Abstract
Background:
Titanium and polyetheretherketone (PEEK) implants have been used in spinal surgery with low rejection rates. Compared to titanium, PEEK has many advantages, including a density more similar to that of bone, radiolucency, and a lack of artifacts in computed tomography (CT) and magnetic resonance imaging (MRI). In this study, we evaluated the effectiveness of PEEK cages as an alternative to titanium for bone fusion after fractures of the thoracolumbar spine. We also propose a classification to the impaction index.
Methods:
We evaluated 77 patients with fractures of the thoracic or lumbar spine who were treated by anterior fixation with titanium cages (TeCorp®) in 46 (59.7%) patients or PEEK (Verte-stak®) in 31 (40.3%) patients from 2006 to 2012 (Neurological Hospital of Lyon).
Results:
The titanium group achieved 100% fusion, and the PEEK group achieved 96.3% fusion. The titanium systems correlated with higher impact stress directed toward the lower and upper plateaus of the fused vertebrae; there were no nonunions for those treated with titanium group. Nevertheless, there was only one in the PEEK group. There was no significant difference in the pain scale outcomes for patients with ±10 degrees of the sagittal angle. Statistically, it is not possible to associate the variation of sagittal alignment or the impaction with symptoms of pain. The complication rate related to the implantation of cages was low.
Conclusions:
Titanium and PEEK are thus equally effective options for the reconstruction of the anterior column. PEEK is advantageous because its radiolucency facilitates the visualization of bone bridges.
Keywords: Fractures of the thoracolumbar spine, implant, polyetheretherketone, titanium
INTRODUCTION
The surgical treatment of spine fractures reduces deformity and restores spinal stability. If the patient has direct spinal cord compression, decompression of the spinal canal is also affected.[14] In the thoracic and lumbar spine, the posterior approach is most common (e.g., laminectomy). Fixation is then accomplished with screws or hooks fixed by titanium rods. A heterologous or autologous bone graft, usually taken from the spinous processes, is added to facilitate bone fusion.[1] Usually, it is necessary to fix more than one segment above and below the fracture site to better distribute the axial load on the screw/rod complex.[14] The disadvantage of long instrumentation is the more limited spinal mobility, that may contribute to residual pain and adjacent segment degeneration. However, a short one segment above/below construct may lead to a fractured vertebra.[9] PEEK is superior to titanium in the long-term for maintaining disc space height and offers a high rate of radiographic fusion.[11] The advantages of PEEK compared to titanium include a density close to that of bone, radiolucency, and a lack of artifacts in magnetic resonance imaging (MRI) and computed tomography (CT). Good kyphosis correction and fusion rates were obtained with PEEK cages in thoracolumbar fractures.[7] PEEK implants have been used since 2009 in nearly all cases. This paper compares PEEK versus titanium implants used to perform thoracic/lumbar fusions.
PATIENTS AND METHODS
Population
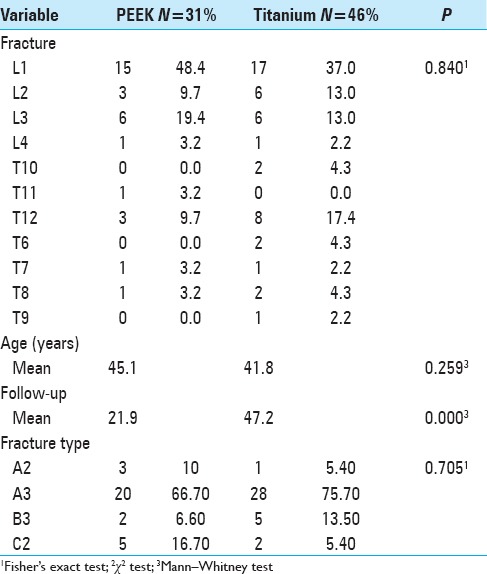
A total of 77 patients [Table 1] with fractures of the thoracic or lumbar spine treated with vertebral body replacement were evaluated between 2006 and 2012 at the Neurological Hospital of Lyon; 46 patients received titanium cages (TeCorp®), and 31 patients received PEEK cages (Vertestak®). There was no significant difference in age between the groups. The mean follow-up period was significantly higher in the titanium group than in the PEEK group.
Table 1.
Characteristics of the study participants
Inclusion criteria
This study evaluated patients with traumatic fractures who underwent vertebral body replacement using a titanium or PEEK cage. The patients were older than 16 years of age, and had a minimum of 8 months of follow-up. The indications for cage placement via the anterior route were spine fractures that affected the anterior and middle segments of the vertebra and where there were signs of substance loss due to vertebral body splintering (e.g., documented on CT).[5,13,16]
Exclusion criteria
Patients with more than one fractured vertebra, reoperations for failed fusion of previous surgery, and children were excluded from the research.
Variables
The following variables were evaluated: age, cause and location of fracture, complications, type of implant, and fracture classification according to Magerl et al.[8] Radiological aspects: all patients underwent radiography preoperatively and postoperatively in the immediate and later periods, and the following four stages (presurgery, after the posterior approach, after the anterior approach, and final follow-up) were evaluated.
Multiple factors were examined:
Coronal and sagittal segmental alignment
Impaction of the implant on the plateaus [Table 2]
Radiographic fusion was considered the presence of an intervertebral bone bridge upon radiographic examination.
Table 2.
New classification for impaction

Surgery
All patients underwent emergent posterior fracture stabilization and, if necessary, spinal decompression. A second-stage surgery was performed to place the cage for vertebral body replacement to reinforce the arthrodesis. The second-stage surgery was performed anteriorly by thoracotomy for thoracic vertebra fractures and by L1 or lumbotomy for fractures to L2, L3, and L4. The minimum interval between the first and second approach was 3 days. All patients underwent partial resection of the vertebral body, followed by placement of a cylindrical titanium or PEEK cage.
RESULTS
In total, 77 patients were studied and classified into two groups: 31 in the PEEK group and 46 in the titanium group.
Sagittal alignment angle
Changes in the sagittal segmental angle of the adjacent vertebrae were analyzed at four time points [Figure 1]. In the second evaluation (postoperative posterior approach), the mean value of this angle significantly decreased from 10.43 to 3.64 (P = 0.001) compared to the preoperative period. There was no significant difference between the second (after posterior approach) and third (after anterior) evaluations (P = 0.729) nor between the first and final follow-up (P = 0.611). Between the postoperative period of the anterior approach and the final follow-up there was loss of the deformity correction from 3.65° to 8.86° (P < 0.0001). This difference did not vary significantly (P = 0.867) between individuals who did or did not have cage impaction on the vertebral plateau.
Figure 1.
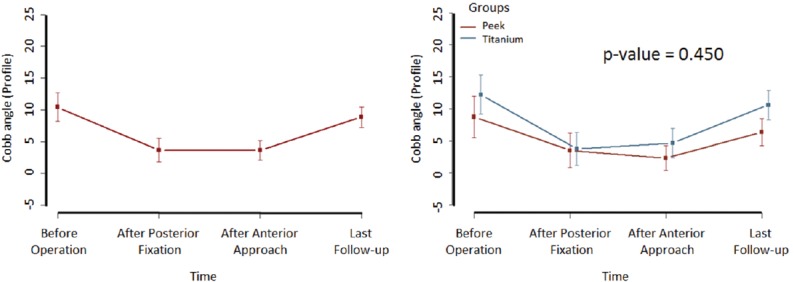
Variation of sagittal alignment among the four evaluated stages and between groups. P value was calculated using the marginal log-linear model based on Chi-square analysis
Analogic visual scale of pain according to sagittal angle
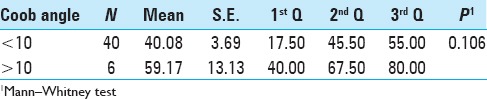
A comparison of the analogic visual scale (AVS) of pain between the categories of sagittal angle (comparing stages 3 and 4) is presented in Table 3. There was no significant difference in pain scale among those patients who had an increase in the sagittal angle bigger or smaller than 10° [Table 3].
Table 3.
Analogic visual scale (AVS) of pain according to sagittal angle
Coronal alignment angle
The mean value of the coronal alignment angle remained close to zero, i.e., close to perfect alignment at all evaluations (P = 0.788), with no large differences between four stages. The coronal alignment angle measurements of the titanium group were significantly higher than those of the PEEK group (P = 0.041). Only the stage after the anterior approach exhibited a significant difference; the mean of the titanium group was higher than that of the PEEK group (P = 0.002).
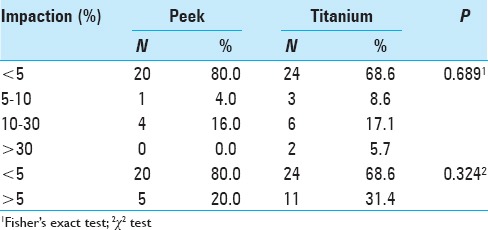
Implant impaction on the lower vertebra
Only 19.0% of the cases had impaction on the lower plateau in the immediate postoperative period of cage placement (Stage 3, Table 4). In the titanium group, 71.0% of the patients had no impaction on the lower plateau and displayed impaction in the final follow-up [Table 4]. Analysis of progress revealed that 20.5% of cages exhibited impaction in the final follow-up. The mean percentage value of impaction increased in the immediate postoperative period compared to the final follow-up, from 4.1% to 7.9% [Figure 2a]. Compared to the immediate postoperative period, there was an increase in impaction in the final follow-up in the titanium group compared to the PEEK group [Figure 2b]. There was no significant difference between the PEEK and titanium groups.
Table 4.
Impaction on the lower plateau in the height affected vertebra
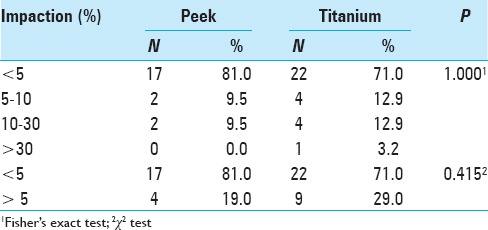
Figure 2.
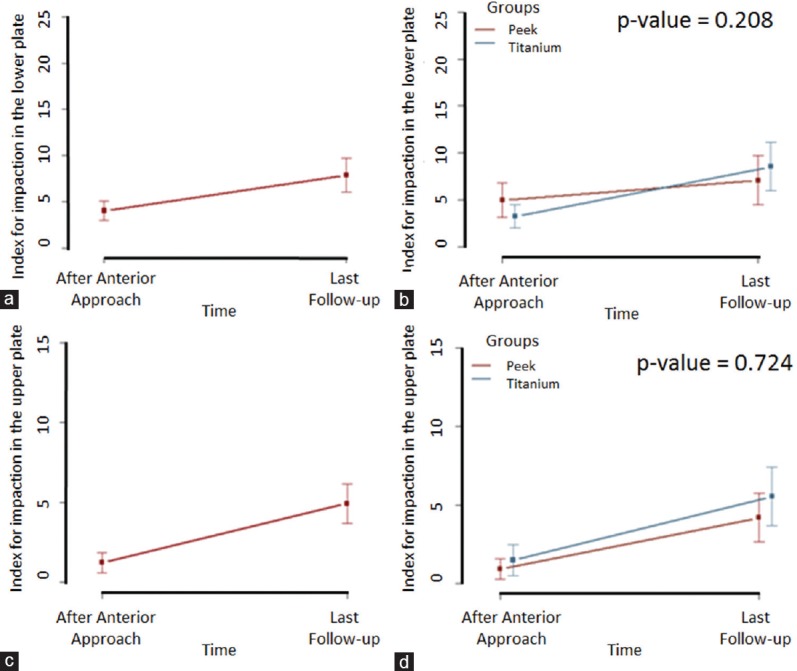
(a) Mean percentage value of impaction on the lower plateau in the cage placement immediate postoperative stage (Stage 3) and at final follow-up. (b) Change in impaction on the lower plateau in the cage placement immediate postoperative stage (third evaluation) and at the final follow-up for the two groups. (c) Mean percentage value of impaction on the upper plateau in the cage placement immediate postoperative stage (Stage 3) and at the final follow-up. (d) Change in impaction in the upper plateau in the cage placement immediate postoperative stage (third evaluation) and at the final follow-up for the two groups. P value was calculated using the marginal log-linear model based on Chi-square analysis
Impaction of the implant on the upper vertebra
In the PEEK and titanium groups, 20 (80.0%) and 24 (68.6%) patients did not exhibit impaction on the upper plateau in the final follow-up, respectively. There was no significant difference between the groups [Table 5]. The overall mean value of percentage impaction increased from 1.2% at the postoperative stage to 4.9% at the final follow-up [Figure 2c], a significant increase (P = 0.012). In the stratification of data into groups [Figure 2d], the titanium group had higher mean impaction at the cage placement immediate postoperative stage and the final follow-up, but the differences between the titanium and PEEK groups were not significant (P = 0.724).
Table 5.
Impaction on the upper plateau in the height affected vertebra
Analogic visual scale of pain according to impaction
A comparison of the AVS of pain according to impaction in stage 4 is presented in Table 6. There was no significant difference in pain scale among those patients with or without compaction [Table 6].
Table 6.
Analogic visual scale (AVS) of pain according to impaction
Bone fusion
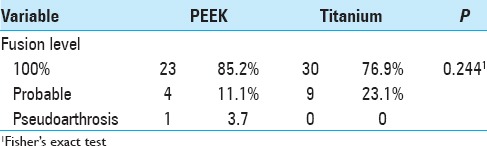
Including cases of probable fusion, the titanium group obtained 100% fusion, and the PEEK group achieved 96.3% fusion. As demonstrated in Table 7, 23.1% of patients in the titanium group were considered probable radiographic fusion. There was no pseudoarthrosis in the titanium group, but one case was detected in the PEEK group.
Table 7.
Descriptive and comparative analysis of groups for percent bone fusion, considering the existence of bone bridges
Complications
The complications connected or not directly linked to the procedure are presented in Tables 8, 9 and 10.
Table 8.
Early complications
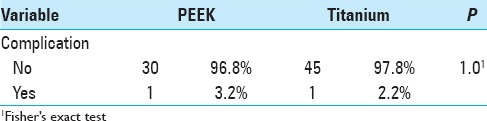
Table 9.
Later complications
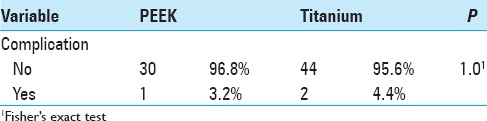
Table 10.
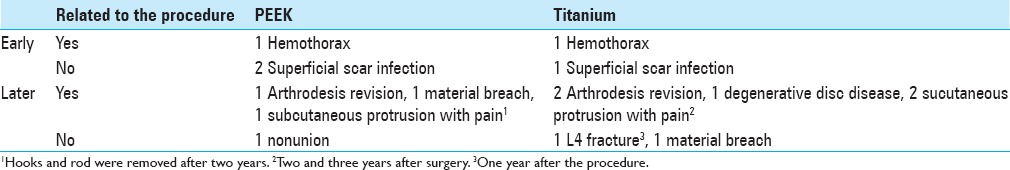
Early and later complications
DISCUSSION
There are still no guidelines or uniformity regarding the treatment of unstable fractures of the thoracolumbar spine. The most common treatment is posterior fixation with a pedicle screw.[16] However, arthrodesis combined with a pedicle screw associated with the placement of the cage anteriorly is a good option, particularly for fractures with burst vertebrae.
As a very rigid material with much greater density than bone, titanium does not have compliance compatible with a large overload area such as the thoracolumbar spine. Theoretically, titanium can, over time, impact the vertebral body and thus can lead the patient to suffer progressive kyphosis. Another drawback of titanium is difficulty in evaluating postoperative control X-ray because it is often not possible to confirm bone fusion due to the difficulty of visualizing the trabecular bones. In some cases, consolidation cannot be confirmed by CT scan due to the strong presence of artifacts.[7] PEEK implants are advantageous in this respect because they are radiolucent, and thus, the evaluation of the degree of fusion can be more precise.[7] Thus, a higher rate of probable fusions was expected in the titanium group. In 23.1% of cases it was not possible to observed bone bridges in the control X-ray, and fusion could only be verified by radiograph in flexion and extension.
Although the osteogenic properties of autologous bone are undeniable, such grafts are not free from fracture and dislocation[10] and are inherently associated with morbidity in their removal, generally requiring another surgical incision.[2,6] In this study, the use of fractured vertebral body bone associated with heterologous apatite hydroxide prevented any significant morbidity related to graft removal. Cotler et al.[3] reported a fusion rate of 93% in 1 year using an autograft to reconstruct the anterior column after thoracic and lumbar vertebrectomy. In the present study, we observed a rate of 100% in the titanium group and 96.3% in the PEEK group.
Bone fusion
Considering as probable fusion a lack of material displacement in the radiograph in flexion and extension and an absence of translucency between the material and vertebral plateau, both implants achieved fusion levels of close to 100%, with 100% in the titanium group and 96.3% in the PEEK group. Viewing of the bone bridges is more difficult with a titanium implant because the implant is not radiolucent and presents artifacts in MRI and CT. Thus, 23.1% of the titanium group patients were classified as requiring probable radiographic arthrodesis because it was not possible to identify bone bridges in the intervertebral space, although this does not indicate failure of the bone fusion.
These data coincide with findings by Dvorak et al.[4] who published a retrospective study of titanium cages in 57 patients who underwent thoracolumbar corpectomy. Bone fusion was identified in 93% of radiographs at the end of follow-up. Schnake, Stavridis, and Kandziora[13] evaluated the long-term results of titanium implants after a minimum follow-up of 5 years and observed a 91% fusion rate. Graillon et al.[5] analyzed 85 patients with fractures between T4 and L4 and obtained a 100% fusion rate at a mean follow-up of 16 months.
Coronal and sagittal alignment
The mean alignment angle measurements on the coronal plane were thus always close to 0 at all studied stages, indicating good alignment of the spine at the time of the trauma that remained up to the final evaluation. The mean coronal plane angle was significantly greater in the titanium group than the PEEK group (P = 0.033); however, this effect depended significantly on the analyzed stage (P = 0.041). A significant difference was observed only after the anterior approach, in which the mean was higher in the titanium group than in the PEEK group (P = 0.002). At other stages, there was no difference. It should be noted that the measurements of the two groups were always close to zero degrees, i.e., near perfect alignment.
In relation to the sagittal plane, some authors have reported a correction loss of 7–16°, with spinal kyphosis during the first postoperative year, in exclusively posterior approach fixations, particularly in fractures with upper plateau injuries of the vertebra.[1,12,17] Verlaan et al.[15] reported a mean correction loss of 10° with kyphosis during the first postoperative year after an isolated posterior approach. Graillon et al.[5] reported no significant correction loss at final follow-up, and complete fusion was documented in all patients at follow-up of 3–12 months. One potential explanation is the accommodation of the implant in the body, with impaction on the plateau and consequent correction loss on the sagittal plane.
Correction loss between the postoperative anterior approach and final follow-up was also observed in our series (P < 0.0001). In absolute numbers, this loss was 5.21°. No significant differences in sagittal alignment were detected when comparing the two implants. The PEEK group exhibited a 4.04° correction loss and the titanium group 5.93°, with no significant difference.
When also analyzing posterior instrumentation combined with a titanium cage, after 5 years of follow-up, Schnake, Stavridis, and Kandziora[13] reported a significant correction loss, from 6.5° to 9.1° (mean 2.6°, P < 0.001) compared to preoperative sagittal angle values. Most of the correction loss (mean 2.4°) and cage subsidence in the vertebral body occurred within the first 12 months postoperatively. There was also a correction loss in this study from 3.65° at the first measurement to 8.86° in the final follow-up (P < 0.0001).
Impaction on the vertebral plateau
A classification to measure the impaction index could assist in the follow-up of patients with potential neurological damage. This is a critical problem mainly in cases in which the impaction is higher than 30%. The suggested index is presented below and the impaction level has a direct correlation with the increase of the symptoms.
Type I ⟶ <5%: no impaction;
Type II ⟶ 5–10%: minimum impaction;
Type III ⟶ 10–30%: moderate impaction;
Type IV ⟶ >30%: severe impaction.
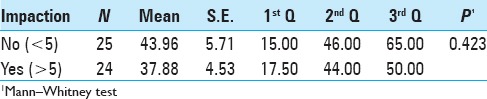
The types of impaction were based on the proposed classification. There was a highest percentage of patients without impaction (type I). The impaction was higher in PEEK cages (80% in upper plateau and 81% in lower plateau) than in titanium (71% in upper plateau and 68.6% in lower plateau) [Tables 4 and 5].
The cases classified as types II and III, for lower impaction, had strictly the same percentage for PEEK cage and titanium, i.e., 9.5% and 12.9%, respectively. The same was not observed for upper vertebral plateau: the percentage of impaction was smaller for PEEK cage group (4% - type II and 16% - type III) than for the titanium group (8.6% - type II and 17% - type III). Patients classified in severe cases, or type IV, were observed only in titanium group: 5.7% in upper plateau and 3.2% in lower plateau [Tables 4 and 5].
Titanium is a more rigid material with less compliance, with more impaction on the vertebral plateau. Titanium tended to have greater impaction on the plateau. Both groups had a significant increase in the number of cases of impaction on the upper vertebra in the final follow-up. This increase was greater in the titanium group, with 31.4% of patients with impaction, compared to 20% in the PEEK group in the final follow-up. This increase in the lower vertebra was not significant.
Graillon et al.[5] observed cage subsidence in the upper or lower vertebral plateau after 1 year in 35% of cases. This subsidence was between 2 mm and 7 mm. As in the present study, the authors observed a high fusion rate of 100%. Schnake, Stavridis, and Kandziora[13] observed subsidence over 1 year of 2.1 mm, measured in profile radiographs, which did not worsen after 5 years of follow-up. In this study, we chose to measure impaction as a percentage because percent values are considered more reliable for comparison of radiographs performed on devices with different configurations. In this study, there was an increase in impaction between anterior implant placement and final follow-up on the upper plateau of 3.7% and on the lower plateau of 3.8%, with a mean vertebral size of 30 mm; thus, the mean value obtained was 1.1 mm, representing a very low level of impaction.
Complications
Schnake, Stavridis, and Kandziora[13] published a series of 80 patients who received titanium cages in which 45 (56%) patients were followed-up over 5 years. As in the present study, there were no complications related to the cage. The authors emphasized a high complication rate related to thoracotomy (26%), but most complications were not clinically significant. Transient pulmonary complications were more frequent (with an overall rate of 18.75%). Revision surgery (repeat thoracotomy) was required in one case (1.25%) for thoracic seroma drainage. Le Huec et al.,[7] in a series of 50 patients that included 30 PEEK cages, highlighted five (10%) cases of pulmonary atelectasis and no cases of pseudoarthrosis. Another series of 43 patients with titanium cages[4] included three (7.3%) cases of deep infection, one case of pseudomeningocele (2.4%), one case of cage breakage, one case of cage migration and six (14.6%) cases of revision surgery.
In our study, there was one case of hemothorax in each group, with no long-term repercussions. One (3.2%) revision surgery was performed in the PEEK group and two (4.4%) in the titanium group. In other cases, where the posterior osteosynthesis material was removed, the reason was not instrumentation failure. Cage placement surgery resulted in no infections. Infections were related to the surgical placement of pedicle screws posteriorly, with one (3.2%) case in the PEEK group and two (4.4%) in the titanium group, and were only superficial scar infections. The posterior approach was generally performed in an emergency and was therefore susceptible to a higher infection rate.
CONCLUSIONS
Titanium and PEEK implants are effective options for the reconstruction of the vertebral body in the case of thoracolumbar spine fracture. Both have a similar fusion rate. PEEK offers the advantage of greater visibility of bone bridges due to its radiolucency. Titanium tends to have higher impaction on the vertebral plateau, but the difference between PEEK and titanium is not significant. A classification to measure the impaction index could assist in the follow-up of patients with potential neurological damage. There was no difference in pain scale for patients with or without compaction. There was no association in pain scale among those patients who had an increase in the sagittal angle bigger or smaller than 10 degrees. For both implants, both initial and later infection and complication rates were low.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Rafael Augusto Castro Santiago Brandão, Email: rafabrand@gmail.com.
Warley Carvalho da Silva Martins, Email: medicalwarley@yahoo.com.br.
Aluízio Augusto Arantes, Jr, Email: aluizio_arantes@uol.com.br.
Sebastião Nataniel Silva Gusmão, Email: sebastiaogusmao@gmail.com.
Gilles Perrin, Email: giperrin@numericable.fr.
Cédric Barrey, Email: cedric.barrey@chu-lyon.fr.
REFERENCES
- 1.Alanay A, Acaroglu E, Yazici M, Oznur A, Surat A. Short-segment pedicle instrumentation of thoracolumbar burst fractures: Does transpedicular intracorporeal grafting prevent early failure? Spine. 2001;26:213–7. doi: 10.1097/00007632-200101150-00017. [DOI] [PubMed] [Google Scholar]
- 2.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–60. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Cotler HB, Cotler JM, Stoloff A, Cohn HE, Jerrell BE, Martinez L, et al. The use of autografts for vertebral body replacement of the thoracic and lumbar spine. Spine. 1985;10:748–56. doi: 10.1097/00007632-198510000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak MF, Kwon BK, Fisher CG, Eiserloh HL, 3rd, Boyd M, Wing PC. Effectiveness of titanium mesh cylindrical cages in anterior column reconstruction after thoracic and lumbar vertebral body resection. Spine. 2003;28:902–8. doi: 10.1097/01.BRS.0000058712.88053.13. [DOI] [PubMed] [Google Scholar]
- 5.Graillon T, Rakotozanany P, Blondel B, Adetchessi T, Dufour H, Fuentes S. Circumferential management of unstable thoracolumbar fractures using an anterior expandable cage, as an alternative to an iliac crest graft, combined with a posterior screw fixation: Results of a series of 85 patients. Neurosurg Focus. 2014;37:E10. doi: 10.3171/2014.5.FOCUS1452. [DOI] [PubMed] [Google Scholar]
- 6.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324–31. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Le Huec JC, Tournier C, Aunoble S, Madi K, Leijssen P. Video-assisted treatment of thoracolumbar junction fractures using a specific distractor for reduction: Prospective study of 50 cases. Eur Spine J. 2010;19(Suppl 1):S27–32. doi: 10.1007/s00586-009-1121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 9.McLain RF, Sparling E, Benson DR. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures. A preliminary report. J Bone Joint Surg Am. 1993;75:162–7. doi: 10.2106/00004623-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Meding JB, Stambough JL. Critical analysis of strut grafts in anterior spinal fusions. J Spinal Disord. 1993;6:166–74. [PubMed] [Google Scholar]
- 11.Niu CC, Liao JC, Chen WJ, Chen LH. Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: Titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech. 2010;23:310–6. doi: 10.1097/BSD.0b013e3181af3a84. [DOI] [PubMed] [Google Scholar]
- 12.Oertel J, Niendorf WR, Darwish N, Schroeder HWS, Gaab MR. Limitations of dorsal transpedicular stabilization in unstable fractures of the lower thoracic and lumbar spine: An analysis of 133 patients. Acta Neurochir. 2004;146:771–7. doi: 10.1007/s00701-004-0284-6. [DOI] [PubMed] [Google Scholar]
- 13.Schnake KJ, Stavridis SI, Kandziora F. Five-year clinical and radiological results of combined anteroposterior stabilization of thoracolumbar fractures. J Neurosurg Spine. 2014;20:497–504. doi: 10.3171/2014.1.SPINE13246. [DOI] [PubMed] [Google Scholar]
- 14.Thongtrangan I, Balabhadra RS, Le H, Park J, Kim DH. Vertebral body replacement with an expandable cage for reconstruction after spinal tumor resection. Neurosurg Focus. 2003;15:E8. doi: 10.3171/foc.2003.15.5.8. [DOI] [PubMed] [Google Scholar]
- 15.Verlaan JJ, Diekerhof CH, Buskens E, van der Tweel I, Verbout AJ, Dhert WJ, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: A systematic review of the literature on techniques, complications, and outcome. Spine. 2004;29:803–14. doi: 10.1097/01.brs.0000116990.31984.a9. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Liu P. Analysis of surgical approaches for unstable thoracolumbar burst fracture: Minimum of five year follow-up. J Pak Med Assoc. 2015;65:201–5. [PubMed] [Google Scholar]
- 17.Wood K, Buttermann G, Mehbod A, Garvey T, Jhanjee R, Sechriest V. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85-A:773–81. doi: 10.2106/00004623-200305000-00001. [DOI] [PubMed] [Google Scholar]


