Abstract
This study aimed to evaluate the associations of serum ferritin with insulin resistance indices, body fat mass/percentage, and all the components of metabolic syndrome (MetS), as well as the risk for MetS according to serum ferritin levels in Korean adolescents and adults.
A total of 15,963 Korean males and females aged 16 to 80 years were analyzed using data from the Korean National Health and Nutrition Examination Survey, 2005 to 2011.
The median serum ferritin concentration was 98.82 ng/mL for males and 38.60 ng/mL for females (P < 0.001). Increased risks of greater waist circumference and elevated glucose levels, elevated triglyceride levels, and reduced high-density lipoprotein cholesterol levels were noted across the serum ferritin quartiles after adjustment for confounders in both genders (P ≤ 0.012 for trend). Insulin resistance indices and abdominal obesity (trunk fat mass/percent) increased across the ferritin concentration quartiles after adjustment for confounders in males and females (P ≤ 0.011 for trend), and the risk of MetS increased across the ferritin quartiles in males (P < 0.001 for trend) and females (P = 0.001 for trend). The highest serum ferritin quartile exhibited a 1.62-fold increased risk of MetS (95% CI, 1.28–2.12) in males and a 1.36-fold increased risk of MetS (95% CI, 1.09–1.69) in females compared with the lowest quartile after adjustment for confounders.
Our results suggest that ferritin is associated with insulin resistance and abdominal obesity.
Keywords: abdominal obesity, ferritin, insulin resistance, metabolic syndrome
1. Introduction
The prevalence of metabolic syndrome (MetS) is 31.4% in male adults and 27.1% in female adults in the USA, according to the National Cholesterol Education Program-Adult Treatment Panel-III,[1] and is 26.6% in men and 21.3% in women, according to the results of a recent Korean study.[2] MetS is related to cardiovascular disease, cerebrovascular disease (CVD), kidney disease, and type 2 diabetes mellitus (T2DM).[3] There are multiple definitions that can be used to determine whether MetS is present. The International Diabetes Federation (IDF) proposed the IDF Consensus Worldwide Definition of MetS as a universally accepted tool for diagnosing individuals with MetS.[4] The IDF recommended that adolescents aged 16 years or older should be treated in accordance with the worldwide IDF definition for adults.[5] However, the primarily studied population comprised adults, and reports in which adolescents and adults were included in the same study population are relatively scarce.
Ferritin, a ubiquitously distributed protein, is essential for regulating iron homeostasis, and serum ferritin concentrations are used as a biomarker for estimating body iron status in clinical settings. Excessive iron accumulation can be responsible for pathologic conditions, such as hemochromatosis and hemosiderosis.[6] In addition, a study reported that moderately elevated body iron concentrations may be associated with a high risk of MetS in US adults.[7] A series of studies demonstrated that elevated serum ferritin concentrations are related to obesity,[8] T2DM,[9] insulin resistance,[10] cardiovascular disease (CVD),[11] MetS, and MetS components.[12,13] Although there are studies investigating the association between serum ferritin and obesity, there are few reports evaluating the association between serum ferritin and body fat mass measured using dual-energy X-ray absorptiometry (DXA).
In the present study, we aimed to evaluate the association between serum ferritin and insulin resistance indices, body fat mass/percentage, and all the components of MetS in Korean adolescents and adults aged 16 to 80 years from a nationwide survey. In addition, we assessed the risk of MetS and its components according to serum ferritin concentrations.
2. Methods
2.1. Subjects
Data from the Korean National Health and Nutrition Examination Survey (KNHANES) from 2008 to 2011 were analyzed in the present study. The KNHANES is a cross-sectional, nation-wide, and representative survey with a multistage and stratified sampling design. The survey is composed of 3 parts, that is, a health interview survey, a health examination survey, and a nutritional survey, and was conducted by the Division of Chronic Disease Surveillance, Korea Centers for Disease Control and Prevention.[14] Additional details regarding the study design and method are provided elsewhere.[15] Of the 37,753 randomly selected individuals who participated in the KNHANES from 2008 to 2011, 28,965 subjects were aged 16 to 80 years. To avoid biasing the results, we excluded subjects with incomplete significant analytical data (n = 13,002). A total of 15,963 Korean adolescent and adults were ultimately included in this study. The database is available to the public at the KNHANES website (http://knhanes.cdc.go.kr).[15] Informed consent was provided by all participants included in the KNHANES.
2.2. Measurements
Height and body weight were measured to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated as weight (kg)/square of height (m2). Waist circumference (WC) was assessed in the horizontal plane at a level midway between the lower rib margin and the iliac crest to the nearest 0.1 cm after normal expiration. Systolic blood pressure (SBP, mm Hg) and diastolic blood pressure (DBP, mm Hg) were assessed 3 times in the right upper arm using a calibrated sphygmomanometer (Baumanometer, Baum, Copiague, NY) with an appropriately sized cuff while the individual was in a seated position after having rested for at least 5 minutes. All BP assessments were taken 2 minutes apart. Then, the mean of the values of the 2nd and 3rd blood pressure measurements was used for analysis.
Blood samples were collected from the antecubital vein after fasting for ≥10 hours year-round. The obtained blood samples were immediately processed, refrigerated, and transported to a central laboratory (NeoDin Medical Institute, Seoul Korea). Serum ferritin and insulin were measured by immuno-radiometric assay using a 1470 Wizard Gamma Counter (Perkin-Elmer, Turku, Finland). Serum fasting glucose (mg/dL), total cholesterol (mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), and triglyceride (TG, mg/dL) concentrations were measured by an enzymatic method using a Hitachi 7600 automatic analyzer (Hitachi; Tokyo, Japan). Hemoglobin (Hb, g/dL) was assessed by the SLS hemoglobin method (NoCyanide) using a Sysmex XE-2100D (Sysmex, Hyogo, Japan).
Whole-body DXA examinations were conducted using a QDR Discovery fan beam densitometer (Hologic, Inc., Bedford, MA). The DXA results were analyzed using Hologic Discovery software (version 13.1). Examinations that revealed the presence of items that could affect the accuracy of the DXA results, such as prosthetic devices, implants, or other extraneous objects, were recorded as missing in the datasets for the regional and global DXA results. The KNHANES provided all DXA data, including whole-body DXA measurements of bone mineral content (g); bone mineral density (g/cm2); fat mass (g); lean mass, including bone mineral content (g); and fat percentage (fat mass/total mass × 100), according to individualized demographic information. The head, arms, legs, trunk, pelvic region, subtotal body (excluding only the head), and whole body were also included as data for several predefined anatomical regions. In the current study, whole-body fat (WBF) mass/percentage and trunk fat (TF) mass/percentage were used in the analyses.
2.3. Collection of data for lifestyle-related and metabolic parameters
Smoking, alcohol intake, and physical activity were included in this study as lifestyle-related parameters. Smokers were defined as individuals who currently smoked or who had smoked ≥5 packs in their lifetime and were divided into the following 2 groups: yes and no. Alcohol intake was defined as drinking ≥2 alcoholic beverages/month during the previous year and was divided into the following 2 groups: yes and no. Physical activity was defined as meeting ≥1 of the following 3 criteria: intense physical activity for 20 minutes for ≥3 days/week, moderate physical activity for 30 minutes for ≥5 days/week, or walking for 30 minutes for ≥5 days/week. Physical activity was also divided into the following 2 groups: yes and no. Education and household income were included as socio-demographic characteristics. Household income was reported in quartiles and was categorized into the following 2 groups: lowest quartile versus ≥2nd quartile. Education level was divided into the following 2 categories: ≤elementary school or ≥elementary school. Residence area was divided into the following 2 categories: rural or city.
Hypertension (HTN) was defined as an SBP ≥140 mm Hg, a DBP ≥90 mm Hg, or current antihypertensive drug use. The presences of dyslipidemia, CVD, and coronary heart disease (CHD) were assessed using a self-reported questionnaire. The response options included yes versus no or present medication use for the indicated diseases. Diabetes mellitus (DM), which included both type 1 DM and T2DM, was diagnosed in individuals who fell into ≥1 of the following 4 categories: individuals who self-reported their disease using a questionnaire comprising questions with yes versus no answers, individuals currently using medications for T2DM, individuals currently receiving insulin to manage type 1 DM or T2DM, or individuals with a fasting glucose ≥126 mg/dL during the national survey.
Dietary intake of nutrients, including total intake, total energy intake, total protein, total fat, and total carbohydrates, was assessed by a trained nutritionist using 24-hour recall. The data regarding general participant characteristics were obtained from the KNHANES.
2.4. Definition of insulin resistance indices, MetS, and its components
Insulin resistance was determined by the homeostasis model assessment for insulin resistance (HOMA-IR)[16] using fasting glucose and insulin. The HOMA-IR was calculated using the following equation:
HOMA-IR = (fasting insulin [μU/mL] × fasting glucose [mmol/L])/22.5
MetS was defined according to the IDF definition for Asia.[17] In this study, MetS was diagnosed according to the presence of abdominal obesity (WC ≥90 cm in men or ≥80 cm in women) and the fulfillment of ≥2/4 of the following criteria: an SBP ≥130 mm Hg, a DBP ≥85 mm Hg, or treatment with antihypertensive medications; a fasting glucose ≥100 mg/dL or a previous diagnosis of T2DM; an HDL-C < 40 mg/dL in men or <50 mg/dL in women; or a TG level ≥150 mg/dL.
2.5. Statistical analyses
All analyses were conducted using SPSS software for Windows (SPSS version 22.0, IBM SPSS Inc., Chicago, IL). Normally distributed variables are presented as means ± standard errors, while categorical variables are presented as percentages (%). Differences in categorical variables and normally distributed variables were analyzed using chi-square tests and analysis of variance, respectively. Analysis of covariance (ANCOVA) was used to evaluate the associations between gender-specific serum ferritin concentrations and MetS components, insulin resistance indices, and body fat assessed by DXA after adjustment for the independent factors related to serum ferritin, such as age, BMI, white blood cell (WBC) count, HTN, dyslipidemia, CVD, CHD, diabetes, residence area, smoking, alcohol intake, physical activity, education level, total intake, total energy intake, protein intake, fat intake, and carbohydrate intake, in both genders. To investigate the associations between gender-specific serum ferritin concentrations and MetS and its components, stepwise multivariate logistic regression analysis was performed after controlling for the previously described confounders, and the corresponding odds ratios (ORs) and 95% confidence intervals (95% CIs) were determined. The ORs for MetS components and MetS were determined by serum ferritin quartiles, with the lowest quartile serving as a reference. Trends across quartiles were assessed for each serum ferritin quartile as continuous variables in the multivariate logistic regression models. All significances were analyzed using a 2-tailed method, and a P value < 0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics of the study population
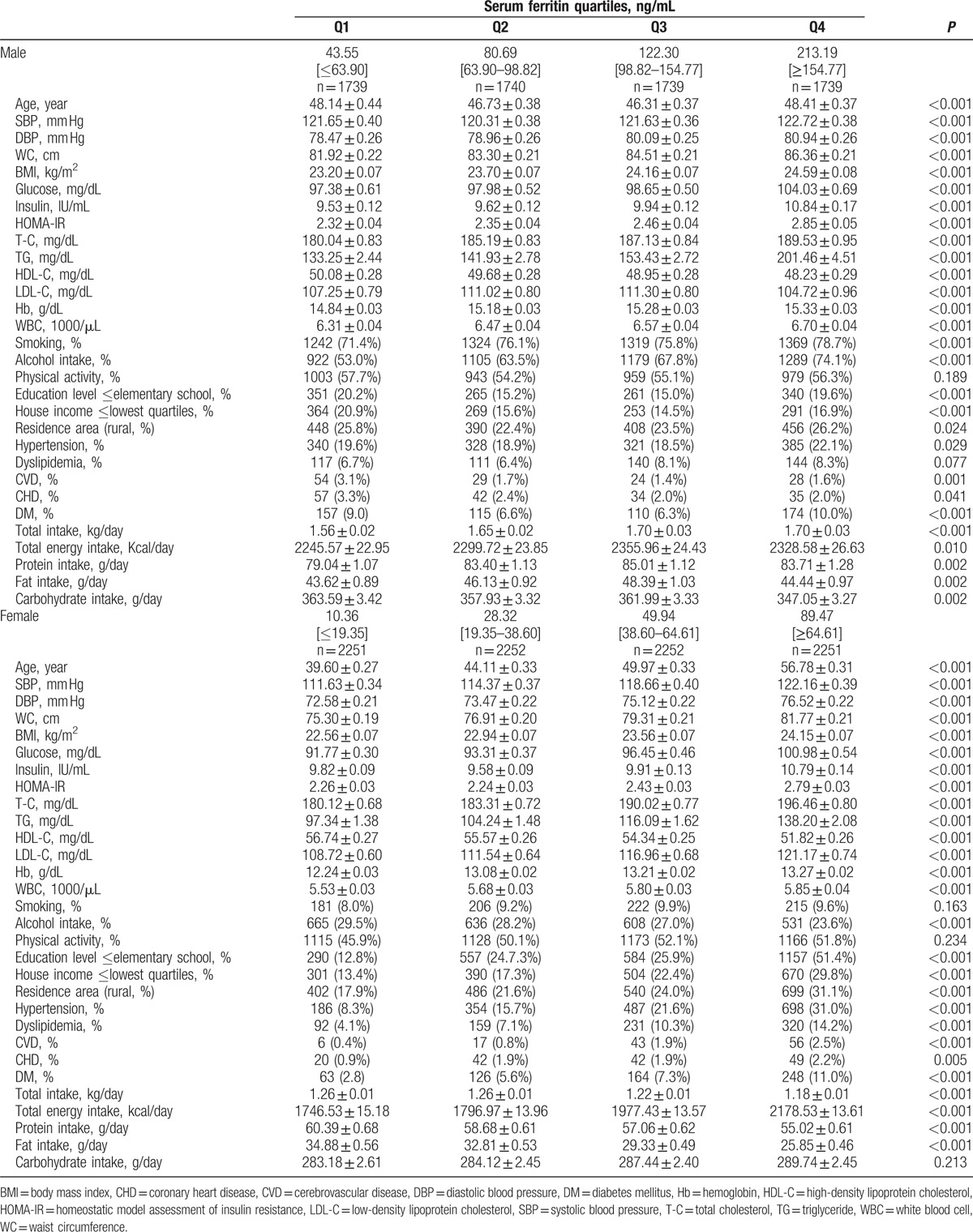
The mean age of the study participants was 47.4 years for men and 47.61 years for women (Table 1). There was a significant difference in serum ferritin concentrations between male and female participants. The median serum ferritin concentration was 122.30 ng/mL for males and 64.61 ng/mL for females (P < 0.001). The median serum ferritin concentrations were 43.55 ng/mL (2.60–63.90 ng/mL) for males and 10.36 ng/mL (0.68–19.35 ng/mL) for females in the lowest quartile, 80.69 ng/mL (63.90–98.82 ng/mL) for males and 28.32 ng/mL (19.35–38.60 ng/mL) for females in the 2nd quartile, 122.30 ng/mL (98.82–154.77 ng/mL) for males and 49.94 ng/mL (38.60–64.61 ng/mL) for females in the 3rd quartile, and 213.19 ng/mL (104.64–6241 ng/mL) for males and 89.47 ng/mL (64.61–2403.00 ng/mL) for females in the highest quartile (P < 0.001). The clinical characteristics of the study population are shown in Table 1. The subjects in the highest serum ferritin quartiles tended to be older and to have increased SBPs, DBPs, WCs, BMIs, glucose levels, insulin levels, HOMA-IRs, total cholesterol levels, TG levels, hemoglobin levels, and WBC counts (P < 0.001) among both male and female participants. Participants in higher serum ferritin quartiles were more likely to have a rural residence (P ≤ 0.024) and were associated with higher prevalences of HTN, dyslipidemia, CVD, CHD, and DM (P ≤ 0.029) in both genders. Participants in higher quartiles were also more likely to have a higher total intake, total energy intake, protein intake, and fat intake than participants in lower quartiles (P ≤ 0.010). Lower serum ferritin quartiles were related to higher mean HDL-C levels in male and female participants (P < 0.001).
Table 1.
Clinical characteristics of the study population according gender-specific serum ferritin concentrations in Korean male (n = 6957) and female (n = 9006) aged 16 years or older.
3.2. Adjusted associations between serum ferritin and metabolic syndrome components, insulin resistance, and body fat
The associations between gender-specific serum ferritin concentrations and MetS components, insulin resistance, and body fat were calculated using ANCOVA after controlling for possible confounding factors, including age, BMI, WBC, HTN, dyslipidemia, CVD, CHD, DM, residence area, smoking, alcohol intake, physical activity, education level, total intake, total energy intake, protein intake, fat intake, and carbohydrate intake, in males and females (Table 2). The adjusted associations between serum ferritin and MetS components, insulin resistance, and body fat are shown in Table 2. There was a significant inverse association between serum ferritin concentrations and SBP in male and female participants (P ≤ 0.010 for trend). SBP was significantly lower in the 2nd serum ferritin quartile than in the lowest serum ferritin quartile (P = 0.002) in males and females without HTN (P < 0.001). SBP was significantly lower in the 3rd quartile than in the lowest quartile in females without HTN (P < 0.001). Additionally, males with HTN, females with HTN, and females without HTN exhibited a lower SBP in the highest quartile than in the lowest quartile (P = 0.007, P < 0.001, and P = 0.013, respectively). A significant inverse association was noted between serum ferritin concentrations and DBP in females without HTN (P < 0.001 for trend). Females without HTN exhibited a lower DBP in the 2nd quartile, 3rd quartile, and highest quartile than in the lowest quartile (P < 0.001). A significant positive linear association was observed between serum ferritin concentrations and WC (P < 0.001 for trend in males and P = 0.003 for trend in females). WCs were significantly higher in the highest serum ferritin quartile than in the lowest (P < 0.001), 2nd (P = 0.001), and 3rd quartiles (P = 0.002) in males and were significantly higher in the highest quartile than in the lowest (P = 0.002) and 2nd quartiles (P = 0.029) in females. There was a significant positive relationship between serum ferritin concentrations and glucose (P < 0.001 for trend in both genders). The highest quartile had significantly higher glucose levels than the lowest (P < 0.001), 2nd (P = 0.005), and 3rd serum ferritin quartiles (P = 0.001) among males and significantly higher glucose levels than the lowest (P < 0.001), 2nd (P < 0.001), and 3rd quartiles (P = 0.027) among females. A significant inverse linear association between serum ferritin and HDL-C was noted (P = 0.012 for trend in males and <0.001 for trend in females). The estimated mean HDL-C was 1.31 mg/dL higher in the lowest quartile than in the highest quartile (P = 0.011) in males, and the estimated mean HDL-C was 2.46 mg/dL higher in the lowest quartile than in the highest quartile (P < 0.001) in females. There was a positive linear relationship between serum ferritin concentrations and TG concentrations (P < 0.001 for trend in both genders). The highest quartile had a significantly higher TG concentration than the lowest (P < 0.001), 2nd (P < 0.001), and 3rd quartiles (P < 0.001) in both male and female participants.
Table 2.
Association of gender-specific serum ferritin concentration with insulin resistance and body fat in Korean male (n = 6957) and female (n = 9006) aged 16 years or older.
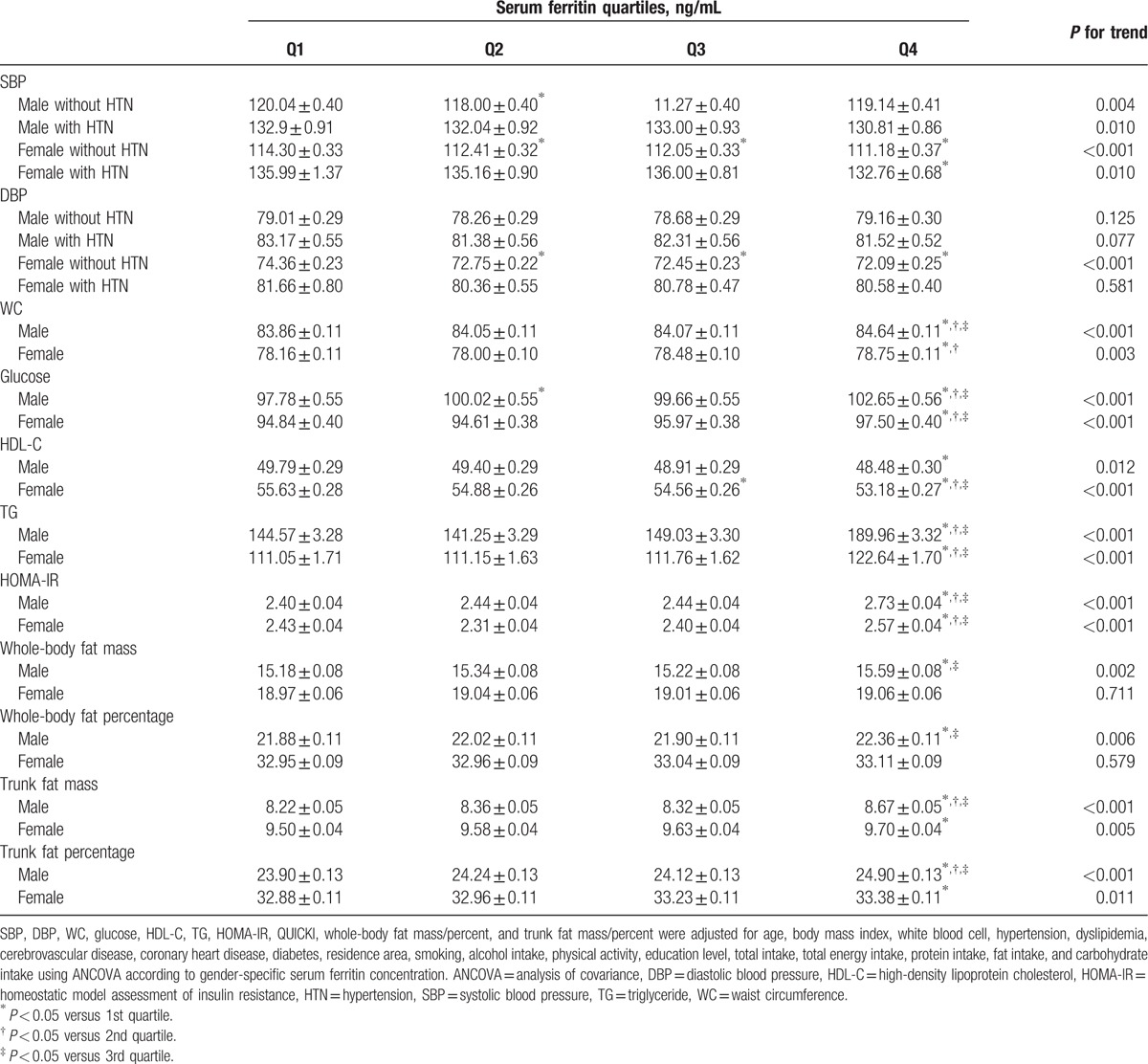
A significant positive linear association was observed between serum ferritin concentrations and insulin resistance indices (P < 0.001 for trend in HOMA-IR). The HOMA-IR was significantly higher in the highest quartile than in the lowest (P < 0.001), 2nd (P < 0.001), and 3rd serum ferritin quartiles (P < 0.001) in males and was significantly higher in the highest quartile than in the 2nd (P < 0.001) and 3rd quartiles (P = 0.016) in females. There were significantly positive associations between serum ferritin concentrations and WBF mass (P = 0.002 for trend) and WBF percentage (P = 0.006 for trend) in males, but not in females (P = 0.711 for trend in WBF mass and P = 0.579 for trend in WBF percentage). WBF mass and percentage in males were significantly higher in the highest quartile than in the lowest (P = 0.002 for WBF mass, P = 0.010 for WBF percentage) and 3rd quartiles (P = 0.008 for WBF mass, P = 0.015 for WBF percentage). In particular, TF mass and percentage were significantly positively associated with serum ferritin concentrations (TF mass, P < 0.001 for trend in males and P = 0.005 for trend in females; TF percent, P < 0.001 for trend in males and P = 0.011 for trend in females). TF mass and percentage were significantly higher in the highest quartile than in lowest (P < 0.001 for TF mass and percentage), 2nd (P < 0.001 for TF mass and P = 0.002 for TF percentage), and 3rd serum ferritin quartiles (P < 0.001 for TF mass and percentage) in males, and TF mass and percentage were significantly higher in the highest quartile than in the lowest quartile (P = 0.003 for TBF mass and P = 0.017 for TBF percentage) in females.
3.3. Adjusted ORs for MetS and its components, according to serum ferritin concentrations
The prevalence of MetS was 17.6% in male participants and 22.8% in female participants (Table 3). The prevalence of MetS in males according to ferritin concentrations was 12.1% in the lowest quartile, 15.1% in the 2nd quartile, 17.4% in the 3rd quartile, and 25.6% in the highest quartile (P < 0.001). The prevalence of MetS in females according to ferritin concentrations was 11.8% in the lowest quartile, 17.1% in the 2nd quartile, 24.9% in the 3rd quartile, and 37.6% in the highest quartile (P < 0.001). The risks of MetS components and MetS according to gender-specific serum ferritin quartiles were assessed using stepwise multivariate logistic regression analysis after adjustment for possible confounders, including age, BMI, WBC, HTN, dyslipidemia, CVD, CHD, DM, residence area, smoking, alcohol intake, physical activity, education level, total intake, total energy intake, protein intake, fat intake, and carbohydrate intake, in males and females. Table 3 shows the adjusted risks of MetS and its components according to serum ferritin concentrations.
Table 3.
Adjusted odds ratio (95% CI) of metabolic syndrome and its components according to gender-specific serum ferritin quartiles in Korean male (n = 6957) and female (n = 9006) aged 16 years or older.
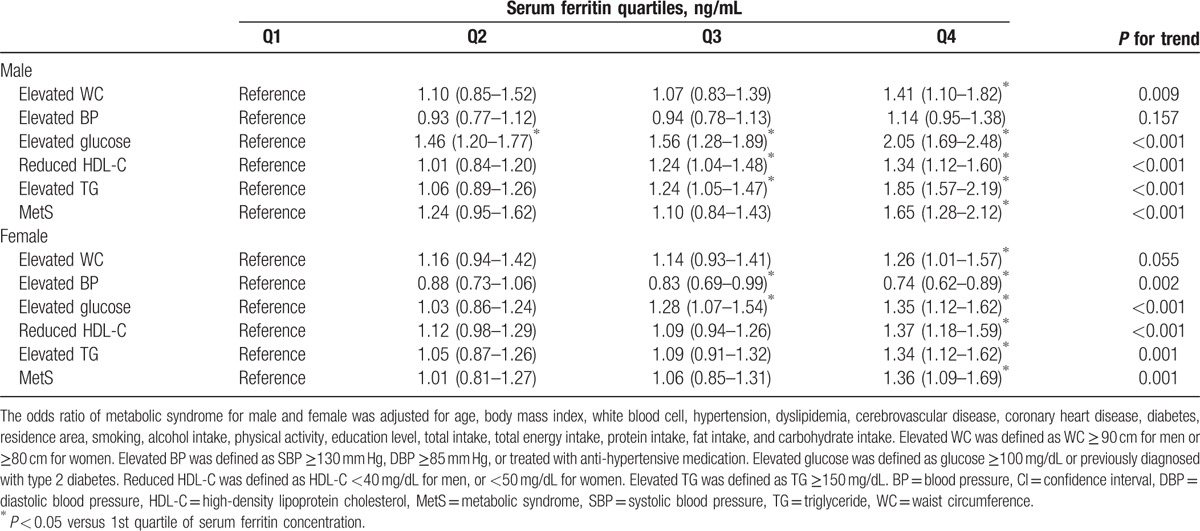
In men, the 2nd quartile had a 1.46-fold increased risk for an elevated glucose level (95% CI, 1.20–1.77) compared with the lowest quartile. The 3rd quartile showed a 1.56-fold increased risk for an elevated glucose level (95% CI, 1.28–1.89), a 1.24-fold increased risk for a reduced HDL-C level (95% CI, 1.04–1.48), and a 1.24-fold increased risk for an elevated TG level (95% CI, 1.05–1.47). There was a 1.41-fold increased risk for elevated WC (95% CI, 1.10–1.82), a 2.05-fold increased risk for an elevated glucose level (95% CI, 1.69–2.48), a 1.34-fold increased risk for a reduced HDL-C level (95% CI, 1.12–1.60), and a 1.85-fold increased risk for an elevated TG level (95% CI, 1.57–2.19) in the highest quartile compared with the lowest quartile. Accordingly, the highest quartile of serum ferritin showed a 1.65-fold increased risk for MetS compared with the lowest quartile (95% CI, 1.28–2.12) in men.
In women, the 3rd quartile had a 17% decreased risk for an elevated BP (95% CI, 0.69–0.99) and a 1.28-fold increased risk for an elevated glucose level (95% CI, 1.07–1.54). There was a 26% decreased risk for an elevated BP, whereas there was a 1.26-fold increased risk for an elevated WC (95% CI, 1.01–1.57), a 1.35-fold increased risk for an elevated glucose level (95% CI, 1.12–1.62), a 1.37-fold increased risk for a reduced HDL-C level (95% CI, 1.18–1.59), and a 1.34-fold increased risk for an elevated TG level (95% CI, 1.12–1.62) in the highest quartile compared with the lowest quartile. Finally, the highest quartile of serum ferritin was associated with a 1.36-fold increased risk of MetS compared with the lowest quartile (95% CI, 1.09–1.69) in females.
4. Discussion
In the current nationwide cross-sectional study, we noted a positive association between serum ferritin concentrations and MetS components, including WC, glucose, and TGs, and an inverse association between serum ferritin concentrations and HDL-C in Korean males and females aged 16 to 80 years after adjustment for possible confounders in covariance analyses. A positive association among serum ferritin concentrations and insulin resistance and trunk body fat mass/percentage determined using DXA was found in men and women after adjustment for possible confounders in ANCOVA. The highest serum ferritin quartile showed an increased risk for MetS compared with the lowest quartile in both genders after adjustment for confounders in multivariate logistic regression analyses.
Ferritin plays a key role in the regulation of body iron homeostasis and is used as a clinical marker for iron deficiency (ID) and hemochromatosis. It has also been demonstrated that ferritin is related to various metabolic diseases, such as obesity,[18] insulin resistance,[19–21] MetS,[19–21] and T2DM.[9] The pathophysiology underlying the relationship between ferritin and MetS is not fully understood. One possible explanation is that excessive iron, which can present as a high ferritin concentration, is related to insulin resistance, which plays a key role in MetS development. Iron is a transition metal that can convert into highly reactive moieties by catalyzing the formation of free radicals, which are associated with oxidative damage to cells and tissue.[22] Increased oxidative stress, which is defined as a persistent disproportion between the production of highly reactive oxidants and the production of antioxidants, is related to abnormal changes in intracellular signaling and gene expression and can lead to pathological conditions, such as insulin resistance.[23] However, the findings of a series of studies have suggested that high ferritin levels are the result of chronic inflammatory reactions, in addition to being the result of increased iron stores.[24,25] The recent finding that increased ferritin levels are related to nonalcoholic steatohepatitis rather than nonalcoholic fatty liver disease supports the notion that ferritin is closely related to chronic inflammation.[26] Various cytokines involved in chronic inflammation are associated with increased levels of NAD (P)H oxidase, which is an important source of free radicals.[27] Increased oxidative stress due to NAD (P)H can lead to activation of intracellular signal pathways that can produce cellular damage-related products.[27] In addition, a recent study demonstrated that ferritin is positively correlated with C-reactive protein (CRP). CRP can be used as a surrogate for inflammation[28] and is positively associated with hepcidin, which can be elevated in inflammation.[29,30] The results of the present study, which indicated that ferritin was associated with insulin resistance after adjustment for confounding factors, are in line with the findings of previous studies.
The association between obesity and iron status that was assessed using ferritin concentrations in usual clinical settings is controversial. In some reports, ID is associated with obesity.[31,32] However, a few reports suggest that iron overload is associated with obesity.[33,34] This controversy may result from differences in the methods used to determine iron status. A recent meta-analysis suggested that the use of a ferritin-based diagnosis of ID is more likely to lead to the conclusion that obesity is not associated with ID development or may even play a protective role with respect to ID development.[35] In this analysis, a significant correlation between ID and obesity was noted in studies without a ferritin-based diagnosis.[35] There are a few studies demonstrating that elevated ferritin concentrations are associated with obesity. A US study demonstrated that ferritin is associated with various body fat distribution and obesity indices.[36] Ferritin was positively independently correlated with visceral fat area and subcutaneous fat area.[37] A Chinese study reported that ferritin is positively associated with total body fat and TF mass.[38] In that study, ferritin levels were positively correlated with TF mass but were inversely correlated with leg fat.[38] A recent prospective cohort study in Korea demonstrated that elevated serum ferritin levels may be a predictive factor for obesity in nonobese men during a 5-year follow-up period.[18] In the present study, total body fat mass/percentage and TF mass/percentage, which were assessed using DXA, were used to evaluate the relationship between ferritin and obesity. Total body fat mass/percentage was significantly associated with ferritin in males, but not in females, while trunk body fat mass/percentage was significantly associated with ferritin in both men and women. Our results, namely, our finding that ferritin is associated with obesity and abdominal obesity, in particular, correspond with those of previous reports.
Unexpectedly, we found that ferritin was significantly inversely associated with SBP in Korean males and females aged 16 to 80 years, regardless of HTN, after adjusting for multiple variables and that ferritin was significantly inversely associated with DBP in women without HTN. Specifically, we noted an inverse association between ferritin and SBP and DBP in women with no HTN. The relationship between ferritin and BP is controversial. Ferritin levels were significantly positively correlated with SBP in healthy individuals within the 2 highest quartiles of serum ferritin but were not correlated with SPB in individuals within the 2 lowest quartiles in a Spanish study.[39] In a Korean prospective study, ferritin was a significant predictor of HTN development in middle-aged Korean men during a 4-year follow-up period.[40] However, most studies have demonstrated that ferritin is not significantly associated with elevated BP as a component of MetS.[41,42,43] Moreover, Belgian men with T2DM and serum ferritin in the highest serum ferritin quartile did not present with a higher BP than those in the 3 lowest serum ferritin quartiles.[44] Rather, men with T2DM within the highest serum ferritin quartiles exhibited a decreased prevalence of macroangiopathy compared with those with the 3 lowest serum ferritin quartiles in their study.[44] These discrepancies may be associated with the mechanisms underlying the relationship between ferritin and BP. The possible impact of ferritin on BP is not fully understood. One possible explanation for the impact of ferritin on BP is that the relationship between the 2 parameters may be mediated by insulin resistance, as opposed to ferritin exerting a direct influence on BP.[40] Nevertheless, it is notable that the highest serum ferritin quartile exhibited an increased risk of 36% for MetS in women, while the highest serum ferritin quartile had a decreased risk of 26% for elevated BP as a component of MetS in the current study.
This study had potential limitations. First, this study was conducted using a cross-sectional design; thus, causality could not be determined, although possible mechanisms underlying the relationship between iron and MetS, such as insulin resistance mediated by iron overload and chronic low-grade inflammation, were described. Second, multivitamin or iron intake amounts were not assessed in this nationwide, population-based study. We could not rule out the possibility that the results were influenced by vitamins or iron supplement intake. Third because data pertaining to physical activity were collected using self-reporting methods in KNHANES, we could not utilize objectively measured data in our study. This may have affected the level of accuracy of the data, potentially leading to recall bias and social desirability bias. However, this study was conducted on a nationwide basis, and physical activity was adjusted for as a confounder in ANCOVA and in the multivariate logistic regression analyses. Finally, serum ferritin can be classified as only 1 acute-phase reactant whose levels may be artificially elevated in the presence of an inflammatory reaction. Because we could not exclude confounding effects due to a failure to adjust for CRP protein, we made an effort to restrict the effects of inflammatory conditions, as we used WBC count as an independent variable in the covariance analysis and multivariate logistic regression model. However, a Chinese study demonstrated that there was little difference in the adjusted ORs for MetS, regardless of whether hs-CRP, which was used to control for inflammation, was adjusted for or not (4.08 vs 4.05 in men and 2.43 and 2.34 in women, respectively).[45]
In conclusion, elevated serum ferritin concentrations were associated with an increased risk of MetS components, such as elevated WCs, elevated glucose levels, reduced HDL-C levels, and elevated TG levels, in Korean adolescents and adults aged 16 to 80 years. Serum ferritin concentrations were also related to insulin resistance indices and abdominal obesity assessed by DXA. Higher serum ferritin levels were associated with a higher risk of MetS in males and females, although higher serum ferritin concentrations were associated with a decreased risk of elevated BP in females. These findings suggest that ferritin is associated with insulin resistance and abdominal obesity.
Footnotes
Abbreviations: ANCOVA = analysis of covariance, BMI = body mass index, CHD = coronary heart disease, CI = confidence interval, CRP = C-reactive protein, CVD = cerebrovascular disease, DBP = diastolic blood pressure, DXA = dual-energy X-ray absorptiometry, HDL-C = high-density lipoprotein cholesterol, HOMA-IR = homeostasis model assessment for insulin resistance, HTN = hypertension, ID = iron deficiency, IDF = International Diabetes Federation, KNHANES = Korean National Health and Nutrition Examination Survey, MetS = metabolic syndrome, OR = odds ratio, SBP = systolic blood pressure, T2DM = type 2 diabetes mellitus, TF = trunk fat, TG = triglyceride, WBC = white blood cell, WBF = whole-body fat, WC = waist circumference.
Authorship: All authors were involved in drafting the article and revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. ITH has full access to all of the data pertaining to the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SYS and JPL participated in the writing of the paper. SYS, MJK, YJO, JWB, SY, and ITH participated in the research design. SYS and SY provided expert advice regarding the statistical design. SYS, MJK, YJO, JWB, SY, and ITH participated in the analysis and interpretation of the data. SYS, MJK, YJO, and JWB participated in the data collection.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999-2006. Diabetes Care 2011;34:216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang JJ, Yoon HS, Lee SA, et al. Metabolic syndrome and sex-specific socio-economic disparities in childhood and adulthood: the Korea National Health and Nutrition Examination Surveys. Diabet Med 2014;31:1399–409. [DOI] [PubMed] [Google Scholar]
- [3].Beltran-Sanchez H, Harhay MO, Harhay MM, et al. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol 2013;62:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome, http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf; 2006. [Accessed April 10, 2016]. [Google Scholar]
- [5].International Diabetes Federation. The IDF consensus definition of the metabolic syndrome in children and adolescents, http://www.idf.org/webdata/docs/Mets_definition_children.pdf; 2007. [Accessed April 10, 2016] [Google Scholar]
- [6].Heeney MM, Andrews NC. Iron homeostasis and inherited iron overload disorders: an overview. Hematol Oncol Clin North Am 2004;18: 1379-1403, ix. [DOI] [PubMed] [Google Scholar]
- [7].Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care 2004;27:2422–8. [DOI] [PubMed] [Google Scholar]
- [8].Jeon YJ, Jung IA, Kim SH, et al. Serum ferritin level is higher in male adolescents with obesity: results from the Korean National Health and Nutrition Examination Survey 2010. Ann Pediatr Endocrinol Metab 2013;18:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sun L, Franco OH, Hu FB, et al. Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly Chinese. J Clin Endocrinol Metab 2008;93:4690–6. [DOI] [PubMed] [Google Scholar]
- [10].Kim CH, Kim HK, Bae SJ, et al. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism 2011;60:414–20. [DOI] [PubMed] [Google Scholar]
- [11].Olesnevich ME, Fanelli Kuczmarski M, Mason M, et al. Serum ferritin levels associated with increased risk for developing CHD in a low-income urban population. Public Health Nutr 2012;15:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park SK, Ryoo JH, Kim MG, et al. Association of serum ferritin and the development of metabolic syndrome in middle-aged Korean men: a 5-year follow-up study. Diabetes Care 2012;35:2521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim JW, Kim DH, Roh YK, et al. Serum ferritin levels are positively associated with metabolically obese normal weight: a nationwide population-based study. Medicine (Baltimore) 2015;94:e2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yi KH, Hwang JS, Kim EY, et al. Prevalence of insulin resistance and cardiometabolic risk in Korean children and adolescents: a population-based study. Diabetes Res Clin Pract 2014;103:106–13. [DOI] [PubMed] [Google Scholar]
- [15].Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- [17].Alberti KG, Zimmet P, Shaw J. The metabolic syndrome – a new worldwide definition. Lancet 2005;366:1059–62. [DOI] [PubMed] [Google Scholar]
- [18].Park SK, Choi WJ, Oh CM, et al. Association between serum ferritin levels and the incidence of obesity in Korean men: a prospective cohort study. Endocr J 2014;61:215–24. [DOI] [PubMed] [Google Scholar]
- [19].Wrede CE, Buettner R, Bollheimer LC, et al. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur J Endocrinol 2006;154:333–40. [DOI] [PubMed] [Google Scholar]
- [20].Chen L, Li Y, Zhang F, et al. Association of serum ferritin levels with metabolic syndrome and insulin resistance in a Chinese population. J Diabetes Complications 2016. [DOI] [PubMed] [Google Scholar]
- [21].Padwal MK, Murshid M, Nirmale P, et al. Association of serum ferritin levels with metabolic syndrome and insulin resistance. J Clin Diagn Res 2015;9:BC11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med 2002;33:457–63. [DOI] [PubMed] [Google Scholar]
- [23].Guo H, Ling W. The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives. Rev Endocr Metab Disord 2015;16:1–3. [DOI] [PubMed] [Google Scholar]
- [24].Zafon C, Lecube A, Simo R. Iron in obesity. An ancient micronutrient for a modern disease. Obes Rev 2010;11:322–8. [DOI] [PubMed] [Google Scholar]
- [25].Esser N, Legrand-Poels S, Piette J, et al. [NLRP3 inflammasome and visceral adipose tissue]. Rev Med Liege 2014;69:57–61. [PubMed] [Google Scholar]
- [26].Manousou P, Kalambokis G, Grillo F, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int 2011;31:730–9. [DOI] [PubMed] [Google Scholar]
- [27].Lin N, Zhang H, Su Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab 2012;38:250–7. [DOI] [PubMed] [Google Scholar]
- [28].Gonzalez AS, Guerrero DB, Soto MB, et al. Metabolic syndrome, insulin resistance and the inflammation markers C-reactive protein and ferritin. Eur J Clin Nutr 2006;60:802–9. [DOI] [PubMed] [Google Scholar]
- [29].Nemeth E, Valore EV, Territo M, et al. a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003;101:2461–3. [DOI] [PubMed] [Google Scholar]
- [30].Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? A population-based study. Obesity (Silver Spring) 2008;16:2356–61. [DOI] [PubMed] [Google Scholar]
- [31].Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, et al. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr 2011;93:975–83. [DOI] [PubMed] [Google Scholar]
- [32].Siddique A, Nelson JE, Aouizerat B, et al. Iron deficiency in patients with nonalcoholic Fatty liver disease is associated with obesity, female gender, and low serum hepcidin. Clin Gastroenterol Hepatol 2014;12:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kordas K, Fonseca Centeno ZY, Pachon H, et al. Being overweight or obese is associated with lower prevalence of anemia among Colombian women of reproductive age. J Nutr 2013;143:175–81. [DOI] [PubMed] [Google Scholar]
- [34].Karl JP, Lieberman HR, Cable SJ, et al. Poor iron status is not associated with overweight or overfat in non-obese pre-menopausal women. J Am Coll Nutr 2009;28:37–42. [DOI] [PubMed] [Google Scholar]
- [35].Zhao L, Zhang X, Shen Y, et al. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev 2015;16:1081–93. [DOI] [PubMed] [Google Scholar]
- [36].Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men – the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord 2001;25:639–45. [DOI] [PubMed] [Google Scholar]
- [37].Iwasaki T, Nakajima A, Yoneda M, et al. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care 2005;28:2486–91. [DOI] [PubMed] [Google Scholar]
- [38].Wu H, Qi Q, Yu Z, et al. Opposite associations of trunk and leg fat depots with plasma ferritin levels in middle-aged and older Chinese men and women. PLoS One 2010;5:e13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fernandez-Real JM, Ricart-Engel W, Arroyo E, et al. Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care 1998;21:62–8. [DOI] [PubMed] [Google Scholar]
- [40].Kim MK, Baek KH, Song KH, et al. Increased serum ferritin predicts the development of hypertension among middle-aged men. Am J Hypertens 2012;25:492–7. [DOI] [PubMed] [Google Scholar]
- [41].Yoo KD, Ko SH, Park JE, et al. High serum ferritin levels are associated with metabolic risk factors in non-obese Korean young adults: Korean National Health and Nutrition Examination Survey (KNHANES) IV. Clin Endocrinol (Oxf) 2012;77:233–40. [DOI] [PubMed] [Google Scholar]
- [42].Tang Q, Liu Z, Tang Y, et al. High serum ferritin level is an independent risk factor for metabolic syndrome in a Chinese male cohort population. Diabetol Metab Syndr 2015;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee BK, Kim Y, Kim YI. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism 2011;60:1416–24. [DOI] [PubMed] [Google Scholar]
- [44].Hermans MP, Ahn SA, Amoussou-Guenou KD, et al. Do high ferritin levels confer lower cardiovascular risk in men with Type 2 diabetes? Diabet Med 2010;27:417–22. [DOI] [PubMed] [Google Scholar]
- [45].Li J, Wang R, Luo D, et al. Association between serum ferritin levels and risk of the metabolic syndrome in Chinese adults: a population study. PLoS One 2013;8:e74168. [DOI] [PMC free article] [PubMed] [Google Scholar]


