Abstract
Objective(s):
Behavioral and neuroimaging studies have shown that transcranial direct current stimulation, as a non-invasive neuromodulatory technique, beyond regional effects can modify functionally interconnected remote cortical and subcortical areas. In this study, we hypothesized that the induced changes in cortical excitability following the application of cathodal or anodal tDCS over the left frontal cortex as pre-training would affect functional connectivity in resting-state circuits of fear memory and consequently could improve or disturb the acquisition of fear memory.
Materials and Methods:
In order to evaluate the polarity-dependent effects of tDCS on the acquisition of fear memory and the functional connectivity, we applied left prefrontal anodal or cathodal stimulation at 200 μA for one session to healthy mice for the durations of 20 and 30 min prior to fear conditioning.
Results:
Our results revealed that the administration of left prefrontal anodal (for both 20 and 30 min durations) and cathodal (at 30 min duration) tDCS impaired the acquisition of both contextual and cued fear memory. In addition, we did not observe a direct correlation between stimulation duration and the efficacy of tDCS on the acquisition of contextual and cued fear memory.
Conclusion:
In this study, the impairments of both contextual and cued memory further confirmed the previous studies reporting that the administration of transcranial stimulation would affect the activity of deeper structures like amygdala and hippocampus as the main components of the fear memory circuit in acquisition, storage, and expression of the memory.
Keywords: Fear, Memory, Prefrontal cortex, tDCS
Introduction
In recent years, non-pharmacological interven-tions such as transcranial direct current stimulation (tDCS) as a non-invasive neuromodulatory technique have gained much attention. This has been introduced as an effective technique both to enhance performance in a variety of learning and memory tasks in healthy participants and to improve cognitive functions in patients with depression (1), Alzheimer’s disease (2, 3), Parkinson’s disease, and stroke (4). The exact mechanisms underlying tDCS effects is not clear, however, it is believed that using a weak current can lead to a shift in ions and molecules through the cell membrane by enhancing its permeability.
tDCS is believed to modulate the spontaneous firing rates of neurons by specific polarity manipulation of resting-membrane potential toward subthreshold depolarization or hyperpolarization, respectively
anodal, cathodal stimulation and induce long-lasting effects characterized by long-term potentiation (LTP) and long- term depression (LTD) like plasticity. However, it must be taken into account that the relationship is so complicated that the enhanced duration of anodal stimulation can lead to decreased cortical excitability and increased intensity of cathodal stimulation (5).
Beyond the regional effects under the electrodes, the activity modifications of the interconnected remote cortical and subcortical areas have also been reported (6, 7). It potentially enables tDCS technique to modulate the function of the structures that are located in the deep brain.
According to these findings, we hypothesized that the changes in the frontal cortex induced by applying tDCS would affect the activity of deeper structures that are connected to this area. Based on behavioral and neuroimaging studies like functional magnetic resonance imaging (fMRI) (8, 9) and positron emission tomography (PET) (10), it is assumed that tDCS not only modulates cortical excitability but also affects the subcortical areas. Demonstrated that tDCS affected the activity of resting-state networks so that the application of anodal stimulation over the left motor cortex led to enhanced functional connectivity between the left motor cortex and the ipsilateral thalamus, caudate nucleus, and parietal association cortex whereas cathodal tDCS induced a reduction in connectivity between the left motor cortex and the contralateral putamen (11). The fMRI tracking of tDCS after- effects on resting-state functional connectivity demonstrated that bilateral tDCS over primary sensorimotor cortex (SM1) induced widespread connectivity changes in primary and secondary motor cortex as well as the prefrontal cortex. In contrast, unilateral tDCS over SM1 predominantly modulated the functional connectivity in prefrontal, parietal, and cerebellar areas (12). According to these findings, we hypothesized that the induced consequent changes of cortical excitability following the application of cathodal or anodal tDCS over the left frontal cortex as pre-training would affect the functional connectivity in resting-state circuits of fear memory and could improve or disturb the acquisition of fear memory.
Pavlovian fear conditioning is an important model for investigating the neural substrates of associative learning and the underlying mechanisms of memory formation in the mammalian brain. The neural circuits of fear conditioning and synaptic plasticity in the circuits have been very well characterized. The neural circuits include medial prefrontal cortex, amygdala, and hippocampus and these structures are reciprocally connected (13-16). The converging lines of evidence revealed that among the involved structures of fear memory, amygdala had a critical role in acquisition, storage, and expression of conditioned fear memory (17, 18). Accordingly, we hypothesized that the induced consequent changes to the cortical excitability following the application of cathodal or anodal tDCS over the left frontal cortex would affect the functional connectivity in resting-state circuits of fear memory and could improve or disturb acquisition of fear memory.
There are no animal studies evaluating the effects of tDCS on the acquisition of fear conditioning memory; hence, in this study, we aimed at indicating whether anodal or cathodal tDCS, which modulates brain cortical excitability and activity, could affect the acquisition of fear memory as implicit memory when administered as pre-training over prefrontal cortex. We expected that the manipulation of prefrontal cortical excitability as an involved structure in fear condition circuits would impact the function of other structures of the fear circuit such as amygdala and hippocampus which are placed in the deep brain and are connected with the cortex.
Materials and Methods
Animals
Male NMRI mice (25-28 g) were obtained from Institute for Cognitive Sciences Studies (ICSS). The animals were placed in polycarbonate cages (4 per cage), given ad libitum access to food and water in a vivarium with a 12: 12 hr light/dark cycle, (lights on at 07.00 hr). All testing was conducted between 09.00 hr and 17.00 hr. All experiments were approved by the guidelines of the Ethical Committee of Tehran University of Medical Sciences and The Declaration of Helsinki (DoH) for the care and use of laboratory animals (publication No. 85-23, revised 2010).
Surgery
The mice were anesthetized by injection of ketamine (50 mg/kg) and xylazine (5 mg/kg) intraperitoneally. By using a stereotaxic apparatus, a custom made epicranial electrode (with 2.1 mm internal diameter and a void space which could provide 3.5 mm effective contact area after filling with saline) was positioned over the left frontal cortex, 1 mm anterior and 1 mm left of the Bregma area and was fixed with glass ionomer cement as described in previous studies (8-10). After surgery, all subjects were allowed to recover for 4 days while they were placed in individual cages.
Transcranial brain stimulation
After recovery, to stimulate the brain, each mouse was kept in a restrainer (manufactured and designed in the lab) to restrict the movements of the mouse and to avoid interference effects of the anesthesia. A 9.5 square cm carbon rubber electrode was placed in the soaked sponge-like cover and placed under the subject’s chest which served as the reference electrode.
Cathodal or anodal tDCS was applied continuously with 0.2 mA current intensity from the epicranial electrode for 20 or 30 min by using a DC stimulator (Active Dose II unit made in Activatek company-Taiwan). In the sham condition, no current was applied. In order to assess the safety of applied current, a group of mice who received anodal or cathodal tDCS with 20 or 30 min duration was sacrificed for extraction of their brains; the prepared slices were stained with hematoxylin and investigated for signs of burning and abnormality in brain in relative shame[A1], the data are not shown here.
Fear conditioning experiments
Training
On the training day, the mice were placed in the training chamber and habituated for 120 sec prior to3 consecutive auditory-foot shock pairings. The conditioned stimulus, CS (white nose, 30 sec, 4 kHz) was then presented for 30 seconds. A mild foot shock as unconditioned stimulus, US (2 sec, 0.5 mA) was administered during the last 2 sec of the tone presentation and was co-terminated with the tone. The pairing of CS-US was repeated three times with a 90 sec interval time. 30 sec after the last shock, the animals were removed from the chamber (12, 19, 20).
Contextual fear memory test
The contextual conditioning fear memory test took place 24 hr after the training. The animals were placed into the training chamber and were allowed to freely explore the chamber in the absence of CS and US, and then the percentage of time spent on freezing was measured continuously for 5 min. In addition, the latency to first contextual freezing, as an index to memory retrieval was determined.
Cued fear memory test
One hour after context test the mice were individually transferred to a new chamber with different shape and color and were allowed to habituate for 180 sec in the absence of CS. The same intensity tone cue used in the conditioning session was then presented for the next 180 sec. The latency to the first cued freezing, as an index to retrieval cued memory, and the percentage of time spent on freezing, as a cued fear memory, was scored continuously during that period.
Hole-board apparatus for assessment of locomotor activity and exploratory behavior
The hole-board apparatus (Borj Sanat Co, Tehran, Iran) consisted of gray Perspex panels (40×40×2.2 cm) with 16 equidistant 3 cm wide holes in the floor. The board was located 15 cm above a table. Animals were individually located in the center of the board facing away from the observer and head-dip numbers and the latency time to first head-dip was recorded during a 5 min period using photocells arranged below the holes. Furthermore, the surface of hole-board apparatus was also designed as 16 equal-sized squares to determine the locomotor activity. The locomotor activity was recorded as the number of crossings from one square to another during the 5 min period by an observer (21, 22).
Experimental design
In this study, the experimental groups were divided into five groups. Group 1(n=9) was the sham group without receiving any current; Groups 2 (n=8) and 3 (n=8) received anodal tDCS for 20 or 30 min prior to fear conditioning; Groups 4 (n=8) and 5 (n=8) received cathodal tDCS for 20 or 30 min prior to fear conditioning. All subjects underwent one tDCS session with 0.2 mA current intensity. (the sham group was placed in the restrainer while the electrodes were connected to them without receiving any current.
Statistical analysis
Statistical analyses were conducted using the statistical package for social sciences (SPSS) for Windows, Version 22.0 (IBM corp., Armonk, NY). P-values less than 0.05 were considered statistically significant. By using the Kolmogorov-Smirnov test, the normality of the data-distribution was determined, then the data were analyzed by using one-way analysis of variance (ANOVA) or by the non-parametric Kruskal-Wallis test followed by Tukey’s or Dunn’s test as post hoc to determine specific inter-group differences, respectively. In addition, a linear regression analysis was conducted on the contextual or cued freezing scores at the stimulation durations.
Results
The application of left prefrontal anodal tDCS at both durations (20 min and 30 min) impaired the acquisition of contextual conditioned fear memory
One way ANOVA and Tukey’s post hoc analysis revealed that pre-training administration of anodal tDCS at both durations (20 or 30 min) impaired contextual fear memory[F(2, 22)=20.66, P<0.001, Figure 2A]. 20 min anodal tDCS significantly reduced the contextual freezing [F (2, 22) = 20.66, P=0.001, Figure 2A] compared to the sham; also 30 min anodal stimulation decreased the contextual freezing significantly [F(2, 22)=20.66, P= 0.005 in compari-son to the sham; but unexpectedly, this reduction was significantly less than that in the 20 min anodal stimulation [F(2, 22)=20.66, P=0.027 Figure 2A]. The linear regression analysis revealed that there was no significant relationship between the anodal durations (0, 20, and 30 min durations) and the freezing scores [F (1, 2) = 0.43, P=0.63, r2=-0.39], indicating that there was no direct relationship between tDCS-induced effects and the duration of stimulation.
Figure 1.
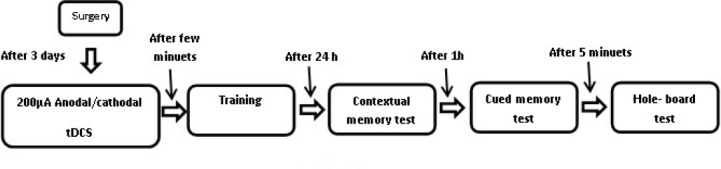
Schematic overview of experimental procedure: Three days after surgery, the animals received anodal or cathodal stimulation (at 20 min or 30 min durations) just prior to training. 24 hr after conditioning, the contextual fear memory was scored continually for 5 min in the training chamber by an observer; one hour after the contextual fear test, the cued fear memory was scored continually for 3 min by an observer. 5 min after the cued test, exploratory behaviors of the subjects were assessed by a hole-board apparatus
Figure 2.
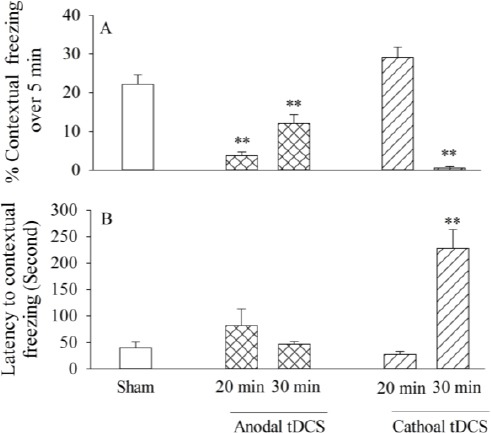
The effects of left prefrontal anodal or cathodal transcranial direct current stimulation (tDCS) on contextual fear memory: the percentage of contextual freezing at 20 min or 30 min duration (A), latency to contextual freezing (B). All bars are expressed as mean±SEM (sham group: n= 9, anodal or cathodal group: n=8; **P<0.01 compared with the sham group)
Regarding the latency to contextual freezing, as an index of memory recalling, a similar analysis showed that neither 20 min [F (2, 22)=1.35, P=0.28, Figure 2B] nor 30 min F(2, 22)=1.35, P=0.96, Figure 2B] anodal stimulation had any statistically signifi-cant effects compared to sham, suggesting that the left prefrontal anodal tDCS did not affect the retrieval of contextual conditioned fear memory.
Application of left prefrontal cathodal tDCS at 30 min duration disrupted acquisition and retrieval of contextual conditioned fear memory but not at 20 min duration
One way ANOVA and Tukey’s post hoc analysis showed that pre-training 30 min with cathodal tDCS impaired contextual fear memory[F(2, 22)=47.54, P=0.001, Figure 2A] while cathodal tDCS at 20 min duration had no effect in comparison to the sham [F(2, 22)=47.57 P=0.07, Figure 2A]. The linear regression analysis revealed that there was no significant relationships between cathodal durations (0, 20, and 30 min) and the freezing scores, indicating that there was no direct relationship between the induced-effects and the stimulation duration [F(1, 2)=1.1, P=0.48, r2=0.05].
Regarding the latency to contextual freezing, the same analysis showed that 20 min cathodal [F (2, 22) =28.36, P=0.99, Figure 2B] stimulation had no statistically significant effect while 30 min cathodal [F(2, 22)= 28.36, P=0.001, Figure 2B] increased contextual freezing latency in comparison to sham, suggesting that left prefrontal 30 min cathodal tDCS, as pretraining, induced contextual fear memory recall impairment.
The effect of left prefrontal anodal and cathodal tDCS on the acquisition of cued fear memory
The application of left prefrontal anodal tDCS at 20 min duration impaired the acquisition of cued fear memory but it had no effects on memory at 30 min duration
One way ANOVA and Tukey’s post hoc analysis showed that pre-training left prefrontal administra-tion of 20 min anodal tDCS significantly decreased the cued freezing [F(2,22)=7.7, P=0.002, Figure 3A] compared to the sham group whereas by increasing the duration up to 30 min, we did not observe any significant effects on the acquisition of cued fear memory[F(2,22)=7.7, P=0.16, Figure 3A] in comparison to the sham, indicating that left prefrontal anodal tDCS impaired acquisition of cued memory at 20 min duration. The linear regression analysis revealed that there was no significant relationship between the anodal durations (0, 20 and 30 min durations) and the freezing scores, [F (1, 2) = 0.3, P=0.67, r2=-0.53].
Figure 3.
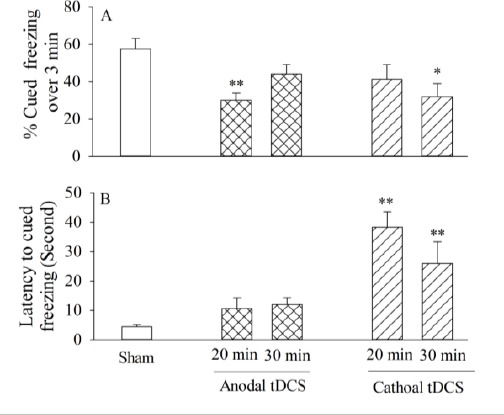
The effects of left prefrontal anodal or cathodal transcranial direct current stimulation (tDCS) on cued fear memory: the percentage of contextual freezing at 20 min or 30 min duration (A), latency to the cued freezing (B). All bars are expressed as mean±SEM (sham group: n=9, anodal or cathodal group: n=8; *P<0.05, **P<0.01 compared with the sham group)
With respect to latency to the cued freezing, no significant differences existed at both 20 min [F (2, 22)=2.73, P=0.19] and 30 min[F(2, 22)=2.73, P= 0.09, Figure 3B] durations in comparison to the sham, suggesting that the application of left prefrontal anodal tDCS (at 20 or 30 min duration) did not impact retrieval cued conditioned fear memory.
Administration of left prefrontal cathodal tDCS impaired the acquisition of cued memory at the 30 min and not the 20 min duration
One-way ANOVA and post hoc analysis revealed that left prefrontal cathodal stimulation at 20 min duration statistically did not alter the acquisition of cued fear memory in comparison to the sham [F (2, 22)=3.6, P=0.23, Figure 3A]; while the 30 min cathodal significantly decreased the acquisition of cued conditioned fear memory compared to the sham[F(2, 22)=3.6, P=0.03, Figure 3A]. In addition, the linear regression analysis revealed that there was no significant relationship between the cathodal durations(0, 20 and 30 min durations) and the freezing scores, [F(1, 2)=0.44, P=0.09, r2=-0.95].
With respect to latency to cued freezing, the similar analysis showed that cathodal tDCS at both 20 min [F (2, 22)=12.04 , P=0.001, Figure 3B] and 30 min [F(2, 22)=12.04, P=0.01, Figure 3B] durations increased the latency compared to the sham, suggesting that pre-training left prefrontal cathodal tDCS would impair the retrieval of cued conditioned fear memory.
Effects of pre-training left prefrontal anodal or cathodal tDCS on exploratory behaviors
One way ANOVA analysis showed that application of anodal or cathodal stimulation at either durations (20 and 30 min) did not alter locomotion activity [F(4, 35)=0.38, P =0.68, Figure 4A], latency to the first head dipping [F(4, 35)= 0.165, P=0.99, Figure 4C], or head dipping number [F(4, 35)=1.64, P=0.68, Figure 4B] compared to the sham control group.
Figure 4.
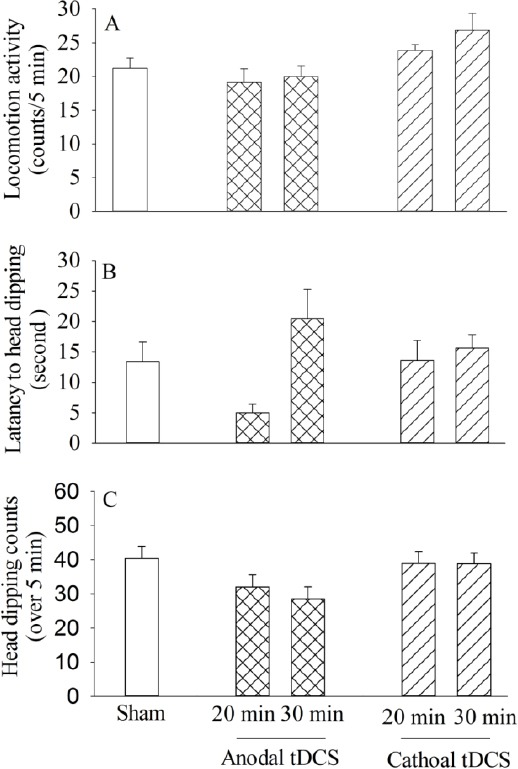
Comparison of exploratory behaviors: the locomotor activity during 5 min (A), head-dip latency (B), head dipping counts for 5 min (C), the exploratory behaviors of subjects were assessed by a hole-board apparatus. All bars are expressed as mean±SEM (sham group: n = 9, anodal or cathodal group: n=8; *P < 0.05, **P<0.01 compared with the sham group)
Discussion
In order to evaluate the polarity-dependent effects of tDCS on the acquisition of fear memory and the functional connectivity, we applied left prefrontal anodal or cathodal stimulation to healthy mice for the durations of 20 and 30 min prior to fear conditioning.
In relation to contextual memory, our results revealed that, although, both the 20 min and 30 min left prefrontal anodal stimulations disrupted signifi-cantly the contextual fear memory in comparison to the sham, the 20 min anodal induced impairment of the fear memory was more than the 30 min anodal’s. In cathodal stimulation, the 20 min cathodal stimulation trended significantly toward an enhanced acquisition of the contextual fear memory compared to anodal stimulation but it was not statistically significant compared with the sham; unexpectedly, increasing the duration of stimulation up to 30 min, impaired the acquisition of contextual fear memory.
Additionally, with respect to cued fear memory, our results indicated that a 20 min anodal tDCS impaired cued fear memory, but a 30 min anodal had no significant effect. Thus, similar to the contextual fear memory, the prolongation of the stimulation duration did not increase tDCS efficacy. Although cathodal stimulation at 30 min duration resulted in cued fear memory impairment, at the 20 min duration, it had no effect on the cued fear memory.
Based on these results, by increasing the duration of anodal or cathodal stimulation, not only the tDCS-induced contextual or cued fear memory effect did not linearly increase but also the effect was diminished and tended to be reversed.
Thus, it seems that this relationship is more complex than once understood in that the prolongation of tDCS-duration does not necessarily increase the induced effect of stimulation. Our findings is consistent with the previous study in which it has been reported that anodal tDCS can actually result in decreased excitability when the stimulation time is increased and it suggests that increasing the duration of stimulation may reverse the anodal augmented cortical excitability to a decreased excitability, resulting in changes in the same direction and effect as those of cathodal tDCS (23)). In addition, consistent with our results, several pharmacological studies have shown that the tDCS effects are none-linear and might be calcium-dependent (6, 23).
In relation to the complexity of tDCS effects, a neuroimaging study demonstrated that a 5-min cathodal tDCS led to 38% reduction in cortical excitability, while 15–20 min stimulation led to a reduction of 28%. In contrast, they did not find any changes in cortical excitability after anodal tDCS (24).
Another study also showed that cathodal tDCS could enhance cortical excitability when intensity increased whereas it conventionally reduced excitability (6). Thus, the relationship between the stimulation and neural response is not dependent on just the stimulating polarity but also on other factors including the duration and the intensity of tDCS, the brain stimulated area, and the position of polarizing reference electrode are also involved in neural response (7).
Up to now, the effect of tDCS on acquisition fear memory has not been investigated, while, more attention has been paid to the consolidation and retrieval of the memory. It has been recently shown that human application of 12 min cathodal stimulation over the left dorsolateral prefrontal cortex (DLPFC) leads to an inhibitory effect on fear memory consolidation compared to anodal and sham stimulation while anodal tDCS has no effect on fear memory consolidation (25).
An important point that should be noted is that previous studies indicated that the consolidation process happened during the time window from minutes to 6 hr (26, 27). On the other hand, several studies have shown that the tDCS-after-effects last at least for 90 min and up to 24 hr or even for days, depending on the used protocols, so there is an overlap between the consolidation time and the durability of tDCS-after effects. Thus, in our study, the application of tDCS as pre-training could affect not only the acquisition but also the consolidation and retrieval of the memory.
Regarding tDCS and fear memory retrieval, our results showed that left prefrontal anodal tDCS at either duration did not affect the retrieval of both contextual and cued memory while cathodal stimulation at 20 min duration disrupted only the retrieval of cue fear memory but at 30 min duration it impaired both the contextual and cued fear memory retrieval.
Differing from our results, a recent report showed that the application of right dlPFC-anodal, left supraorbital-cathodal tDCS immediately after fear memory retrieval for 20 min at 1mA current intensity increased fear memory (28). It should be noted that in the present study the location of the reference electrode and the application time of tDCS were different from theirs. We applied non-cephalic reference as a substitute for the scalp reference used in most studies which allowed us to evaluate selectively the effect of scalp polarization over a well-defined cortical area, escaping conceivable interference due to the polarization of other cortical structures near the reference scalp electrode.
According to our results, it seems that cathodal tDCS at both durations increased latency to cued freezing in comparison to sham while in respect with contextual freezing, the latency was increased only at 30 min duration. In addition, anodal tDCS application however, statistically did not affect neither latency to cued nor contextual freezing, but in all of our results, reduced freezing was accompanied by increased latency to freezing and versa[A2], indicating a long-lasting delay in memory retrieval causes memory formation impairment.
Thus, it seems that frontal application of tDCS has different effects on the processing of acquisition, consolidation, and recall of fear memory. Previous studies have shown that cortical excitability by anodal or cathodal stimulation is affected by elements including the polarity, the session duration, and intervals between the stimulations, the strength and timing of stimulation, the location of the reference electrode, the stimulated region, and the type of implied tasks (7).
In addition to the evaluation of conditioning fear memory, we also examined the effects of tDCS on the locomotion activity and exploratory behavior on the hole-board apparatus; the hole-board test has been used to evaluate emotionality, anxiety, and/or responses to stress in animals. Several studies have shown that changes in head-dipping behavior in the hole-board test might reflect the anxiogenic and/or anxiolytic state of mice(29, 30). It has been previously reported that the exposure of animals to various stressful stimuli changed some exploratory behaviors(31). A significant decrease of head dipping and increased latency to head dipping was observed in animals that had been exposed to stressful stimuli (32). It is noted that only the drugs with high values of head dips and normal values of locomotion behavior are considered as anxiolytics. It should also be noted that enhanced locomotor activity would result in reduced freezing and increased latency to the first cued or contextual freezing(33). In our study, lack of difference between interventional and normal groups in related locomotion, head dipping and latency to the first head dipping behaviors were evidence that all subjects were in normal and not anxiety state on the test day, which could influence the freezing behaviors of animals, and application of tDCS did not probability exert anxiogenic activity.
Also, the impairment of both contextual and cued fear memory provided further evidence in support of our hypothesis and the findings of other studies that the cortical excitability-induced changes of the prefrontal applied tDCS would affect the activity of deeper structures that are functionally connected directly or indirectly to this area. To understand the mechanism of the effect of tDCS on the amygdala and hippocampus in reducing the fear conditioned learning as well as the role of tDCS in modulating neurotransmitter systems and the concentration and expression changes in some ions and proteins involved in the memory signaling pathways, it is suggested that, together with applying tDCS, intra-amygdala and hypothalamic injections of agonists or antagonists in neurotransmitter systems involved in learning and the memory system including cholinergic and dopaminergic ones can also be done which may determine on which transmitters, tDCS exerts its effect. Moreover, at the molecular level, the impact of tDCS on gene and protein expression profiles involved in memory can also be evaluated.
Conclusion
Our results revealed that the administration of left prefrontal anodal (for both 20 and 30 min durations) and cathodal (at 30 min duration) tDCS impaired the acquisition of both contextual and cued fear memory indicating that transcranial prefrontal stimulation would affect the activity of deeper structures like amygdala and hippocampus as the main components of fear memory circuit in acquisition, storage, and expression of memory. Consistent with previous reports, we did not observe a direct correlation between stimulation duration and the efficacy of tDCS on the acquisition of contextual and cued fear memory. Regarding fear memory retrieval, cathodal tDCS would modify the retrieval while anodal stimulation had no effect.
Acknowledgment
The authors would like to express their appreciation to Dr Fariborz Manteghi for designing the electrode.
References
- 1.Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, Giacopuzzi M, et al. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord. 2009;118:215–219. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Boggio PS, Valasek CA, Campanhã C, Giglio AC, Baptista NI, Lapenta OM, et al. Non-invasive brain stimulation to assess and modulate neuroplasticity in Alzheimer’s disease. Neuropsychol Rehabil. 2011;21:703–716. doi: 10.1080/09602011.2011.617943. [DOI] [PubMed] [Google Scholar]
- 3.Boggio PS, Fregni F, Valasek C, Ellwood S, Chi R, Gallate J, et al. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:444–447. doi: 10.1136/jnnp.2007.141853. [DOI] [PubMed] [Google Scholar]
- 4.Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, et al. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79:451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- 5.Teo F, Hoy KE, Daskalakis ZJ, Fitzgerald PB, et al. Investigating the role of current strength in tDCS modulation of working memory performance in healthy controls. Front Psychiatry. 2011;2:3389. doi: 10.3389/fpsyt.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014;37:42–753. doi: 10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, Löscher W, et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47:1216–1224. doi: 10.1111/j.1528-1167.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 9.Liebetanz D, Fregni F, Monte-Silva KK, Oliveira MB, Amâncio-dos-Santos A, Nitsche MA, et al. After-effects of transcranial direct current stimulation (tDCS) on cortical spreading depression. Neurosci Lett. 2006;398:85–90. doi: 10.1016/j.neulet.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 10.Paxinos G, Franklin kB. The mouse brain in stereotaxic coordinates. Gulf Professional Publishing; 2004. [Google Scholar]
- 11.Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stapley NW, Guariglia SR, Chadman KK. Cued and contextual fear conditioning in BTBR mice is improved with training or atomoxetine. Neurosci Lett. 2013;549:120–124. doi: 10.1016/j.neulet.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson CW. Role of amygdala–prefrontal cortex circuitry in regulating the expression of contextual fear memory. Neurobiol Learn Mem. 2011;96:315–323. doi: 10.1016/j.nlm.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 14.McDonald A, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 15.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 16.Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: a review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 17.Kochli DE, Thompson EC, Fricke EA, Postle AF, Quinn JJ. The amygdala is critical for trace, delay, and contextual fear conditioning. Learn Mem. 2015;22:92–100. doi: 10.1101/lm.034918.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 19.Nasehi M, Zamanparvar M, Ebrahimi-Ghiri M, Zarrindast MR. Modulation of cannabinoid signaling by hippocampal 5-HT4 serotonergic system in fear conditioning. J Psychopharmacol. 2016;30:936–944. doi: 10.1177/0269881116652584. [DOI] [PubMed] [Google Scholar]
- 20.Nasehi M, Davoudi K, Ebrahimi-Ghiri M, Zarrindast MR. Interplay between serotonin and cannabinoid function in the amygdala in fear conditioning. Brain Res. 2016;1636:142–151. doi: 10.1016/j.brainres.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 21.Nasehi M, Kamali-Dolatabadi L, Torabi-Nami M, Zarrindast MR. Possible involvement of the CA1 GABAA receptors upon acquisition and expression of the ACPA-induced place preference in mice. Physiol Behav. 2016;161:155–165. doi: 10.1016/j.physbeh.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Nasehi M, Meskarian M, Khakpai F, Zarrindast MR. Harmaline-induced amnesia: Possible role of the amygdala dopaminergic system. Neuroscience. 2016;312:1–9. doi: 10.1016/j.neuroscience.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6:424–432. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Baudewig J, Nitsche MA, Paulus W, Frahm J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med. 2001;45:196–201. doi: 10.1002/1522-2594(200102)45:2<196::aid-mrm1026>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Asthana M, Nueckel K, Mühlberger A, Neueder D, Polak T, Domschke K, et al. Effects of transcranial direct current stimulation on consolidation of fear memory. Front Psychiatry. 2013;4:107. doi: 10.3389/fpsyt.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 27.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 28.Mungee A, Kazzer P, Feeser M, Nitsche MA, Schiller D, Bajbouj M. Transcranial direct current stimulation of the prefrontal cortex: a means to modulate fearmemories. Neuroreport. 2014;25:480–484. doi: 10.1097/WNR.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji M, Takeda H, Matsumiya T. Different effects of 5-HT1A receptor agonists and benzodiazepine anxiolytics on the emotional state of naive and stressed mice: a study using the hole-board test. Psychopharmacology. 2000;152:157–166. doi: 10.1007/s002130000514. [DOI] [PubMed] [Google Scholar]
- 30.Takeda H, Tsuji T, Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharm. 1998;350:21–29. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- 31.Stone EA, Trullas R, Platt JE. The effect of acute and chronic administration of desmethylimipramine on responses to stress in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 1984;8:587–592. doi: 10.1016/0278-5846(84)90019-8. [DOI] [PubMed] [Google Scholar]
- 32.Echandia ER, Broitman S, Foscolo M. Effect of the chronic ingestion of chlorimipramine and desipramine on the hole board response to acute stresses in male rats. Pharmacol Biochem Behav. 1987;26:207–210. doi: 10.1016/0091-3057(87)90106-7. [DOI] [PubMed] [Google Scholar]
- 33.Vazdarjanova A, McGaugh JL. Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proc Natl Acad Sci. 1998;95:15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]


