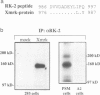
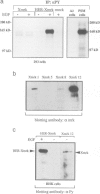
Abstract
Xmrk encodes a putative transmembrane glycoprotein of the tyrosine kinase family and is a melanoma-inducing gene in Xiphophorus. We attempted to investigate the biological function of the putative Xmrk receptor by characterizing its signalling properties. Since a potential ligand for Xmrk has not yet been identified, it has been difficult to analyse the biochemical properties and biological function of this cell surface protein. In an approach towards such analyses, the Xmrk extracellular domain was replaced by the closely related ligand-binding domain sequences of the human epidermal growth factor receptor (HER) and the ligand-induced activity of the chimeric HER-Xmrk protein was examined. We show that the Xmrk protein is a functional receptor tyrosine kinase, is highly active in malignant melanoma and displays a constitutive autophosphorylation activity possibly due to an activating mutation in its extracellular or transmembrane domain. In the focus formation assay the HER-Xmrk chimera is a potent transforming protein equivalent to other tyrosine kinase oncoproteins.
Full text
PDF
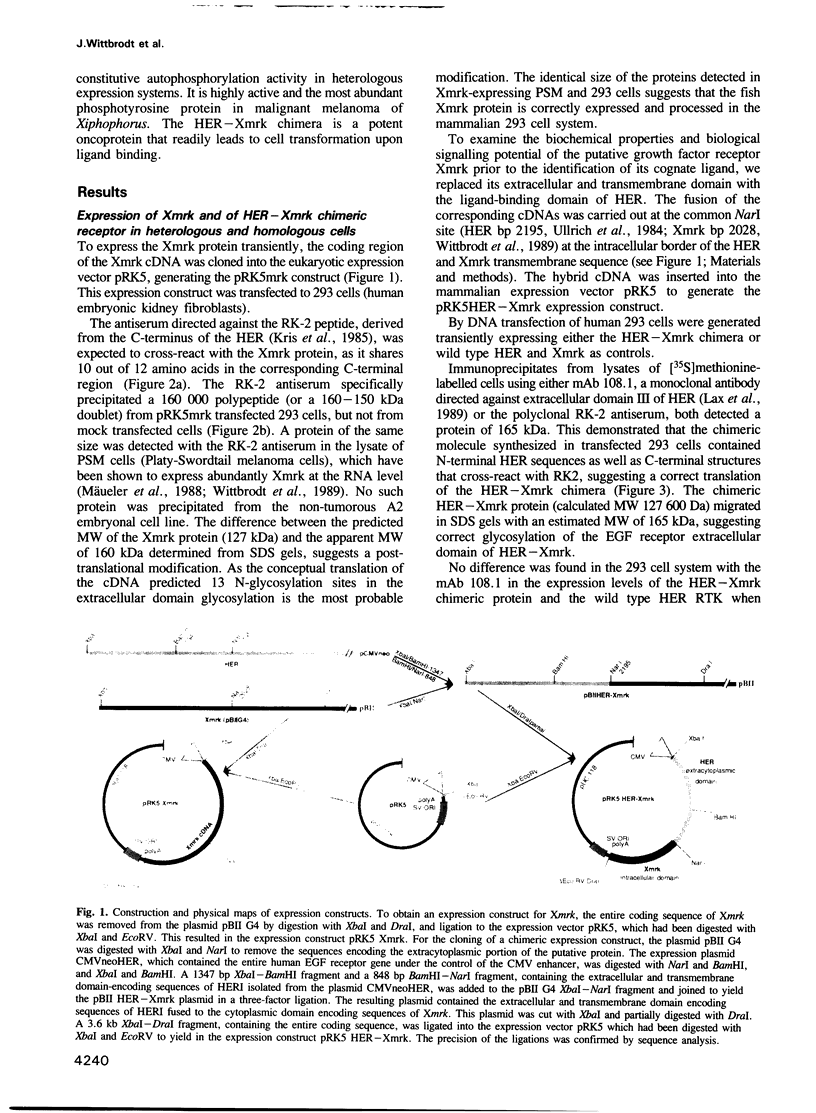






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam D., Mäueler W., Schartl M. Transcriptional activation of the melanoma inducing Xmrk oncogene in Xiphophorus. Oncogene. 1991 Jan;6(1):73–80. [PubMed] [Google Scholar]
- Aroian R. V., Koga M., Mendel J. E., Ohshima Y., Sternberg P. W. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990 Dec 20;348(6303):693–699. doi: 10.1038/348693a0. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K., Hafen E. Sevenless and Drosophila eye development: a tyrosine kinase controls cell fate. Trends Genet. 1988 Mar;4(3):74–79. doi: 10.1016/0168-9525(88)90044-3. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. L., Wood W. I., Eaton D., Hass P. E., Hollingshead P., Wion K., Mather J., Lawn R. M., Vehar G. A., Gorman C. Construction and characterization of an active factor VIII variant lacking the central one-third of the molecule. Biochemistry. 1986 Dec 30;25(26):8343–8347. doi: 10.1021/bi00374a001. [DOI] [PubMed] [Google Scholar]
- Friedenreich H., Schartl M. Transient expression directed by homologous and heterologous promoter and enhancer sequences in fish cells. Nucleic Acids Res. 1990 Jun 11;18(11):3299–3305. doi: 10.1093/nar/18.11.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Honegger A., Dull T. J., Bellot F., Van Obberghen E., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Biological activities of EGF-receptor mutants with individually altered autophosphorylation sites. EMBO J. 1988 Oct;7(10):3045–3052. doi: 10.1002/j.1460-2075.1988.tb03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammers R., Gray A., Schlessinger J., Ullrich A. Differential signalling potential of insulin- and IGF-1-receptor cytoplasmic domains. EMBO J. 1989 May;8(5):1369–1375. doi: 10.1002/j.1460-2075.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I., Bellot F., Howk R., Ullrich A., Givol D., Schlessinger J. Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J. 1989 Feb;8(2):421–427. doi: 10.1002/j.1460-2075.1989.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Dull T. J., Lax I., Schlessinger J., Ullrich A. HER2 cytoplasmic domain generates normal mitogenic and transforming signals in a chimeric receptor. EMBO J. 1989 Jan;8(1):167–173. doi: 10.1002/j.1460-2075.1989.tb03361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehväslaiho H., Lehtola L., Sistonen L., Alitalo K. A chimeric EGF-R-neu proto-oncogene allows EGF to regulate neu tyrosine kinase and cell transformation. EMBO J. 1989 Jan;8(1):159–166. doi: 10.1002/j.1460-2075.1989.tb03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Li N., Koch A., Mohammadi M., Hurwitz D. R., Zilberstein A., Ullrich A., Pawson T., Schlessinger J. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. EMBO J. 1990 Dec;9(13):4375–4380. doi: 10.1002/j.1460-2075.1990.tb07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mäueler W., Raulf F., Schartl M. Expression of proto-oncogenes in embryonic, adult, and transformed tissue of Xiphophorus (Teleostei: Poeciliidae). Oncogene. 1988 May;2(5):421–430. [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Price J. V., Clifford R. J., Schüpbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 1989 Mar 24;56(6):1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- Riedel H., Dull T. J., Schlessinger J., Ullrich A. A chimaeric receptor allows insulin to stimulate tyrosine kinase activity of epidermal growth factor receptor. Nature. 1986 Nov 6;324(6092):68–70. doi: 10.1038/324068a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Lindquist P. B., Bringman T. S., Goeddel D. V., Derynck R. Expression in rat fibroblasts of a human transforming growth factor-alpha cDNA results in transformation. Cell. 1986 Jul 18;46(2):301–309. doi: 10.1016/0092-8674(86)90747-6. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Schartl M., Adam D. Molecular cloning, structural characterization, and analysis of transcription of the melanoma oncogene of xiphophorus. Pigment Cell Res. 1992;Suppl 2:173–180. doi: 10.1111/j.1600-0749.1990.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Schejter E. D., Shilo B. Z. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989 Mar 24;56(6):1093–1104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Stevens L. M., Nüsslein-Volhard C. The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature. 1989 Apr 6;338(6215):478–483. doi: 10.1038/338478a0. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Kamps M. P., Cao H. Oncogenic activation of p185neu stimulates tyrosine phosphorylation in vivo. Mol Cell Biol. 1988 Sep;8(9):3969–3973. doi: 10.1128/mcb.8.9.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Nishibe S., Suh P. G., Rhee S. G., Carpenter G. Epidermal growth factor stimulates tyrosine phosphorylation of phospholipase C-II independently of receptor internalization and extracellular calcium. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1568–1572. doi: 10.1073/pnas.86.5.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. E., Eisenman J., Baird A., Rauch C., Van Ness K., March C. J., Park L. S., Martin U., Mochizuki D. Y., Boswell H. S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990 Oct 5;63(1):167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Wirth M., Bode J., Zettlmeissl G., Hauser H. Isolation of overproducing recombinant mammalian cell lines by a fast and simple selection procedure. Gene. 1988 Dec 20;73(2):419–426. doi: 10.1016/0378-1119(88)90506-9. [DOI] [PubMed] [Google Scholar]
- Wittbrodt J., Adam D., Malitschek B., Mäueler W., Raulf F., Telling A., Robertson S. M., Schartl M. Novel putative receptor tyrosine kinase encoded by the melanoma-inducing Tu locus in Xiphophorus. Nature. 1989 Oct 5;341(6241):415–421. doi: 10.1038/341415a0. [DOI] [PubMed] [Google Scholar]
- Woolford J., McAuliffe A., Rohrschneider L. R. Activation of the feline c-fms proto-oncogene: multiple alterations are required to generate a fully transformed phenotype. Cell. 1988 Dec 23;55(6):965–977. doi: 10.1016/0092-8674(88)90242-5. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]