Abstract
The united airways concept suggests that patients with asthma typically exhibit parallel inflammation in the upper airway. The resulting nasal symptoms should reduce quality of life and substantially affect the evaluation of asthma control among these patients. This study aimed to assess the association of nasal symptoms with the evaluation of asthma control.
Fifty-eight patients with asthma and persistent nasal symptoms were prospectively recruited for evaluations of their sinonasal symptoms and asthma control in a cross-sectional study from August 2013 to June 2016. Participants underwent thorough nasal endoscopy, sinus computed tomography, pulmonary function testing, the asthma control test (ACT), and the Sino-Nasal Outcome Test-22 (SNOT-22) questionnaires to evaluate their asthma control and sinonasal symptoms.
There was a significant association between ACT and SNOT-22 scores. Among patients with asthma and chronic rhinosinusitis, ACT scores were closely related to the symptoms of cough, post-nasal discharge, dizziness, waking up at night, absence of a good night's sleep, and waking up tired. Among patients with asthma and chronic rhinitis, the forced expiratory volume in 1 second was closely related to the symptoms of needing to blow nose, runny nose, and cough. Patients with emergency clinic visits during the previous 3 months had relatively high SNOT-22 scores, especially for the symptoms of sneezing, runny nose, nasal blockage, cough, and dizziness.
Sinonasal symptom severity was closely associated with measured asthma control status among patients with asthma and persistent nasal symptoms. Therefore, upper and lower airway inflammations should be considered and treated simultaneously.
Keywords: airway, allergy, asthma, chronic rhinitis, chronic rhinosinusitis
1. Introduction
Asthma is a common, chronic, and inflammatory disease of the lower airway, which currently affects 235 million people throughout the world.[1] The global prevalence of asthma and its related complications have created significant burdens on patients, employers, and socioeconomic systems.[2] During recent decades, disease status has been evaluated using patient-reported outcomes, such as the patient's self-perceived experience of their symptoms and the effects of their illness.[3,4] Thus, the concept of asthma control has replaced the concept of asthma severity, and asthma control is now considered the main outcome during asthma treatment.[5] Furthermore, the Global Initiative for Asthma (GINA) Guidelines suggest that asthma control includes 2 domains: symptom control and future risk of adverse outcomes. In this context, periodic assessments of the patient's asthma control and risk factors are an essential part of asthma management5. The asthma control test (ACT) is one of the most commonly used instruments for evaluating asthma control, and this tool includes 4 symptom/relief questions and a self-assessment of asthma control.[6,7] A low forced expiratory volume in 1 second (FEV1) is the most important risk factor for poor asthma outcomes[5] and has been added to other tools for evaluating asthma control, such as the asthma control questionnaire.[8]
Patients with asthma have a higher incidence of allergic or nonallergic chronic rhinitis (CR)[9–11] and chronic rhinosinusitis (CRS).[11–13] Moreover, based on the united airways concept, these conditions exhibit associations with sinonasal inflammation and related nasal symptoms.[11,14] In this context, nasal symptoms are expected to reduce quality of life (QoL) and substantially affect the evaluation of asthma control among patients with asthma. However, to the best of our knowledge, no studies have investigated the effect of nasal symptoms on the evaluation of asthma control. Therefore, the present study aimed to assess the correlations between questionnaire-based nasal symptom scores and asthma control outcomes (e.g., the ACT score, FEV1, and acute exacerbation) among patients with asthma and persistent nasal symptoms.
2. Methods
2.1. Patients
Between August 2013 and June 2016, we prospectively recruited patients with asthma and persistent nasal symptoms who were being treated at our Thoracic Medicine Department. Figure 1 shows the study flow chart. The inclusion criteria were patients with asthma who fulfilled the diagnostic criteria from the GINA guidelines[5] and had failed 3 months of medical treatment for their nasal symptoms. Participants received initial medical therapy including: at least either 3 months course of topical corticosteroids or 1 week course of oral corticosteroids, and at least 2 weeks of culture-directed or broad spectrum antibiotics per the standard of care. The failed medical therapy was based upon persistent symptoms reported by participants and/or physical examination. All patients’ asthma had been treated based on the GINA guidelines for ≥ 6 months and the antiasthma medication was showed on Table 1. We excluded patients with major medical disorders, such as diabetes, nephrotic diseases, autoimmune disorders, immunodeficiency, malignancy, and other chronic illnesses. The institutional review board of Chang Gung Memorial Hospital approved this study's design ((IRB number: 103-7085B), and all patients provided their informed consent.
Figure 1.

Study flow chart. ACT = asthma control test, CR = chronic rhinitis, CRSsNP = chronic rhinosinusitis without nasal polyps, CRSwNP = chronic rhinosinusitis with nasal polyps, SNOT-22 = Sino-nasal Outcome Test-22.
Table 1.
Clinical characteristics of the study groups.

2.2. Objective and subjective measurements
All patients were referred to the Otolaryngology Department and underwent thorough nasal endoscopy and sinus computed tomography (CT). The findings from these examinations were interpreted using the Lund–Kennedy endoscopy score[15] and the Lund–Mackay CT score,[16] respectively. Pulmonary function testing includes the evaluation of forced vital capacity (FVC), FEV1, and the FEV1/FVC ratio. Percentages of the predicted values for these tests can be calculated according to age, sex, height, and ethnicity.[17] Participants completed the ACT and the Sino-Nasal Outcome Test-22 (SNOT-22) questionnaires[18] at the same time to evaluate their asthma control and sinonasal symptoms. Additional data regarding the patients’ clinical characteristics were collected from their medical records.
The ACT includes 5 items regarding asthma control: activity limitations, shortness of breath, waking up because of asthma symptoms, use of asthma relief medication, and a global evaluation of asthma control. The ACT items evaluate symptoms that were experienced during the last 4 weeks and are scored from 1 to 5, with a maximum score of 25 indicating perfectly controlled asthma.[7]
The SNOT-22 questionnaire is the most widely used and validated self-reported measure of nasal symptom severity and health-related QoL among patients with sinonasal conditions. The SNOT-22 questionnaire evaluates various symptoms, physical problems, functional limitations, and emotional consequences of having a sinonasal disorder.[18]
2.3. Diagnoses of chronic rhinosinusitis, chronic rhinitis, and nasal polyps
Cases of CRS were diagnosed using the criteria from the European position paper,[19] which requires the presence of 2 or more related symptoms for at least 12 weeks, as well as relevant endoscopic signs and/or CT findings. The related symptoms include facial pain/pressure, reduction or loss of smell, nasal blockage, or nasal discharge. At least one of the related symptoms should be nasal blockage/obstruction/congestion or nasal discharge. Cases of CR were defined as non-CRS cases with persistent nasal symptoms. Nasal polyps were identified using nasal endoscopy.
2.4. Statistical analyses
Data were reported as mean ± standard error or number (%), and were analyzed using GraphPad Prism software (version 5; GraphPad Prism Software Inc., San Diego, CA). Categorical variables were analyzed using the chi-square test or Fisher's exact test, as appropriate. Continuous variables were compared between 2 or 3 groups using the Mann–Whitney U test or the Kruskal–Wallis test, respectively. Correlations between 2 items were evaluated using Spearman's correlation coefficient. A P-value of < 0.05 was considered statistically significant.
3. Results
3.1. The participants’ clinical characteristics
Figure 2A shows the final diagnoses of the upper airway inflammation among patients with asthma and persistent nasal symptoms, based on their symptoms, endoscopy findings, and CT findings. Among the 58 participants, 30 patients had CRS (52%), which included 19 cases (33%) of CRS without nasal polyps (CRSsNP) and 11 cases (19%) of CRS with nasal polyps (CRSwNP). Table 1 summarizes the participants’ clinical characteristics. The patients with and without CRS did not exhibit any significant differences in the values for age, atopy, serum immunoglobulin E (IgE) levels, SNOT-22 score, ACT score, pulmonary function, and antiasthma medication. However, patients with CRS exhibited significantly higher endoscopy and CT scores, which indicated that these tests were the most reliable for diagnosing CRS.
Figure 2.
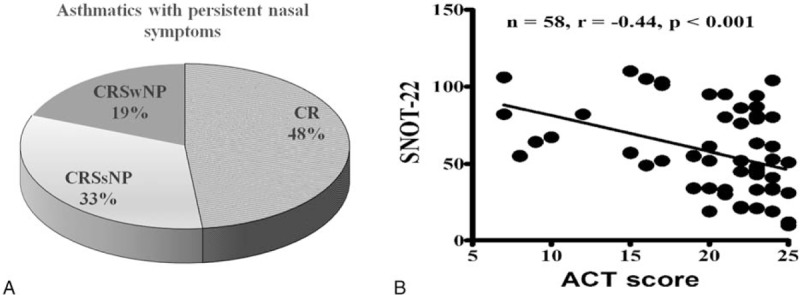
(A) Distribution of the upper airway diseases among patients with asthma and persistent nasal symptoms. (B) A good correlation was observed between the ACT and SNOT-22 scores. ACT = asthma control test, CR = chronic rhinitis, CRSsNP = chronic rhinosinusitis without nasal polyps, CRSwNP = chronic rhinosinusitis with nasal polyps, SNOT-22 = Sino-nasal Outcome Test-22.
3.2. Correlations between SNOT-22, ACT, and FEV1 outcomes
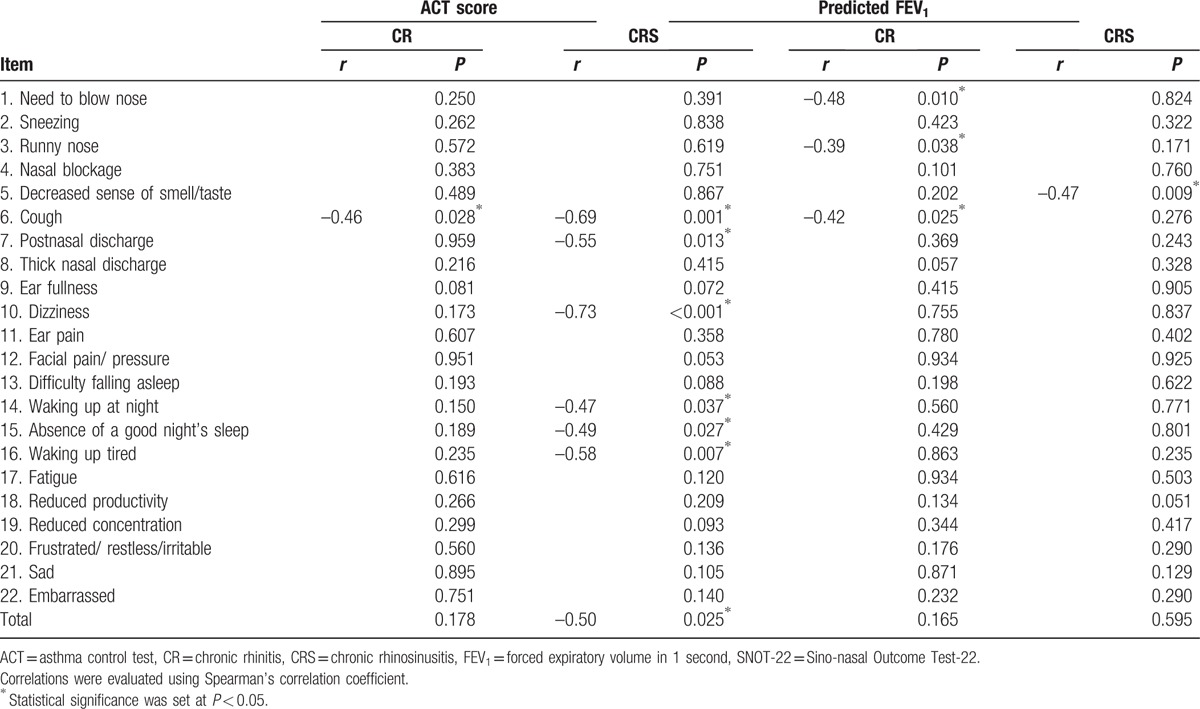
We observed a good correlation between the ACT and SNOT-22 scores (Fig. 2B). The SNOT-22 nasal symptoms score had a crucial effect on asthma evaluation using the ACT questionnaire. Among patients with asthma and CRS, the ACT score was closely related to the SNOT-22 items regarding cough, postnasal discharge, dizziness, waking up at night, absence of a good night's sleep, and waking up tired. Among patients with asthma and CR, the ACT score was closely related to cough symptoms in the SNOT-22 questionnaire (Table 2). We also evaluate the correlations between the predicted FEV1 and the SNOT-22 items (Table 2). Among patients with CR, the predicted FEV1 was closely related to the symptoms of needing to blow nose, runny nose, and cough. Among patients with CRS, the predicted FEV1 was closely related to a decreased sense of smell/taste.
Table 2.
The associations of SNOT-22 items with ACT score and predicted FEV1 among patients with asthma and persistent nasal symptoms.
3.3. Comparing SNOT-22 items among patients with CR, CRSsNP, or CRSwNP
When we compared the SNOT-22 items between patients with asthma and CR, CRSsNP, or CRSwNP, we found that patients with asthma and CRSwNP had higher scores for a decreased sense of smell/taste and facial pain/pressure. In addition, patients with CRS had more severe symptoms for ear fullness, compared to patients without CRS. Nevertheless, the total SNOT-22 scores were not significantly different when we compared the 3 subgroups (Fig. 3).
Figure 3.
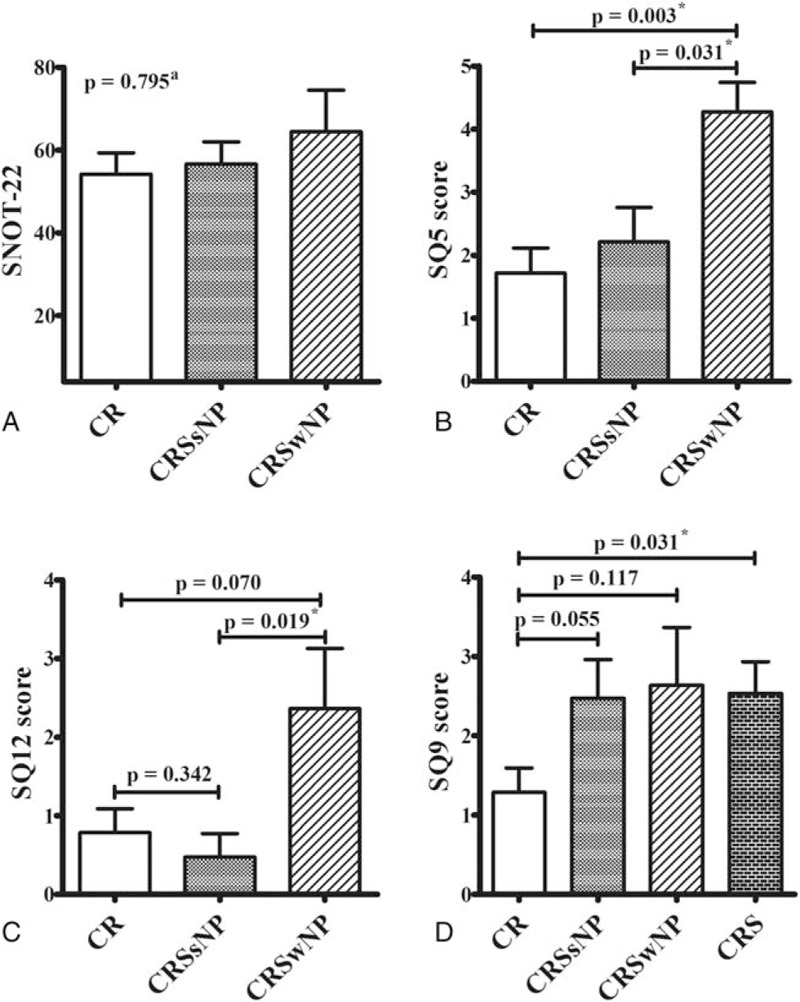
Comparing the SNOT-22 scores from patients with asthma and different sinonasal conditions. There were no significant differences in the total SNOT-22 scores when we compared patients who had asthma and CR, CRSsNP, or CRSwNP (A). Patient with CRSwNP exhibited higher scores for decreased sense of smell/taste (SQ5, B) and facial pain/pressure (SQ12, C), and patients with CRS exhibited higher scores for ear fullness (SQ9), compared to patients without these conditions (D). a The Kruskal–Wallis test was used to compare the 3 groups. ∗Statistical significance was set at P < 0.05. ACT, asthma control test, CR = chronic rhinitis, CRS = chronic rhinosinusitis, CRSsNP = chronic rhinosinusitis without nasal polyps, CRSwNP = chronic rhinosinusitis with nasal polyps, SNOT-22 = Sino-nasal Outcome Test-22.
3.4. Nasal symptoms and emergency clinic visits for acute asthma exacerbation
We retrospectively reviewed the participants’ medical records and found that 16 patients had visited an emergency clinic because of acute asthma exacerbation during the previous 3 months. When we compared the patients with and without an emergency clinic visit, we found that patients with an emergency clinic visit had relatively high SNOT-22 scores, especially for the symptoms of sneezing, runny nose, nasal blockage, cough, and dizziness (Fig. 4).
Figure 4.
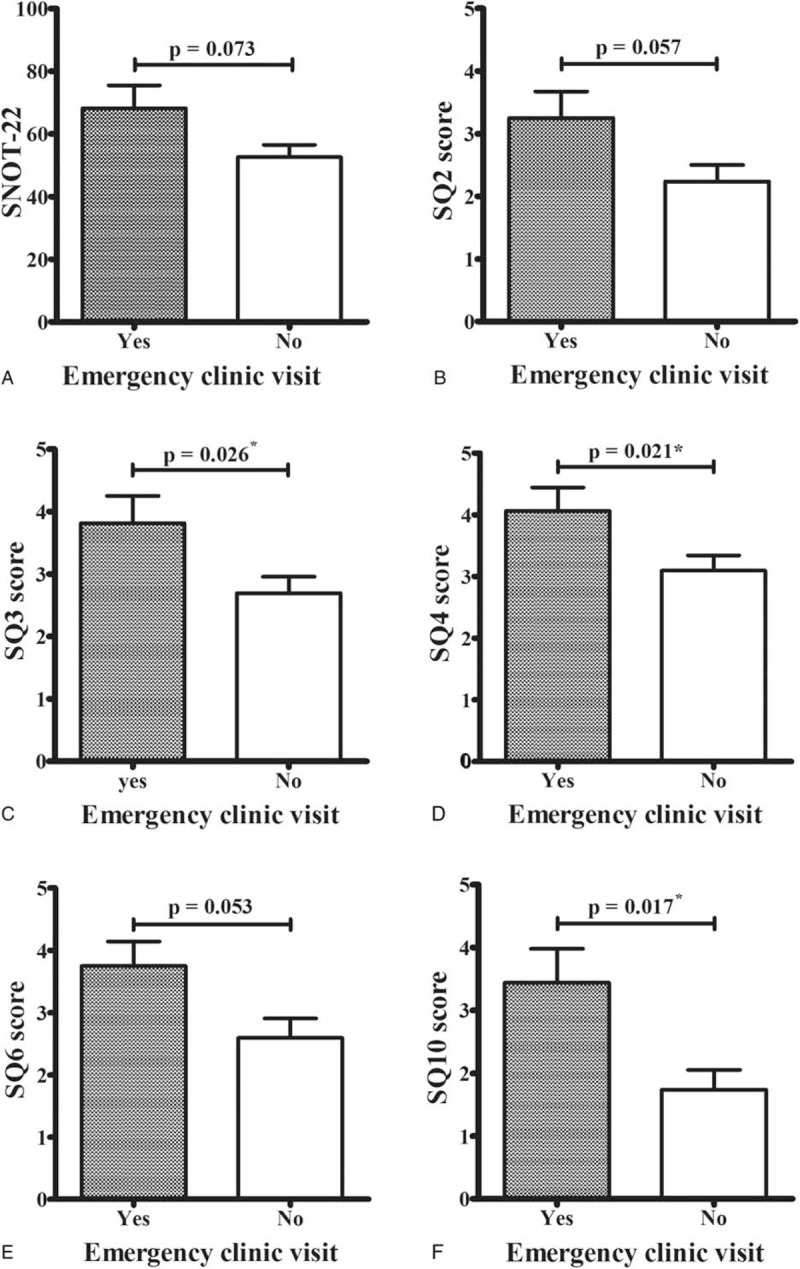
Nasal symptoms and emergency clinic visits for acute asthma exacerbation. Patients with emergency clinic visits during the previous 3 months had higher SNOT-22 scores (A), especially for the symptoms of sneezing (SQ2, B), runny nose (SQ3, C), nasal blockage (SQ4, D), cough (SQ6, E), and dizziness (SQ10, F). ∗Statistical significance was set at P < 0.05.
4. Discussion
To the best of our knowledge, this is the first study to examine the effect of nasal symptoms on the evaluation of asthma control using disease-specific questionnaires. This article described the impact of nasal symptoms on the evaluation of asthma control including QoL, pulmonary function, and emergency clinic visits in a study population with persistent nasal symptoms, even after maximal medical therapy, to emphasize the importance of simultaneous care of nose during management of asthma. In the present study, we used the questionnaires to evaluate nasal symptoms and asthma control among patients with asthma and persistent nasal symptoms, and found that more severe sinonasal symptoms were associated with poorly controlled asthma. ACT scores among patients with asthma and CRS were closely related to the symptoms of cough, post-nasal discharge, dizziness, waking up at night, absence of a good night's sleep, and waking up tired. Furthermore, the predicted FEV1 among patients with asthma and CR was closely associated with the symptoms of needing to blow nose, runny nose, and cough. In this context, greater amounts of post-nasal discharge, as well as the associated cough and worsened sleep quality, may contribute to reductions in QoL and asthma control among patients with asthma and CRS. However, nose-related allergy symptoms were associated with FEV1 among patients with asthma and CR. Therefore, our findings appear to indicate that asthma stability is closely related to control of the sinonasal disorder, and vice versa. The nasal symptoms have significant effects on the subjective and objective evaluations of asthma control.
Several tools have been developed to assess the patients’ quality of life and symptoms’ severity for asthma and for rhinitis, or both.[20–22] However, there is no questionnaire assess CRS and asthma simultaneously.[23] Besides, it is difficult to distinguish between CRS and CR using only symptomatic evaluations of patients with asthma and nasal symptoms, although this evaluation is important for managing the patients’ sinonasal conditions.[19] Among the 58 participants in the present study, for example, approximately half of the patients had CRS (52%). Based on the united airways concept, patients with lower airway inflammation typically exhibit parallel inflammation in the upper airway. Most of the inflammation in the upper airway can be managed well with or without medication for nasal symptoms when the asthma were being treated. However, in this study, we prospectively recruited patients with asthma and “persistent” nasal symptoms who were being treated at Thoracic Medicine Department for ≥3 months. The enrolled population was those with more severe symptoms and required surgical intervention. That would be the reason for the high incidence of CRS comorbidity in this study population.
Our results revealed no significant differences in the SNOT-22 total scores and most single-item scores when we compared the subgroups with the 3 different sinonasal conditions. The notable exceptions were patients with CRS exhibiting higher scores for fullness, and the patients with CRSwNP exhibiting higher scores for decreased sense of smell/taste and facial pain/pressure, compared to their counterparts who did not have these conditions. Therefore, endoscopy and CT appear to be the most reliable methods for diagnosing these sinonasal conditions.
Emergency clinic visits because of acute asthma exacerbation is another important indicator of asthma control, risk of adverse outcomes, and increased healthcare utilization.[24] In the present study, patients with an emergency clinic visit because of acute asthma exacerbation during the previous 3 months exhibited relatively high SNOT-22 scores, especially for the symptoms of sneezing, runny nose, nasal blockage, cough, and dizziness. Therefore, it appears that it is important to simultaneously evaluate and treat nose-related conditions in patients with asthma.
Given that the airway is a continuous structure that extends from the nose to the alveolar units of the lung, which is constantly exposed to the outside world, inflammation throughout the airway may be induced by exposure to various irritants, allergens, micro-organisms, and other environmental factors.[25–27] Furthermore, upper and lower airway inflammation may be triggered by a systemic response to mediators of inflammation. Moreover, changes in the inspired air (e.g., dry air, cold air, or nitric oxide-depleted air) can be caused by nasal obstruction or aspiration of sinus secretions into the lower airways, which would interfere with the function of the lower airway.[11,28,29] Therefore, although asthma and sinonasal disorders (e.g., CRS and CR) appear to be distinct diseases, we believe that they should be viewed and treated as common airway diseases.
The present study has several limitations that warrant consideration. The first limitation is the absence of strict inclusion criteria, which created a heterogeneous group of patients with various sinonasal diseases, asthma severities, and treatment modalities. Nevertheless, our patient population's heterogeneity reflects the reality of daily clinical practice at an outpatient clinic, and it is important to note that we found that sinonasal symptoms had significant effects on the subjective and objective aspects of asthma control. The second limitation is that we performed a cross-sectional analysis and are unable to comment on the dynamic changes in the evaluation and control of the upper and lower airways (e.g., before and after nasal surgery or medical treatment). The cross-sectional design also precludes any conclusions regarding the causality of the relationships that we observed. Therefore, longitudinal cohort studies are needed to validate our findings regarding the connections between nasal symptoms and asthma control evaluation.
5. Conclusion
We found that sinonasal symptom severity was closely associated with asthma control status (i.e., ACT score, FEV1, and acute exacerbation) among patients with asthma and persistent nasal symptoms. This result highlights the importance of simultaneously managing sinonasal disorders during the evaluation and treatment of patients with asthma. Furthermore, based on our findings, we believe that upper and lower airway inflammation should be considered and treated concurrently.
Acknowledgment
The authors thank Ms. Meng-Chieh Tsai for her contributions.
Footnotes
Abbreviations: ACT = Asthma Control Test, CR = chronic rhinitis, CRS = chronic rhinosinusitis, CRSsNP = chronic rhinosinusitis without nasal polyps, CRSwNP = chronic rhinosinusitis with nasal polyps, CT = computed tomography, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, GINA = Global Initiative for Asthma, IgE = immunoglobulin E, QoL = quality of life, SNOT-22 = Sino-Nasal Outcome Test-22.
C-CH and P-HC contributed equally to the study.
Financial support: Research grants from Chang Gung memorial hospital (CMRPG3F0741, CMRPG3E0122, and CMRPG391991).
The authors have no conflicts of interest to disclose.
References
- [1].World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach, Geneva: World Health Organization; 2007. Available at: http://www.who.int.proxy.lib.cgu.edu.tw:81/gard/publications/GARD_Manual/en/. [cited 2016 Aug 3], accessed August 3, 2016. [Google Scholar]
- [2].Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469–78. [DOI] [PubMed] [Google Scholar]
- [3].Asadi-Lari M, Tamburini M, Gray D. Patients’ needs, satisfaction, and health related quality of life: towards a comprehensive model. Health Qual Life Outcomes 2004;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Apfelbacher CJ, Hankins M, Stenner P, et al. Measuring asthma-specific quality of life: structured review. Allergy 2011;66:439–57. [DOI] [PubMed] [Google Scholar]
- [5].From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2016 [cited 2016 Aug 3]. Available at: http://www.ginasthma.org/ Accessed August 3, 2016 [Google Scholar]
- [6].Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006;117:549–56. [DOI] [PubMed] [Google Scholar]
- [7].Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59–65. [DOI] [PubMed] [Google Scholar]
- [8].Juniper EF, O’Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902–7. [DOI] [PubMed] [Google Scholar]
- [9].Bousquet J, Schünemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol 2012;130:1049–62. [DOI] [PubMed] [Google Scholar]
- [10].Wang Q, Ji J, Xie Y, et al. Lower airway inflammation and hyperresponsiveness in non-asthmatic patients with non-allergic rhinitis. J Thorac Dis 2015;7:1756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dixon AE, Kaminsky DA, Holbrook JT, et al. Allergic rhinitis and sinusitis in asthma: differential effects on symptoms and pulmonary function. Chest 2006;130:429–35. [DOI] [PubMed] [Google Scholar]
- [12].Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy 2012;67:91–8. [DOI] [PubMed] [Google Scholar]
- [13].Habib AR, Javer AR, Buxton JA. A population-based study investigating chronic rhinosinusitis and the incidence of asthma. Laryngoscope 2016;126:1296–302. [DOI] [PubMed] [Google Scholar]
- [14].Håkansson K, Bachert C, Konge L, et al. Airway inflammation in chronic rhinosinusitis with nasal polyps and asthma: The United Airways Concept Further Supported. Cormier SA, ed. PLoS One 2015;10:e0127228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg 1997;117:S35–40. [DOI] [PubMed] [Google Scholar]
- [16].Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology 1993;31:183–4. [PubMed] [Google Scholar]
- [17].Marks GB. Are reference equations for spirometry an appropriate criterion for diagnosing disease and predicting prognosis? Thorax 2012;67:85–7. [DOI] [PubMed] [Google Scholar]
- [18].Toma S, Hopkins C. Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhinology 2016;54:129–33. [DOI] [PubMed] [Google Scholar]
- [19].Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50:1–2. [DOI] [PubMed] [Google Scholar]
- [20].Baiardini I, Pasquali M, Giardini A, et al. Rhinasthma: a new specific QoL questionnaire for patients with rhinitis and asthma. Allergy 2003;58:289–94. [DOI] [PubMed] [Google Scholar]
- [21].Fonseca JA, Nogueira-Silva L, Morais-Almeida M, et al. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy 2010;65:1042–8. [DOI] [PubMed] [Google Scholar]
- [22].Nogueira-Silva L, Martins SV, Cruz-Correia R, et al. Control of allergic rhinitis and asthma test – a formal approach to the development of a measuring tool. Respir Res 2009;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yatera K, Yamasaki K, Noguchi S, et al. Prevalence of sinusitis and efficacy of intranasal corticosteroid treatment on asthmatic symptoms in asthmatic patients with rhinosinusitis: a pilot study. Int Forum Allergy Rhinol 2016;6:398–406. [DOI] [PubMed] [Google Scholar]
- [24].Fuhlbrigge A, Peden D, Apter AJ, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012;129(3 suppl):S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kariyawasam HH, Rotiroti G. Allergic rhinitis, chronic rhinosinusitis and asthma: unravelling a complex relationship. Curr Opin Otolaryngol Head Neck Surg 2013;21:79–86. [DOI] [PubMed] [Google Scholar]
- [26].Huang CC, Wang CH, Fu CH, et al. The link between chronic rhinosinusitis and asthma: A questionnaire-based study. Medicine (Baltimore) 2016;95:e4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nascimento-Sampaio FS, Leite Mdos S, Leopold DA, et al. Influence of upper airway abnormalities on the control of severe asthma: a cross-sectional study. Int Forum Allergy Rhinol 2015;5:371–9. [DOI] [PubMed] [Google Scholar]
- [28].Rosati MG, Peters AT. Relationships among allergic rhinitis, asthma, and chronic rhinosinusitis. Am J Rhinol Allergy 2016;30:44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang CC, Wang CH, Fu CH, et al. Association between cigarette smoking and interleukin-17A expression in nasal tissues of patients with chronic rhinosinusitis and asthma. Medicine (Baltimore) 2016;95:e5432. [DOI] [PMC free article] [PubMed] [Google Scholar]


