Abstract
Background:
Giant internal carotid artery (ICA) aneurysms extending into the sellar region, mimicking pituitary tumors, and causing pituitary dysfunction are relatively rare. Open surgery or endovascular treatment can treat these aneurysms, but achieving recovery of endocrine function is difficult.
Case Description:
A 56-year-old man presented with giant aneurysm of the ICA causing pituitary impairment, leading to disturbance of consciousness due to hyponatremia. High-flow bypass from the cervical external carotid artery to the middle cerebral artery and ligation of the ICA at the cervical portion were performed. One year after the operation, his pituitary function recovered well; he was followed up as an outpatient without hormonal replacement therapy for 8 years after the operation.
Conclusion:
Giant ICA aneurysm causing pituitary dysfunction is relatively rare, but it is important to consider in the differential diagnosis for hypopituitarism. Treatment with high-flow bypass using radial artery graft can achieve both prevention of aneurysm rupture and recovery of pituitary function.
Keywords: Giant aneurysm, high-flow bypass, internal carotid artery aneurysm, pituitary dysfunction
INTRODUCTION
Giant internal carotid artery (ICA) aneurysms extending into the sellar region, mimicking pituitary tumors, and causing pituitary dysfunction are rare but occasionally reported.[5,7,11,16,17,23,25,26,28] Such aneurysms are the cause of hypopituitarism in 0.17% of patients.[13] Open surgery or endovascular treatment is indicated for these aneurysms, but achieving recovery of endocrine function is difficult.[5,7,11] We describe a case of giant aneurysm of ICA causing pituitary impairment, which was successfully treated with high-flow bypass using radial artery graft (RAG), followed by ligation of the ICA at the cervical portion with subsequent recovery of pituitary function after treatment.
CASE REPORT
A 56-year-old man with past medical history of dyslipidemia had been complaining of sleep disturbance, loss of motivation and appetite, and had lost 8 kg in weight in the last 6 months. He was diagnosed with depression and began treatment with antidepressant agents, sulpiride 150 mg and paroxetine 10 mg, at a local hospital. His symptoms deteriorated despite treatment, and he was admitted to a local mental hospital. Head computed tomography (CT) disclosed a mass suggestive of tumor near the sellar region and he was referred to our hospital [Figure 1a].
Figure 1.
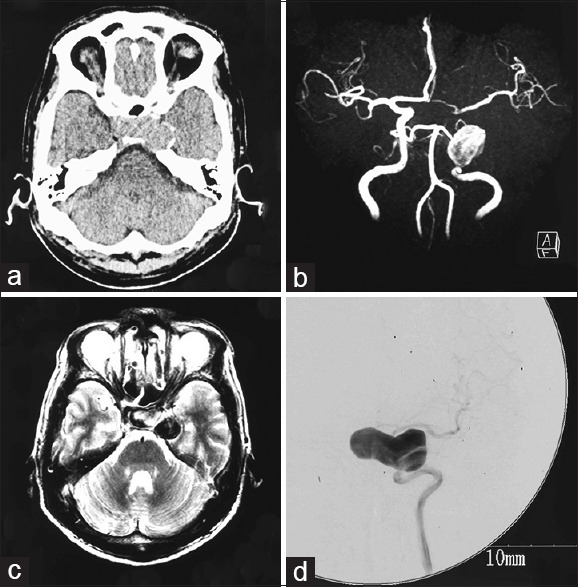
(a) Computed tomography scan showing a mass suggestive of tumor near the sellar region. (b) Magnetic resonance angiogram showing a large aneurysm of the left internal carotid artery (ICA). (c) T2-weighted magnetic resonance image revealing the aneurysm extending to the suprasellar region. (d) Cerebral digital subtraction angiogram of the left ICA revealing a giant aneurysm at the cavernous portion extending in the medial direction
On admission, he was drowsy and biochemistry revealed severe hyponatremia, with serum sodium level of 117 mEq/l. Treatment with intravenous saline and 200 mg hydrocortisone was started immediately. Magnetic resonance (MR) angiography demonstrated a large aneurysm of the left ICA, and T2-weighted MR imaging revealed the aneurysm extending to the suprasellar region [Figure 1b and c]. Basal pituitary hormone levels were as follows: morning cortisol, 2.2 μg/dl; adrenocorticotrophic hormone (ACTH), 12.9 pg/ml; free thyroxine, 0.40 ng/dl; thyroid-stimulating hormone (TSH), 1.47 μIU/ml; growth hormone (GH), 0.06 ng/ml; follicle-stimulating hormone (FSH), 1.0 mIU/ml; luteinizing hormone (LH), 0.4 mIU/ml; and prolactin 6.2 ng/ml. After treatment with intravenous saline, hydrocortisone 100 mg, and levothyroxine 50 μg, his impaired consciousness recovered and hyponatremia normalized in a couple of days; his depressive symptoms disappeared within a week.
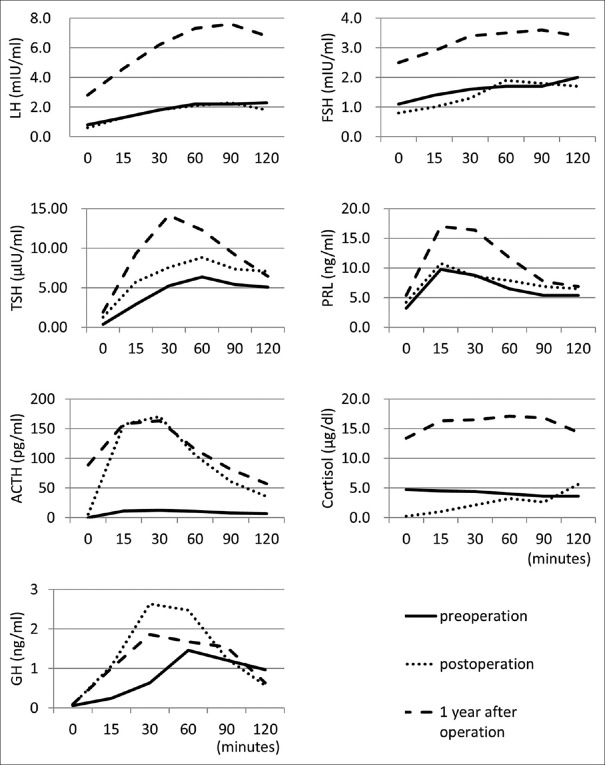
Several hormone load tests were conducted [Figure 2]. The LH-releasing hormone load test showed poor reactions of LH and FSH. The thyrotropin-releasing hormone load test showed delayed TSH reaction. The corticotropin-releasing hormone load test showed poor reactions of ACTH and cortisol. The GH-releasing factor load test showed delayed reaction of GH. Cerebral digital subtraction angiography of the left ICA revealed a giant aneurysm at the cavernous portion extending in the medial direction [Figure 1d]. Based on these findings, we diagnosed pituitary dysfunction secondary to mass effect of giant suprasellar ICA aneurysm. To decrease the mass effect on the pituitary gland and prevent aneurysm rupture, we decided to perform high-flow bypass from the cervical external carotid artery (ECA) to the middle cerebral artery (MCA) using RAG and ligation of the cervical portion of the ICA.
Figure 2.
Time-series graphs of hormone load tests. Luteinizing hormone (LH)-releasing hormone load test: after intravenous injection of LH-releasing hormone (0.1 mg), LH and follicle-stimulating hormone (FSH) levels were examined. Thyrotropin-releasing hormone load test: after intravenous injection of thyrotropin-releasing hormone (0.5 mg), thyroid-stimulating hormone (TSH) and prolactin (PRL) levels were examined. Corticotropin-releasing hormone load test: after intravenous injection of corticotropin-releasing hormone (0.1 mg), adrenocorticotrophic hormone (ACTH) and cortisol levels were examined. Growth hormone (GH)-releasing factor load test: after intravenous injection of GH-releasing factor (0.1 mg), GH level was examined. Solid, dotted, and dashed lines indicate the data for preoperation, postoperation (3 weeks after operation), and 1 year after operation
Operation
Operation was conducted as described in our previous report.[19] Neuroanesthesia was induced under monitoring with somatosensory evoked potentials (SSEPs) of the right extremities. The left cervical carotid bifurcation was exposed, a curvilinear frontotemporal skin incision was made, and the superficial temporal artery (STA) was meticulously prepared under the operating microscope. The RAG was harvested concurrently by another neurosurgeon. Frontotemporal craniotomy was performed, and a subzygomatic tunnel was formed for the RAG. The sylvian fissure was split under the operating microscope, and the M2 and M3 portions of the MCA were exposed. First, an “insurance” STA-M3 bypass was made distal to the M2 portion for RAG anastomosis. Then, the harvested RAG was gently pulled through the subzygomatic tunnel, that is, between the lateral pterygoid muscle and the temporal muscle from the cranium to the neck through the lateral corridor of the stylohyoid muscle and the posterior belly of the digastric muscle toward the external carotid artery (ECA).[15] The distal end of the RAG was anastomosed to the M2 of the MCA, and the proximal end was anastomosed to the ECA. The patency of the anastomosis was confirmed with microvascular Doppler flowmetry. The cervical ICA was permanently ligated after confirming no change in the SSEPs 5 minutes after temporary ligation. Microvascular Doppler flowmetry confirmed anterograde bypass flow from the STA and RAG. No significant SSEP changes were observed throughout the operation.
CT obtained on the day after the operation disclosed thrombosis of the aneurysm and cerebral angiography performed at 8 days after the operation demonstrated good patency of the bypasses and disappearance of flow to the aneurysm [Figure 3a and b]. The patient did not develop diabetes insipidus or visual field defect throughout the course. Hormone load tests conducted 3 weeks after the operation during administration of hydrocortisone 10 mg daily showed recovery of the rapid reactions of ACTH and GH [Figure 2]. Follow-up examination at 1 year showed normal pituitary hormone levels, which were elevated by the hormone load test without hormonal replacement therapy [Figure 2]. Therefore, his pituitary dysfunction was considered to have recovered well after the operation. He has been followed up as an outpatient without hormonal replacement therapy, and the latest MR angiography and MR imaging findings at 8 years after the operation disclosed good patency of the RAG and shrinkage of the aneurysm [Figure 3c and d].
Figure 3.
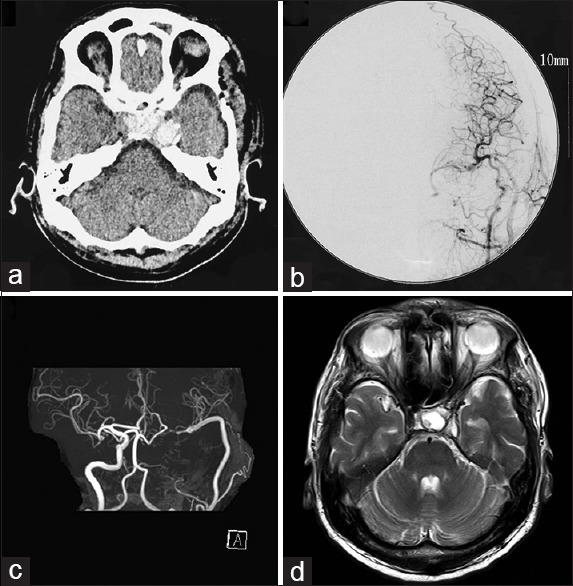
(a) Computed tomography scan on the day after the operation showing thrombosis of the aneurysm. (b) Cerebral angiogram performed at 8 days after the operation demonstrating good patency of the bypasses and disappearance of flow to the aneurysm. (c, d) Magnetic resonance angiogram (c) and image (d) obtained at 8 years after the operation showing good patency of the radial artery graft and shrinkage of the aneurysm
DISCUSSION
Pituitary dysfunction is estimated to have an annual incidence of 4.2 cases per 100,000, and a prevalence of 45.5 per 100,000.[24] The most common cause of hypopituitarism is the mass effect of tumors, such as pituitary adenomas, and nonpituitary tumors such as craniopharyngiomas, meningiomas, and gliomas. Large cerebral aneurysms may also cause extrinsic compression of the pituitary gland and stalk resulting in pituitary insufficiency. However, such large aneurysms are a very rare cause of hypopituitarism and are difficult to identify in the differential diagnosis because they resemble pituitary tumors in terms of imaging and laboratory findings.[13] The mechanisms of endocrine dysfunction may involve either mechanical compression of the pituitary gland and stalk, or vascular compression of the artery supplying the pituitary gland resulting in pituitary ischemia.[26,27] Our present patient was first treated under a diagnosis of depression associated with hyponatremia induced by secondary adrenal insufficiency. Subsequently, our patient was referred to our hospital after discovery of the intracranial mass suggestive of tumor. MR angiography disclosed this mass as a cerebral aneurysm; this differentiation between tumor and aneurysm is crucial to decide the treatment strategy. Treatment is intended to prevent aneurysm rupture and reduce the mass effect on the pituitary gland to recover endocrine function. The options are open surgery such as clipping or trapping, with or without bypass, and endovascular treatment; however, the treatment of large ICA aneurysm is very difficult. Moreover, recovery of endocrine function is rare even after surgery.[26,28]
Endovascular coil embolization with or without stenting for giant aneurysms has unfortunately resulted in high recanalization and re-treatment rates.[18,22] Even if the treatment can prevent rupture, the mass effect to the pituitary gland persists due to the coil in the aneurysm, thus requiring hormone replacement therapy.[7,11] Recently, flow diverting stents have offered treatment for formidable and complex aneurysms, but previous trials showed complete aneurysm occlusion of 73.6% at 6 months and 86.8% at 1 year, and 5.6% had major complications such as major ipsilateral thrombosis, intraparenchymal hemorrhage, and neurologic death.[2,3] Good occlusion of the aneurysm and recovery of pituitary function was reported in one case,[26] but pituitary hormone replacement therapy was necessary after aneurysm treatment in another case.[5] Delayed pan-hypopituitarism was observed after treatment of ICA aneurysm with flow diverting stent, partly caused by compromise to the blood supply of the pituitary gland as a result of the flow diversion to the ICA.[12]
Direct and indirect open surgery methods are available for treating giant ICA aneurysms projecting into the sellar region.[13] Direct surgical options, such as neck clipping or trapping, offer definitive treatment of such aneurysms and pituitary function recovery,[28] but are technically challenging and carry higher risks of cranial nerve palsy and ICA compromise. Indirect surgical treatments, such as proximal ligation of the ICA with or without construction of bypass to the MCA, avoid invasion of the cavernous sinus.[1,6,9] High-flow bypass with ICA ligation achieved aneurysm obliteration with acceptable rates of morbidity and mortality, as well as graft patency in previous reports. For example, Ishishita reported 94.7% graft patency and 100% aneurysm obliteration, with no mortality.[15,20,21]
In this case, proximal ICA ligation was performed at the cervical portion with high-flow bypass using a RAG from the cervical ECA to the MCA, which achieved long-term prevention of aneurysm rupture without ischemic complication. Moreover, the aneurysm shrank and pituitary function recovered completely, making hormone replacement therapy unnecessary.
To assess the tolerance of ligating the ICA, balloon occlusion test (BOT) is commonly used. However, there is still no universal standard of this procedure, the accuracy of this test is complicated and controversial. Sometimes BOT causes complication such as cerebral infarction.[1,8] Therefore, we employed graft bypass without performing preoperative BOT. We chose the radial artery as graft, not the saphenous vein, because the RAG is associated with long-term patency,[4,14] and in this case, the perfusion area of the sacrificed ICA was not so wide, that is, anterior cerebral arteries was also perfused by contralateral ICA and left posterior cerebral artery was supplied via vertebrobasilar artery. Moreover, Allen test was positive.
Duration of pituitary impairment and extent of pituitary compression may also affect endocrine recovery after treatment.[26] Three previous patients who recovered pituitary function after treatment had symptoms for 3, 6, and 7 months.[10,26,28] We cannot be sure exactly when hypopituitarism started in our patient, but his symptoms continued for about 6 months, so this relatively short period may have been favorable for the good outcome.
Giant ICA aneurysm causing pituitary dysfunction is rare and not well characterized, but is important to consider in the differential diagnosis for hypopituitarism. Treatment with high-flow bypass using RAG is effective for achieving both prevention of aneurysm rupture and recovery of pituitary function.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Hideaki Ono, Email: hideono-tky@umin.ac.jp.
Tomohiro Inoue, Email: t.inouenttnsu@gmail.com.
Naoto Kunii, Email: nkunii-tky@umin.ac.jp.
Takeo Tanishima, Email: tt918j_ns@fa2.so-net.ne.jp.
Akira Tamura, Email: tamura-nsu@umin.ac.jp.
Isamu Saito, Email: saito-kyr@umin.ac.jp.
Nobuhito Saito, Email: nsaito-tky@umin.net.
REFERENCES
- 1.Barnett DW, Barrow DL, Joseph GJ. Combined extracranial-intracranial bypass and intraoperative balloon occlusion for the treatment of intracavernous and proximal carotid artery aneurysms. Neurosurgery. 1994;35:92–7. doi: 10.1227/00006123-199407000-00014. discussion 97-8. [DOI] [PubMed] [Google Scholar]
- 2.Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013;267:858–68. doi: 10.1148/radiol.13120099. [DOI] [PubMed] [Google Scholar]
- 3.Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg. 2016 doi: 10.3171/2015.6.JNS15311. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Cao C, Ang SC, Wolak K, Peeceeyen S, Bannon P, Yan TD. A meta-analysis of randomized controlled trials on mid-term angiographic outcomes for radial artery versus saphenous vein in coronary artery bypass graft surgery. Ann Cardiothorac Surg. 2013;2:401–7. doi: 10.3978/j.issn.2225-319X.2013.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding D, Mehta GU, Liu KC. Pituitary insufficiency from large unruptured supraclinoid internal carotid artery aneurysm. Br J Neurosurg. 2014;28:290–2. doi: 10.3109/02688697.2013.829559. [DOI] [PubMed] [Google Scholar]
- 6.Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656–65. doi: 10.3171/jns.1994.81.5.0656. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Real JM, Fernandez-Castaner M, Villabona C, Sagarra E, Gomez-Saez JM, Soler J. Giant intrasellar aneurysm presenting with panhypopituitarism and subarachnoid hemorrhage: Case report and literature review. Clin Investig. 1994;72:302–6. doi: 10.1007/BF00180045. [DOI] [PubMed] [Google Scholar]
- 8.Field M, Jungreis CA, Chengelis N, Kromer H, Kirby L, Yonas H. Symptomatic cavernous sinus aneurysms: Management and outcome after carotid occlusion and selective cerebral revascularization. AJNR Am J Neuroradiol. 2003;24:1200–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Gelber BR, Sundt TM., Jr Treatment of intracavernous and giant carotid aneurysms by combined internal carotid ligation and extra- to intracranial bypass. J Neurosurg. 1980;52:1–10. doi: 10.3171/jns.1980.52.1.0001. [DOI] [PubMed] [Google Scholar]
- 10.Giustina A, Scalvini T, Cerudelli B, Bossoni S, Bodini C, Orlandini A, et al. Hypopituitarism secondary to suprasellar giant carotido-ophthalmic aneurysm. Normalization of the hypophyseal function after neurosurgical depression of the aneurysm. Minerva Endocrinol. 1989;14:255–8. [PubMed] [Google Scholar]
- 11.Gungor A, Gokkaya N, Bilen A, Bilen H, Akbas EM, Karadeniz Y, et al. Pituitary insufficiency and hyperprolactinemia associated with giant intra- and suprasellar carotid artery aneurysm. Case Rep Med 2015. 2015 doi: 10.1155/2015/536191. 536191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall J, Caputo C, Chung C, Holt M, Wang YY. Delayed pan-hypopituitarism as a complication following endovascular treatment of bilateral internal carotid artery aneurysms. A case report and review. Br J Neurosurg. 2015;29:303–5. doi: 10.3109/02688697.2014.969681. [DOI] [PubMed] [Google Scholar]
- 13.Heshmati HM, Fatourechi V, Dagam SA, Piepgras DG. Hypopituitarism caused by intrasellar aneurysms. Mayo Clin Proc. 2001;76:789–93. doi: 10.1016/S0025-6196(11)63222-9. [DOI] [PubMed] [Google Scholar]
- 14.Houkin K, Kamiyama H, Kuroda S, Ishikawa T, Takahashi A, Abe H. Long-term patency of radial artery graft bypass for reconstruction of the internal carotid artery. Technical note. J Neurosurg. 1999;90:786–90. doi: 10.3171/jns.1999.90.4.0786. [DOI] [PubMed] [Google Scholar]
- 15.Ishishita Y, Tanikawa R, Noda K, Kubota H, Izumi N, Katsuno M, et al. Universal extracranial-intracranial graft bypass for large or giant internal carotid aneurysms: Techniques and results in 38 consecutive patients. World Neurosurg. 2014;82:130–9. doi: 10.1016/j.wneu.2013.02.063. [DOI] [PubMed] [Google Scholar]
- 16.Kayath MJ, Lengyel AM, Nogueira R, Tella Junior O, Czepielewski MA. Giant aneurysms of the sellar region simulating pituitary adenomas: A diagnosis to be considered. J Endocrinol Invest. 1991;14:975–9. doi: 10.1007/BF03347127. [DOI] [PubMed] [Google Scholar]
- 17.Lawson EA, Buchbinder BR, Daniels GH. Hypopituitarism associated with a giant aneurysm of the internal carotid artery. J Clin Endocrinol Metab. 2008;93:4616. doi: 10.1210/jc.2008-0596. [DOI] [PubMed] [Google Scholar]
- 18.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 19.Ono H, Inoue T, Suematsu S, Tanishima T, Tamura A, Saito I, et al. Middle cerebral artery dissection causing subarachnoid hemorrhage and cerebral infarction: Trapping with high-flow bypass preserving the lenticulostriate artery. Surg Neurol Int Forthcoming. 2017 doi: 10.4103/sni.sni_154_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel HC, Teo M, Higgins N, Kirkpatrick PJ. High flow extra-cranial to intra-cranial bypass for complex internal carotid aneurysms. Br J Neurosurg. 2010;24:173–8. doi: 10.3109/02688690903531075. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan D, Temkin N, Kim LJ, Ghodke B, Sekhar LN. Cerebral bypasses for complex aneurysms and tumors: Long-term results and graft management strategies. Neurosurgery. 2012;70:1442–57. doi: 10.1227/NEU.0b013e31824c046f. discussion 1457. [DOI] [PubMed] [Google Scholar]
- 22.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 23.Raymond LA, Tew J. Large suprasellar aneurysms imitating pituitary tumour. J Neurol Neurosurg Psychiatry. 1978;41:83–7. doi: 10.1136/jnnp.41.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369:1461–70. doi: 10.1016/S0140-6736(07)60673-4. [DOI] [PubMed] [Google Scholar]
- 25.Seok H, Park HN, Kim GH, Son HS, Sohn TS. A giant carotid aneurysm with intrasellar extension: A rare cause of panhypopituitarism. Korean J Intern Med. 2015;30:265–6. doi: 10.3904/kjim.2015.30.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan LA, Sandler V, Todorova-Koteva K, Levine L, Lopes DK, Moftakhar R. Recovery of pituitary function following treatment of an unruptured giant cavernous carotid aneurysm using Surpass flow-diverting stents. BMJ Case Rep 2014. 2014 doi: 10.1136/bcr-2014-011233. bcr2014011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tungaria A, Kumar V, Garg P, Jaiswal AK, Behari S. Giant, thrombosed, sellar-suprasellar internal carotid artery aneurysm with persistent, primitive trigeminal artery causing hypopituitarism. Acta Neurochir (Wien) 2011;153:1129–33. doi: 10.1007/s00701-010-0931-z. [DOI] [PubMed] [Google Scholar]
- 28.Verbalis JG, Nelson PB, Robinson AG. Reversible panhypopituitarism caused by a suprasellar aneurysm: The contribution of mass effect to pituitary dysfunction. Neurosurgery. 1982;10:604–11. doi: 10.1227/00006123-198205000-00011. [DOI] [PubMed] [Google Scholar]