 82.73 | 3.23 | 29.36 | ||
| 19 | 6 | 0 | –1 | 0 | 0 | 11.28 | 94.00 | 3.00 | 26.60 | 4.00 | 35.46 | ||
| 27 | 7 | 0 | 0 | 0 | 0 | 9.63 | 80.25 | 8.43 | 87.54 | 4.00 | 41.54 | ||
| 16 | 8 | 1 | 1 | 1 | 1 | 10.18 | 84.83 | 8.60 | 84.50 | 3.00 | 29.47 | ||
| 4 | 9 | 1 | 1 | –1 | –1 | 11.47 | 95.58 | 10.47 | 91.28 | 6.20 | 54.05 | ||
| 21 | 10 | 0 | 0 | –1 | 0 | 10.00 | 83.33 | 8.70 | 87.00 | 5.34 | 53.40 | ||
| 22 | 11 | 0 | 0 | 1 | 0 | 10.24 | 85.33 | 5.47 | 53.42 | 3.00 | 29.30 | ||
| 11 | 12 | –1 | 1 | –1 | 1 | 11.77 | 98.08 | 11.54 | 98.05 | 9.75 | 82.84 | ||
| 8 | 13 | 1 | 1 | 1 | –1 | 10.69 | 89.08 | 9.30 | 87.00 | 7.02 | 65.67 | ||
| 18 | 14 | 1 | 0 | 0 | 0 | 9.57 | 79.75 | 9.30 | 97.19 | 5.84 | 61.02 | ||
| 2 | 15 | 1 | –1 | –1 | –1 | 11.69 | 97.42 | 9.95 | 85.12 | 8.47 | 72.46 | ||
| 28 | 16 | 0 | 0 | 0 | 0 | 11.22 | 93.46 | 8.74 | 77.96 | 3.47 | 30.94 | ||
| 10 | 17 | 1 | –1 | –1 | 1 | 11.47 | 95.58 | 10.31 | 89.89 | 9.44 | 82.30 | ||
| 26 | 18 | 0 | 0 | 0 | 0 | 11.00 | 91.67 | 9.00 | 81.82 | 3.00 | 27.27 | ||
| 1 | 19 | –1 | –1 | –1 | –1 | 11.75 | 97.92 | 10.79 | 91.83 | 8.62 | 73.36 | ||
| 29 | 20 | 0 | 0 | 0 | 0 | 11.40 | 95.00 | 9.20 | 80.70 | 3.30 | 28.95 | ||
| 6 | 21 | 1 | –1 | 1 | –1 | 10.87 | 90.58 | 5.00 | 46.00 | 3.70 | 34.04 | ||
| 17 | 22 | –1 | 0 | 0 | 0 | 10.82 | 90.17 | 5.47 | 50.55 | 5.00 | 46.21 | ||
| 30 | 23 | 0 | 0 | 0 | 0 | 10.98 | 91.50 | 9.70 | 88.34 | 4.28 | 38.98 | ||
| 9 | 24 | –1 | –1 | –1 | 1 | 10.25 | 85.42 | 9.88 | 96.39 | 9.30 | 90.74 | ||
| 3 | 25 | –1 | 1 | –1 | –1 | 11.68 | 97.29 | 9.50 | 81.37 | 7.60 | 65.10 | ||
| 14 | 26 | 1 | –1 | 1 | 1 | 11.47 | 95.58 | 10.56 | 92.07 | 10.20 | 88.93 | ||
| 5 | 27 | –1 | –1 | 1 | –1 | 10.72 | 89.33 | 4.91 | 45.80 | 3.11 | 29.01 | ||
| 15 | 28 | –1 | 1 | 1 | 1 | 8.86 | 73.79 | 5.77 | 65.16 | 3.00 | 33.88 | ||
| 12 | 29 | 1 | 1 | –1 | 1 | 11.00 | 91.67 | 10.04 | 91.27 | 9.76 | 88.73 | ||
| 7 | 30 | –1 | 1 | 1 | –1 | 10.35 | 86.25 | 7.00 | 67.63 | 3.69 | 35.65 | ||
Coded values –1 and +1 for A (alginate) and B (soy protein isolate) correspond to actual values of 1 and 4% (by mass), respectively, for C (CaCl2) to actual values of 0 and 1% (by mass), respectively, and for D (inlet temperature) to 90 and 150 °C, respectively
The viability of L. casei 01 in the designed formulations after preparation procedure was in a range of 8.86–11.77 log CFU/g, or expressed in percentage of the initial cell count in the formulation for preparation of microparticles, 74 to 98%. The influence of the examined variables on these response values was described by a linear model expressed in log CFU/g (Eq. 6) and percentage values (Eq. 7), respectively:
where A, B, C and D represent alginate, SPI, CaCl2 and IT, respectively.
With the increase in the alginate concentration, the viability/encapsulation efficacy of the probiotic increased, while the opposite effect was observed when the concentrations of cross-linking agent, soy protein isolate and IT increased. One-way ANOVA indicated that the formulation parameter C was a significant model term for both response values.
The viability of L. casei 01 in SGJ (pH=1.5) after 3 h was in the range of 3.00–11.54 log CFU/g or expressed in percentage of the initial cell count in the microparticles after preparation, from 27 to 98%. The reduced quadratic model was used to describe the relation between the probiotic viability in SGJ and studied factors (Eq. 8 for viability in log CFU/g and Eq. 9 for viability in percentage):
 |
where A, B and C represent alginate, SPI, CaCl2 and IT, respectively.
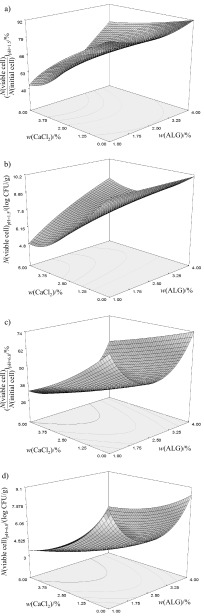
One-way ANOVA indicated that the variables C and C2 (results expressed in log CFU/g; Fig. 1a) and C (results expressed in percentage; Fig. 1b) should be considered as statistically significant. With the increase in the concentration of Ca2+ in the cross-linking solution, the viability of L. casei 01 significantly decreased.
Fig. 1.
Dependence of the viability of Lactobacillus casei 01 encapsulated in optimised soy protein-alginate microparticles on the mass fractions of CaCl2 and alginate (ALG) at inlet temperature of 132 °C and 1% (by mass) of soy protein isolate. The viability is expressed in percentage of the initial cell count and in log CFU/g at pH=1.5 (a,b) and pH=6.8 (c,d), respectively
The viability of the probiotic after 3 h in SIJ (pH=6.8) was in the range of 3.00–10.20 log CFU/g or from 29 to 89% of the initial cell count entering the SGJ. The reduced cubic model was used for description of the relations between these response values and studied factors (Eqs. 10 and 11 for viability expressed in log CFU/g and percentage, respectively):
 |
 |
where A, B, C and D represent alginate, SPI, CaCl2 and IT, respectively.
Individial variables A, C, A2 and interactions between A and C, B and D, and B, C and D (results expressed in log CFU/g; Fig. 1c) and individual variables A, C, A2 and interactions between A and C, A, B and D, and B, C and D (results expressed in percentage; Fig. 1d) were indicated as significant model terms.
According to the ANOVA results for the fitted models for all responses, the predicted and the adjusted R2 were within 0.2 of each other, while adequate precision values were greater than 6, thus signifying satisfactory model fit. The derived correlations enabled optimisation of the formulation by means of maximisation of all responses. The optimal batches with the predicted responses were identified and they are presented in Table 2. The cross-validation of the model, confirmation report and the percentage of relative error of the predicted and experimental values pointed to the optimal IT for spray drying (132 °C) and the optimal formulation for preparation of microparticles (4 and 1%, by mass of alginate and soy protein isolate, respectively), which preserve probiotic viability above the required minimum under all conditions. Insignificant differences of 0.27, 0.04 and 1.06 log CFU/g between the predicted and obtained viabilities were observed when the viability tests of the probiotic encapsulated in the optimised microparticles after their preparation and placement in the SGJ and SIJ (data presented below) were performed, indicating acceptable relative errors of approx. 2.37, –0.39 and 12.60%, respectively (Table 2).
Table 2. Composition of the optimised samples with estimated responses and cross-validation of the model.
| Value | After preparation | t=3 h | Desir- ability |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH=1.5 | pH=6.8 | ||||||||||||
|
w(ALG) % |
w(SPI) % |
w(CaCl2) % |
tinlet °C |
N(viable cell) log CFU/g |
N(viable cell) log CFU/g |
N(viable cell) log CFU/g |
|||||||
| Predicted Actual Bias/% |
1.01 1 |
1.27 1.3 |
0 0 |
90 90 |
11.55 11.41 –1.21 |
96.21 95.05 –1.21 |
9.99 9.47 –5.21 |
87.99 83.03 –5.64 |
9.50 9.00 –5.26 |
85.24 78.91 –7.43 |
0.883 | ||
| Predicted Actual Bias/% |
4 4 |
3.95 4 |
0 0 |
140.74 141 |
10.84 11.55 6.55 |
90.34 96.23 6.52 |
10.57 9.43 –10.79 |
93.26 81.17 –12.96 |
8.31 8.63 3.85 |
71.34 74.74 4.77 |
0.856 | ||
| Predicted Actual Bias/% |
4 4 |
1 1 |
0 0 |
131.23 132 |
11.40 11.67 2.37 |
95.00 97.25 2.37 |
10.09 10.05 –0.39 |
92.49 86.12 –6.89 |
8.41 9.47 12.60 |
74.02 81.15 9.63 |
0.811 | ||
ALG=alginate, SPI=soy protein isolate
The major drawback of the spray drying method for probiotic microencapsulation is the limited survival of the probiotics due to the mechanical stress and heat treatment involved (26). This can explain the opposite effect of IT on L. casei 01 viability. However, the number of reports addressing this issue is limited and they are usually related to the amount and type of coating materials, pointing out that the viability of encapsulated bacteria increases in direct proportion to the amount of coating material, which temporarily reduces the heat transfer into the cells (27). Similar results were observed in a previous study, where L. casei 01 was exposed to high IT of 120 °C during microencapsulation in chitosan-coated alginate microparticles prepared by spray drying (16). The highest amounts of coating materials were used to obtain microparticles with favourable physicochemical properties and acceptable probiotic viability, but at the expense of encapsulation efficiency, suggesting that a balanced ratio of materials is essential to provide functional groups available for binding bacterial cells.
The ability of cells to bind alginate chains and form network structure has been widely explored in cell and tissue engineering (28, 29). Alginate is not cell interactive and hence, when the cells are added to non-modified alginate solutions in the absence of chemical cross-linker, the cell-cell interactions dominate, leading to cell aggregation and creation of non-homogeneous gel network. However, in the presence of adhesion ligand-modified alginate, they generate homogeneous cross-linked network owing to specific receptor-ligand interactions. There is a great possibility that the soy protein isolate serves as a cell adhesion ligand (and signalling molecule for cell proliferation), considering its potential for steric and electrostatic interactions and short range forces such as van der Waals, acid-base, hydrogen bonding and biospecific interactions with both alginate and cells (30). Additionally, the cross- -linking segments of alginate by soy protein isolate can change the conformation of alginate and increase the local charge density, thus promoting adsorption of soy protein isolate and probiotic cells. The same was observed when a well-known adhesive ligand arginine-glycine-aspartic acid (RGD) was conjugated to the alginate backbone (31). When fibroblast cells were added to the RGD-modified alginate solution without the use of calcium, a larger number of cells were mobilised due to the more available bonds between the cells and cell-interactive polymers. In addition, several extracellular matrix proteins have also been coupled with alginate to promote cell attachment (e.g. collagen), as was reviewed by Bidarra et al. (32). This can explain why the increase in the alginate concentration and by that increased content of soy protein isolate provided higher probiotic viability after microencapsulation and the opposite effect of CaCl2 was observed. The effect on probiotic viability of freeze-drying process, used to improve the shelf stability and handling properties of microparticles cross-linked with Ca2+, cannot be excluded. Despite the ability of Ca2+ in the alginate system to protect the cell envelopes and secondary proteins of the probiotics after freeze-drying (33), this effect was probably hindered by the detrimental effect of freeze-drying on the constituents of the cell wall due to the ice crystal formation. This was evident for several probiotics when microencapsulation and freeze-drying were combined (34, 35).
One of the biggest concerns in using the cell-cross- -linked microparticles for preserving the viability of L. casei 01 in the GIJ was their mechanical strength. Therefore, for the cross-linking density to be increased, series of microparticles cross-linked with Ca2+ were prepared. A stronger molecular framework of Ca-alginate, formed by cooperative binding of Ca2+ with the aligned polyguluronate ribbons and partial covalent bonds between Ca2+ and oxygen atom of C-O-C groups of alginate (33), was expected to compensate for viability loss during freeze-drying and provide higher probiotic viability in low pH and controlled release at pH≥6.8. This expectation was supported by a previous study that pointed to significant improvement of the L. casei 01 stability/viability in the SGJ and SIJ with the increase in CaCl2 concentration, when chitosan-coated alginate microparticles were additionally cross-linked with Ca2+ (16).
On the contrary, with the increase in the concentration of the cross-linking agent, the probiotic viability at pH=1.5 decreased and this effect was evident in both response values expressed in CFU/g and in percentage of viable cells from the initial cell count entering the SGJ. The opposite effect was observed when the concentrations of alginate and soy protein isolate increased, suggesting that the compact network of the soy protein isolate-alginate microparticles with encapsulated probiotic is able to preserve the probiotic viability in acidic media effectively and thus provide high cell count at pH≥6.8. There is a possibility that the higher concentration of CaCl2 in the cross-linking medium competitively displaced the soy protein from its bonding site with alginate, leading to the formation of microparticles with more junction zones and/or increased porosity. If so, the probiotic exposure to the harsh SGJ conditions as well as the susceptibility of the microparticles to disintegration was increased (12). Therefore, the higher adhesion capacity and possibly the lower vulnerability of the cell-cross- -linked SPI-alginate microparticles to the environmental stress (acidic pH, bile salts and digestive enzymes) could explain the higher probiotic viability in the simulated GIJ. Similar were the findings of Smilkov et al. (14), where the viability of L. casei 01 in the SGJ and SIJ was positively (dominantly) affected by the concentration of whey protein when incorporated in Ca2+-cross-linked whey protein-alginate microparticles, with the effect of alginate and Ca2+ being insignificant. In addition, there is a possibility that the stability of the SPI-alginate complex was additionally increased by the thermal denaturation of the SPI during spray drying due to increased number of hydrophobic interactions, hydrogen bonding and structure folding that cause irreversible changes in the functional properties of the complex. This was confirmed in a study of Rajam et al. (36) when L. plantarum MTCC 5422 was microencapsulated in undenatured and denatured whey protein isolate with alginate using spray drying and freeze- -drying techniques, respectively. Cells entrapped in the denatured whey protein isolate were more stable in simulated acidic and bile acid conditions than those entrapped in the undenatured protein.
Physicochemical properties of L. casei 01 encapsulated in SPI-alginate microparticles
The optimised SPI-alginate microparticles with encapsulated probiotic were easily recovered from the spray-dryer, showing no tendency to stick to the spray drying chamber and cyclone. They were produced in relatively high yield ((63.8±1.7) %), with d50=(9.7±0.5) µm and very narrow size distribution (span=0.47). The particle size distribution corresponded to the data reported in the literature (10–20 µm) for probiotic microparticles obtained by spray drying (26, 27, 36).
The SEM micrographs of L. casei 01 (as obtained from the producer) and of blank and microparticles containing the probiotic are shown in Fig. 2. As the images show, the blank and the microparticles with the probiotic have similar morphology and tendency to agglomerate. Spherical but also flattened disk-shaped particles are presented, which is the usual appearance of the microparticles prepared by spray drying. The surface appears wrinkled with scarcely visible porosity.
Fig. 2.
Scanning electron microscope images of: a) Lactobacillus casei 01, b) blank soy protein isolate-alginate microparticles, and c and d) soy protein isolate-alginate microparticles with encapsulated L. casei 01
The probiotic encapsulated in the microparticles produced using optimal formulation showed low level of humidity, (0.8±0.1) %, solubility of approx. (95.0±0.2) % and acceptable wettability of 100% in 5 min. These data are similar to the ones of maltodextrin microparticles with encapsulated L. casei 01 obtained by spray drying (23), confirming that soluble complexes are formed in mixed solutions with anionic polysaccharide in excess, as was previously suggested by Xiao et al. (37) as well. The water content in the microparticles was (8.1±0.2) %, not exceeding 10%, which is suggested as maximum for the stability of microencapsulated probiotics during storage (38). The viability of the microencapsulated L. casei 01 during 4- -month storage at 4 °C remained higher than the required minimum ((9.20±0.03) log CFU/g, approx. 82% preserved viability).
The stability of L. casei 01 during microencapsulation was evaluated by rough assignment of the corrected FTIR-ATR spectra of the probiotic released from the microparticles and non-encapsulated one. Generally, the FTIR- -ATR spectrum of L. casei 01 before encapsulation was comparable to the spectra reported for other lactic acid bacteria, identified by the FTIR fingerprint method (39, 40). As in previous studies, in which emulsion and spray drying were used for microencapsulation, the spectra of non-encapsulated and L. casei 01 released from the SPI-alginate microparticles were almost identical, with an additional band at 1127 cm–1 in the spectrum of released cells relevant for lactic acid (14, 16).
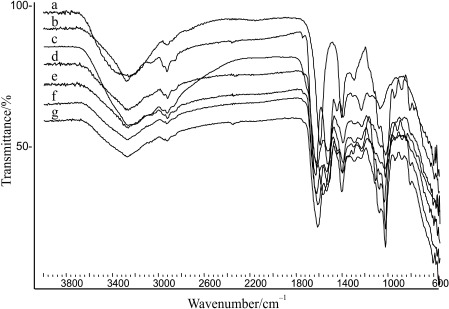
The FTIR-ATR spectrum of alginate used for microencapsulation has been previously investigated (14, 16). The spectrum obtained in this work is presented in Fig. 3, spectrum a. Generally, the spectrum of SPI (Fig. 3, spectrum b) corresponded to the spectrum already described in the literature (41). Amide I (from the C=O group within the peptide bond) occurred at 1633 cm–1, amide II (from the coupled vibrations of ν(C-N) and δ(О-Н)) at 1529 cm–1, while the band at around 3277 cm–1 corresponds to the ν(N-H) vibrations (wide signal due to the hydrogen bonds). The region 3110–3000 cm–1 is associated with the aromatic valent ν(C-H) vibrations originating from the aromatic amino acids in the SPI, while at 2990–1850 cm–1, the aliphatic ν(C-H) vibrations are assigned. In the spectra of the physical mixtures (Fig. 3, spectra d and e), the characteristic spectrum of SPI dominated, plus an intensive band at around 1025 cm–1, characteristic for skeletal vibrations of C-C and C-O of alginate. The same was also observed in the FTIR-ATR spectra of samples with different SPI to alginate ratio (1:4 to 4:1; data not shown), suggesting no interactions between the polymers, and between the polymers and the probiotic.
Fig. 3.
FTIR-ATR spectra of alginate (ALG; a), soy protein isolate (SPI; b), Lactobacillus casei 01 (c), physical mixture of ALG and SPI at 4:1 (d), physical mixture of ALG, SPI and L. casei 01 at 4:1:0.05 (e), blank SPI-alginate microparticles (f), and L. casei 01 encapsulated in SPI-alginate microparticles (g)
In the spectra of the blank and microparticles with encapsulated probiotic (Fig. 3, spectra f and g, respectively), the characteristic bands of alginate dominated. In addition, bands of amide I (at 1632 cm–1 in the SPI) and of valence vibrations of –COOH (at 1596 cm–1 in alginate) overlapped, and the characteristic band of amide II originating from the SPI at around 1528 cm–1 was observed. All these spectral data associated with the complex content of the probiotic and SPI suggest that hydrogen bonds as well as electrostatic and hydrophobic interactions between the polymers and the probiotic exist. The complexity of SPI used in the study was previously confirmed with sodium dodecyl sulfate polyacrylamide gel electrophoresis (data not shown). The presence of proteins with molecular mass below 113 kDa was detected, dominantly 21 and 35 kDa, and protein sub-domain in a range of 53–100 kDa. This is in accordance with the literature data pointing that the principal polypeptides of glycinin can be detected in a range between 20 and 29 kDa, while the principal protein subunits of α-conglycinin are between 56 and 97 kDa (42). Due to the complexity and possibly the low content of the probiotic in the microparticles, the spectral data of the encapsulated probiotic were not clearly identified in the spectrum of microparticles. However, the spectral characteristics of L. casei 01 released from the SPI-alginate microparticles pointed to preserved structural stability and fermentative activity, which was also observed in previous studies (14, 16).
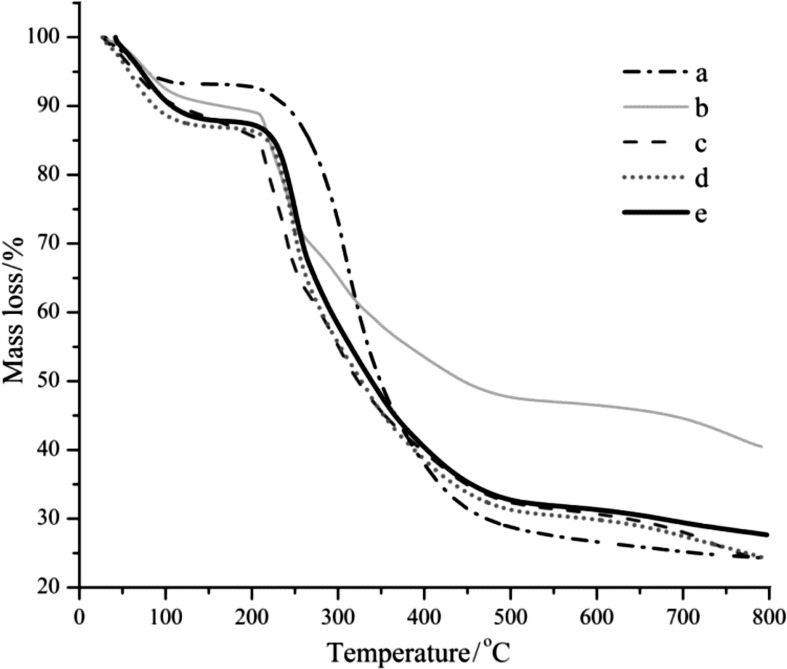
The FTIR-ATR findings were consistent with the thermal stability studies. Characteristic thermal curves of the studied samples obtained by differential scanning calorimetry and thermogravimetric analysis are shown in Figs. 4 and 5, respectively, while the characteristic parameters determined from the obtained thermograms are shown in Table 3. According to the obtained results, the blank and SPI-alginate microparticles with the probiotic were characterised by similar values of the glass transition temperature (tg) (60–70 °C) compared to the neat polymers and their corresponding physical mixtures (Fig. 4 and Table 3). According to the TG thermograms, in all samples initial mass loss of approx. 2–4% was observed, corresponding to the water desorption at temperature (td0) between 47–60 °C (Table 3 and Fig. 5). The TG thermograms of neat alginate (sample I in Table 3) and SPI (sample II in Table 3 and curve a in Fig. 5) showed one- -step degradation at a temperature (td1) of 209.0 and 249.4 °C, respectively, unlike those of the physical mixtures, which showed three-step degradation (samples IV and V in Table 3 and curves b and c in Fig. 5). For the polymers and their physical mixtures (including the one with L. casei 01) intensive degradation started above 200 °C. Considering the microparticles, the TG thermograms of both blank (sample VI in Table 3 and curve d in Fig. 5) and microparticles with encapsulated probiotic (sample VII in Table 3 and curve e in Fig. 5) showed similar mass loss and higher thermal stability than the neat alginate and SPI, with initial dehydration starting at 250 °C, followed by two overlapping processes in the first derivative of TG curve (DTG) and final degradation at around 600 °C. The higher decomposition temperature of the microparticles with encapsulated probiotic confirms the existing interactions between the polymers (and the probiotic) in the microparticles. The higher thermal stability of the microparticles might be due to the lower chance for elimination of small molecules like CO2 and CO, with the formation of electrostatic bonds between the positively charged amine and negatively charged carboxylic groups of soy protein isolate and alginate, respectively.
Fig. 4.
Differential scanning calorimetry of alginate (ALG; a), soy protein isolate (SPI; b), Lactobacillus casei 01 (c), physical mixture of ALG and SPI at 4:1 (d), physical mixture of ALG, SPI and L. casei 01 at 4:1:0.05 (e), blank SPI-alginate microparticles (f), and L. casei 01 encapsulated in SPI-alginate microparticles (g)
Fig. 5.
Thermogravimetric analysis of soy protein isolate (SPI; a), mixture of alginate and SPI at 4:1 (b), physical mixture of alginate, SPI and Lactobacillus casei 01 at 4:1:0.05 (c), blank SPI-alginate microparticles (d), and L. casei 01 encapsulated in SPI-alginate microparticles (e). Note: Starting from 300 °C alginate is subjected to thermal degradation, hence its thermogram is excluded from the figure
Table 3. Characteristic temperatures of neat polymers, their physical mixtures, blank and soy protein isolate-alginate microparticles with encapsulated Lactobacillus casei 01.
| Sample | tg/°C | td0/°C | DTG0/°C | td1/°C | DTG1/°C | td2/°C | DTG2/°C | td3/°C | DTG3/°C |
|---|---|---|---|---|---|---|---|---|---|
| I | 70.0 | n.d. | n.d. | 209.0 | n.d. | n.d. | n.d. | n.d. | n.d. |
| II | 60.0 | 51.0 | 64.1 | 249.4 | 306.8 | n.d. | n.d. | n.d. | n.d. |
| III | n.d. | n.d. | n.d. | 292.8 | n.d. | 336.4 | n.d. | n.d. | n.d. |
| IV | 68.0 | 54.2 | 79.2 | 211.2 | 218.5 | 315.7 | 304.0 | 670.1 | 756.0 |
| V | 61.0 | 46.6 | 64.2 | 208.3 | 217.0 | 283.8 | 301.0 | 689.8 | 728.0 |
| VI | 63.0 | 46.0 | 67.7 | 225.7 | 248.1 | 307.0 | 334.1 | 640.6 | 695.1 |
| VII | 66.0 | 59.9 | 77.2 | 227.4 | 250.4 | n.d. | n.d. | 656.3 | 691.0 |
I=alginate, II=soy protein isolate, III=Lactobacillus casei 01, IV=physical mixture of alginate and soy protein isolate (4:1), V=physical mixture of alginate, soy protein isolate and L. casei 01 (4:1:0.05), VI=blank soy protein-alginate microparticles, VII=soy protein-alginate microparticles with encapsulated L. casei 01, tg=glass transition temperature, td0=temperature of water desorption, td1–3=temperatures of thermal degradation, DTG=first derivative of TG curve, n.d.=not determined
Biological properties of SPI-alginate microparticles with encapsulated L. casei 01
In accordance with the solubility and wettability data for the microparticles, negligible change in d50 from the initial value was observed after 0.5 h in pH=1.5 (approx. 10% increase) when the swelling studies were performed. However, after 3 h in the same SGJ, a tenfold increase in the particle size was detected (d50=111 µm vs. 9.7 µm at zero time point) and maximum swelling was obtained. When the microparticles were subsequently placed in the SIJ (pH=6.8), the structure began to loosen and the microparticles to disintegrate. Such a swelling behaviour was expected, considering the sensitivity of the microparticle structure to the swelling medium pH and susceptibility of the soy protein isolate to cell/enzyme proteolytic activity. At pH=1.5, all negatively charged groups are neutralised and there are fewer positive amine than carboxyl groups. As a result, the electrostatic repulsion is lower and the polymer chain relaxation increases to be comparable with the medium diffusion, resulting in slower swelling. However, at pH=6.8, the polymer chain relaxation is reduced because of electrostatic repulsion among negative charges, so that the medium diffusion is faster than the relaxation of the polymer chains, pointing to diffusion-controlled swelling (43, 44).
The probiotic release profile was consistent with the swelling behaviour of the SPI-alginate microparticles with encapsulated probiotic, suggesting that the probiotic release is governed by a combination of diffusion, dissolution and degradation processes. After 3 h at pH=1.5, an insignificant loss of viability was observed, approx. 14% (Table 2). In SIJ (pH=6.8), after 3 h, approx. 81% of the initial number of viable cells (9.45 log CFU/g) entering the SGJ were preserved, while after 4 h at pH=7.4, approx. 67% of the initial viable cells (7.82 log CFU/g) encapsulated in the microparticles formed under optimal conditions survived the simulated GI passage. At the same time, the viability of the free non-encapsulated probiotic cells was significantly reduced, by approx. 50%, and additional 24% after 0.5 and 3 h in SGJ (pH=1.5), respectively.
Besides viability, for the probiotics to exert their beneficial effect by the proved mechanisms (interference with the adherence of pathogens to the intestinal mucosal cells, immunomodulation, stimulation of epithelial cell proliferation and differentiation and fortification of the intestinal barrier), they should permanently colonise the human intestinal tract (45). Bacteria adhere to GI surfaces by non--specific physical interactions, promoted by the protein and carbohydrate constituents of the bacterial cells (46, 47). Therefore, it is reasonable that a probiotic with potent intestinal mucosal cell adhesion properties be used and/or probiotic carrier exhibit prolonged residence time in the lower intestine. In this regard, the binding of pig mucin to probiotic cells and optimised microparticles in media with different pH values was also evaluated.
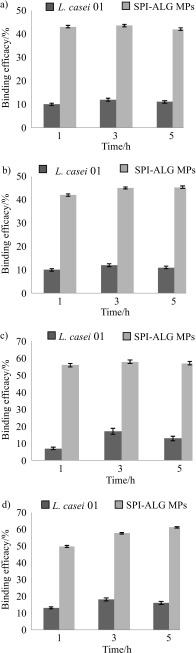
Specifically, after 5 h in all buffer media, the binding efficacy of pig mucin on probiotic cells alone did not exceed 18% and it was significantly lower than the one of the microparticles with the encapsulated probiotic (109–143% difference) (Fig. 6). The reason for such a binding capacity can be the repulsion between the negatively charged probiotic used in the actual study (16) and negatively charged mucin. Under similar pH conditions, low adherence capacity of mucin to other probiotics was detected as well (Bifidobacterium 5%, Lactobacillus rhamnosus GG 10%, B. lactis Bb12 18% and B. animalis 31%) (48). After 5 h of incubation, the lowest binding efficacy of pig mucin on the microparticles in the medium with pH=2 (approx. 42%) was measured, while at pH=4.5, 6.8 and 7.4, it increased to approx. 45, 57 and 61%, respectively. These data can be explained by the forces involved in the process of mucin-polymer adhesion. Most of the literature data report of the improvement in adhesion of microencapsulated probiotics based on alginate coating; however, the mucoadhesion can significantly increase when alginate is blended with mucoadhesive polymers (49). When the carboxylate groups of alginate are exposed to the acidic environment of the SGJ, they become protonated and hence, their bonding with mucin glycoproteins through carboxyl-hydroxyl interactions are reduced. However, the interactions between the microparticles and mucin in the soy protein isolate are stronger due to the protonation of NH3+ ions and their interaction with the functional groups of the mucin. With the increase in the pH, the carboxylate groups of alginate become deprotonated, allowing the formation of hydrogen bonds with the hydrogen part of the mucus layer, thus increasing the mucoadhesivity. The same happens with the free carboxylate groups of SPI. Therefore, the enhanced mucoadhesion and thus, site-specific delivery and prolonged clearance time of optimised cross-linked SPI-alginate microparticles containing probiotic may be dominantly attributed to soy protein isolate, i.e. its functional hydroxyl, amino and thiol groups.
Fig. 6.
Binding efficacy of pig mucin to Lactobacillus casei 01 and L. casei 01 encapsulated in soy protein-alginate microparticles (SPI-ALG MPs) at pH: a) 2, b) 4.5, c) 6.8 and d) 7.4. Bars represent standard deviation
Conclusion
This study presents a simple method for encapsulation of the probiotic Lactobacillus casei 01 in soy protein isolate-alginate microparticles by spray drying. The optimisation of the process and formulation parameters resulted in the production of novel probiotic microparticles with preserved stability and viability of the probiotic above the minimum level of 107 CFU/g at point of delivery, after microencapsulation and in simulated gastrointestinal juice, and high potential for targeted release of probiotic and prolonged residence in the small intestine. In addition, microparticles with acceptable physicochemical properties for further incorporation into food or pharmaceutical products were prepared.
Acknowledgements
This research was financially supported by the Ministry of Education and Science of the Republic of Macedonia (Project no. 13-3583/1). The authors would like to express gratitude to FMC Biopolymer UK Ltd. (Girvan, UK) and Chr. Hansen (Copenhagen, Denmark) for the donation of sodium alginate and Lactobacillus casei 01, respectively.
References
- 1.Harshitha K, Kulkarni PK, Vaghela R, Varma VKSN, Deshpande DR, Hani U. Probiotic and prebiotic-probiotic PEC microparticles for sustaining and enhancing intestinal probiotic growth. Curr Drug Deliv. 2015;12:299–307. 10.2174/1567201812666150120123800 [DOI] [PubMed] [Google Scholar]
- 2.Korada SK, Yarla NS, Bishayee A, Aliev G, Lakshmi KA, Arunasree MK, et al. Can probiotics cure inflammatory bowel diseases? Curr Pharm Des. 2016;22:904–17. 10.2174/1381612822666151209153249 [DOI] [PubMed] [Google Scholar]
- 3.Haukioja A. Probiotics and oral health. Eur J Dent. 2010;4:348–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Waigankar SS. Patel V. Role of probiotics in urogenital healthcare. J Midlife Health. 2011;2:5–10. 10.4103/0976-7800.83253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–28. 10.4161/19490976.2014.983775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu AQ, Li L. The potential role of probiotics in cancer prevention and treatment. Nutr Cancer. 2016;68:535–44. 10.1080/01635581.2016.1158300 [DOI] [PubMed] [Google Scholar]
- 7.Mekkes MC, Weenen TC, Brummer RJ, Claassen E. The development of probiotic treatment in obesity: a review. Benef Microbes. 2014;5:19–28. 10.3920/BM2012.0069 [DOI] [PubMed] [Google Scholar]
- 8.Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–71. 10.1111/all.12700 [DOI] [PubMed] [Google Scholar]
- 9.Gilbert JA, Krajmalnik-Brown R, Porazinska DL, Weiss SJ, Knight R. Toward effective probiotics for autism and other neurodevelopmental disorders. Cell. 2013;155:1446–8. 10.1016/j.cell.2013.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rokka S, Rantämaki P. Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur Food Res Technol. 2010;231:1–12. 10.1007/s00217-010-1246-2 [DOI] [Google Scholar]
- 11.Yeung TW, Arroyo-Maya IJ, McClements DJ, Sela DA. Microencapsulation of probiotics in hydrogel particles: enhancing Lactococcus lactis subsp. cremoris LM0230 viability using calcium alginate beads. Food Funct. 2016;7:1797–804. 10.1039/C5FO00801H [DOI] [PubMed] [Google Scholar]
- 12.Sosnik A. Alginate particles as platform for drug delivery by the oral route: state-of-the-art. ISRN Pharmaceutics. 2014;2014:Article ID 926157. 10.1155/2014/926157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi Y, Su R, Fan DD, Zhu XL, Zhang WN. Preparation of N,O-carboxymethyl chitosan coated alginate microcapsules and their application to Bifidobacterium longum BIOMA 5920. Mater Sci Eng C Mater Biol Appl. 2013;33:3047–53. 10.1016/j.msec.2013.03.035 [DOI] [PubMed] [Google Scholar]
- 14.Smilkov K, Petreska Ivanovska T, Petrushevska-Tozi L, Petkovska R, Hadzieva J, Popovski E, et al. Optimization of the formulation for preparing Lactobacillus casei loaded whey protein-Ca-alginate microparticles using full-factorial design. J Microencapsul. 2014;31:166–75. 10.3109/02652048.2013.824511 [DOI] [PubMed] [Google Scholar]
- 15.Smilkov K, Dodov Glavas M, Hadzieva J, Pavlova MJ, Ivanovska TP, Ristoski T, et al. Anti-inflammatory properties of L. casei loaded whey protein-alginate microparticles in animal model of colitis. Book of Proceedings of the 9th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, Lisbon, Portugal; 2014. p. 112. [Google Scholar]
- 16.Petreska-Ivanovska T, Petrushevska-Tozi L, Grozdanov A, Petkovska R, Hadjieva J, Popovski E, et al. From optimization of synbiotic microparticles prepared by spray drying to development of new functional carrot juice. Chem Ind Chem Eng Q. 2014;20:549–64. 10.2298/CICEQ130218036P [DOI] [Google Scholar]
- 17.Jurhar Pavlova M, Mladenovska K, Petreska Ivanovska T, Petrushevska-Tozi L, Korneti P, Karchev V, et al. Formulation of synbiotic soy-based food product with antihypertensive potential. Maced Pharm Bull. 2014;60:39–50. [Google Scholar]
- 18.Almeida CC, Lorena SLS, Pavan CR, Akasaka HMI, Mesquita MA. Beneficial effects on long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr Clin Pract. 2012;27:247–51. 10.1177/0884533612440289 [DOI] [PubMed] [Google Scholar]
- 19.Fang J, Liao L, Yin H, Nakamura H, Shin T, Maeda H. Enhanced bacterial tumor delivery by modulating the EPR effect and therapeutic potential of Lactobacillus casei. J Pharm Sci. 2014;103:3235–43. 10.1002/jps.24083 [DOI] [PubMed] [Google Scholar]
- 20.Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr. 2008;138:1244S–9S. [DOI] [PubMed] [Google Scholar]
- 21.Tansaz S. Boccaccini AR. Biomedical applications of soy protein: a brief overview. J Biomed Mater Res A. 2016;104:553–69. 10.1002/jbm.a.35569 [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Wang XJ, Liu YX, Ching CB. Thermal and FTIR investigation of freeze-dried protein-excipient mixtures. J Therm Anal Calorim. 2007;89:913–9. 10.1007/s10973-006-7598-y [DOI] [Google Scholar]
- 23.Dos Santos RC, Finkler L, Finkler CLL. Microencapsulation of Lactobacillus casei by spray drying. J Microencapsul. 2014;31:759–67. 10.3109/02652048.2014.932026 [DOI] [PubMed] [Google Scholar]
- 24.Hébrard G, Hoffart V, Beyssac E, Cardot JM, Alric M, Subrade M. Coated whey protein/alginate microparticles as oral controlled delivery systems for probiotic yeast. J Microencapsul. 2010;27:292–302. 10.3109/02652040903134529 [DOI] [PubMed] [Google Scholar]
- 25.European directorate for the quality of medicines and healthcare. European Pharmacopoeia. 9th ed. 01/2017. [Google Scholar]
- 26.Behboudi-Jobbehdar S, Soukoulis C, Yonekura L, Fisk I. Optimization of spray drying process conditions for the production of maximally viable microencapsulated L. acidophilus NCIMB 701748. Dry Technol. 2013;31:1274–83. 10.1080/07373937.2013.788509 [DOI] [Google Scholar]
- 27.Mandal S. Hati S, Puniya AK, Khamrui K, Singh K. Enhancement of survival of alginate-encapsulated Lactobacillus casei NCDC 298. J Sci Food Agric. 2014;94:1994–2001. 10.1002/jsfa.6514 [DOI] [PubMed] [Google Scholar]
- 28.Andersen T, Auk-Emblem P, Dornish M. 3D cell culture in alginate hydrogels. Microarrays (Basel). 2015;4:133–61. 10.3390/microarrays4020133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Tan H. Alginate-based biomaterials for regenerative medicine applications. Materials (Basel). 2013;6:1285–309. 10.3390/ma6041285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgain J, Gaiani C, Francius G, Revol-Junelles AM, Cailliez-Grimal C, Lebeer S, et al. In vitro interactions between probiotic bacteria and milk proteins probed by atomic force microscopy. Colloids Surf B Biointerfaces. 2013;104:153–62. 10.1016/j.colsurfb.2012.11.032 [DOI] [PubMed] [Google Scholar]
- 31.Park H, Kang SW, Kim BS, Mooney DJ, Lee KY. Shear-reversibly crosslinked alginate hydrogels for tissue engineering. Macromol Biosci. 2009;9:895–901. 10.1002/mabi.200800376 [DOI] [PubMed] [Google Scholar]
- 32.Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014;10:1646–62. 10.1016/j.actbio.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 33.Dianawati D, Mishra V, Shah NP. Role of calcium alginate and mannitol in protecting Bifidobacterium. Appl Environ Microbiol. 2012;78:6914–21. 10.1128/AEM.01724-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Araújo Etchepare M, Raddatz GC, Cichoski AJ, Flores EMM, Barin JS, Zepka LQ, et al. Effect of resistant starch (Hi-maize) on the survival of Lactobacillus acidophilus microencapsulated with sodium alginate. J Funct Foods. 2016;21:321–9. 10.1016/j.jff.2015.12.025 [DOI] [Google Scholar]
- 35.Pop OL, Brandau T, Schwinn J, Vodnar DC, Socaciu C. The influence of different polymers on viability of Bifidobacterium lactis 300b during encapsulation, freeze-drying and storage. J Food Sci Technol. 2015;52:4146–55. 10.1007/s13197-014-1441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajam R, Kartnik P, Parthasarathi S, Joseph GS, Anandharamakrishnan C. Effect of whey protein-alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J Funct Foods. 2012;4:891–8. 10.1016/j.jff.2012.06.006 [DOI] [Google Scholar]
- 37.Xiao JX, Yu HY, Yang J. Microencapsulation of sweet orange oil by complex coacervation with soybean protein isolate/gum Arabic. Food Chem. 2011;125:1267–72. 10.1016/j.foodchem.2010.10.063 [DOI] [Google Scholar]
- 38.Ying D, Sun J, Sanguansri L, Weerakkody R, Augustin MA. Enhanced survival of spray dried microencapsulated Lactobacillus rhamnosus GG in the presence of glucose. J Food Eng. 2012;109:597–602. https://doi.org 10.1016/j.jfoodeng.2011.10.017 [DOI] [Google Scholar]
- 39.Vodnar DC, Paucean A, Dulf FV, Socaciu C. HPLC Characterization of lactic acid formation and FTIR fingerprint of probiotic bacteria during fermentation processes. Not Bot Hort Agrobot Cluj. 2010;38:109–13. [Google Scholar]
- 40.Papadimitriou K, Boutou E, Zoumpopoulou G, Tarantilis PA, Polissiou M, Vorgias CE, et al. RNA arbitrarily primed PCR and fourier transform infrared spectroscopy reveal plasticity in the acid tolerance response of Streptococcus macedonicus. Appl Environ Microbiol. 2008;74:6068–76. 10.1128/AEM.00315-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin B, Zhou X, Li B, Chen C, Zhang X, Chen S. Structure and antioxidant activity of soy protein isolate-dextran conjugates obtained by TiO2 photocatalysis. BioMed Res Int. 2015;•••:150603. 10.1155/2015/150603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SK, Bae DH, Rhee KC. Soy protein biopolymers cross-linked with glutaraldehyde. J Am Oil Chem Soc. 2000;77:879–84. 10.1007/s11746-000-0140-3 [DOI] [Google Scholar]
- 43.Devi N, Kakati DK. Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. J Food Eng. 2013;117:193–204. 10.1016/j.jfoodeng.2013.02.018 [DOI] [Google Scholar]
- 44.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–26. 10.1586/erd.11.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomes AM, Pintado MM, Freitas AC, Sousa e Silva JP. Gastrointestinal tract: microflora and transit aspects. In: Freitas AC, editor. Probiotic bacteria: fundamentals, therapy and technological aspects. Boca Raton FL, USA: CRC Press Taylor & Francis Group; 2014. p. 7. https://doi.org/ 10.1201/b15676-3 [DOI] [Google Scholar]
- 46.Van Tassell ML, Miller MJ. Lactobacillus adhesion to mucus. Nutrients. 2011;3:613–36. 10.3390/nu3050613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valeriano VD, Bagon BB, Balolong MP, Kang DK. Carbohydrate-binding specificities of potential probiotic Lactobacillus strains in porcine jejunal (IPEC-J2) cells and porcine mucin. J Microbiol. 2016;54:510–9. 10.1007/s12275-016-6168-7 [DOI] [PubMed] [Google Scholar]
- 48.Laparra JM, Sanz Y. Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Lett Appl Microbiol. 2009;49:695–701. 10.1111/j.1472-765X.2009.02729.x [DOI] [PubMed] [Google Scholar]
- 49.Solanki HK, Shah DA. Formulation optimization and evaluation of probiotic Lactobacillus sporogenes-loaded sodium alginate with carboxymethyl cellulose mucoadhesive beads using design expert software. J Food Process. 2016; Article ID 6041671 10.1155/2016/6041671 [DOI] [Google Scholar]