Abstract
Objective(s):
To investigate the efficiency of a novel series of coumarin derivatives bearing benzoheterocycle moiety as novel cholinesterase inhibitors.
Materials and Methods:
Different 7-hydroxycoumarin derivatives were synthesized via Pechmann or Knoevenagel condensation and conjugated to different benzoheterocycle (8-hydroxyquinoline, 2-mercaptobenzoxazole or 2-mercaptobenzimidazole) using dibromoalkanes 3a-m: Final compounds were evaluated against acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) by Ellman’s method. Kinetic study of AChE inhibition and ligand-protein docking simulation were also carried out for the most potent compound 3b.
Results:
Some of the compounds revealed potent and selective activity against AChE. Compound 3b containing the quinoline group showed the best activity with an IC50 value of 8.80 μM against AChE. Kinetic study of AChE inhibition revealed the mixed-type inhibition of the enzyme by compound 3b. Ligand-protein docking simulation also showed that the flexibility of the hydrophobic five carbons linker allows the quinoline ring to form π-π interaction with Trp279 in the PAS.
Conclusion:
We suggest these synthesized compounds could become potential leads for AChE inhibition and prevention of AD symptoms.
Keywords: Acetylcholinesterase, Alzheimer’s disease, Benzoheterocycles, Butyrylcholinesterase, Coumarin
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder leading to loss of memory and cognitive ability. It was estimated that more than 18 million people suffer from AD worldwide and the number of patients will be increased to 70 million by 2050 (1). It has been reported that reduction of the acetylcholine level in the brain, accumulation of beta-amyloid (βA) plaques, neurofibrillary tangles, and oxidative stress are the most common causes of AD (2). The detailed mechanism of AD is still ambiguous and there is a progressive attention to discover and explain the pathogenesis of AD (3, 4). The most common approach for the treatment of AD is application of cholinesterase inhibitors (ChEIs) increasing the synaptic levels of ACh in the brain (5–7). Galantamine, donepezil, and rivastigmine are the mainstay of AD patient management (8, 9). The AChE has a nearly 20 Aº deep narrow gorge with
two binding sites of the catalytic active site (CAS) at the bottom of the gorge and peripheral anionic site (PAS) near the entry of the gorge. PAS as Aβ binding domain leads formation of the stable and toxic AChE-βA complex (10-11). It has been revealed that the activity of BuchE is more than AChE in patients suffering AD and plays an important role in regulation of ACh level in the neurosynaptic area (12).
Coumarins are natural plant species with diverse biological activities including antiinflammatory (13), anti-tumor (14), anti-oxidant (15), and anti-diabetic (16). Several studies demonstrated that coumarins can bind to the PAS of AChE by their aromatic ring preventing the formation of the Aβ-AChE adducts (16-20). Coumarin derivatives have been also considered as neuroprotective agents against oxidative stress and free radical generation (21).
On the other hand, benzoheterocyclic frame-works such as benzoxazole (A) (19) and 8-hydroxy-quinoline (B) (20) analogs have been described as multifunctional anti-AD agents with the ability to target cholinesterase and prevent deposition of amyloid aggregation as well as promote the clearance of Aβ by neutralizing deposits (Figure 1).
Figure 1.
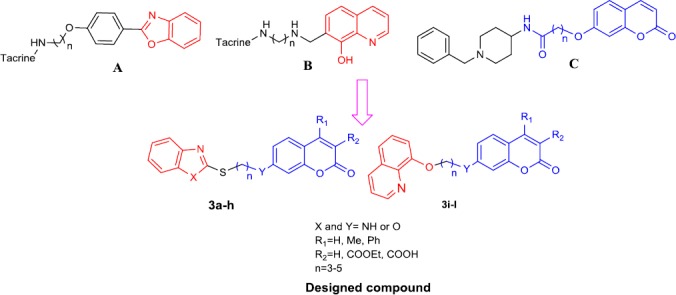
Some previously reported anti-AChE compounds bearing benzoheterocycle moiety (A and B), 7-hydroxycoumarin derivatives as AChE inhibitors reported in our previous study (C) and new designed AChE inhibitors 3a-l
Considering the above results and in continuation of our previous works on coumarin derivatives (Figure 1, C) (21-25), herein we report preparation, evaluation, and molecular modeling study of a series of novel coumarin hybrids (3a-l) bearing different benzoheterocycles as ChE inhibitors (Figure 1).
Materials and Methods
Chemistry
All commercially available chemical reagents were used without further purification. The progress of the reactions was monitored by TLC on silica gel 250 micron, F254 plates. Melting points were measured on a Kofler hot stage. The IR spectra were taken using Nicolet FT-IR Magna 550 spectrograph (KBr discs). 1H NMR spectra were recorded on Brucker 500 MHz. The chemical shifts (δ) and coupling constants (J) are expressed in parts per million and Hertz, respectively. The atoms numbering of the target compounds used for 1H NMR is shown in Scheme 1. Elemental analyses were carried out by a CHN- Rapid Heraeus elemental analyzer and the results were within ±0.5% of the calculated values.
Scheme 1.
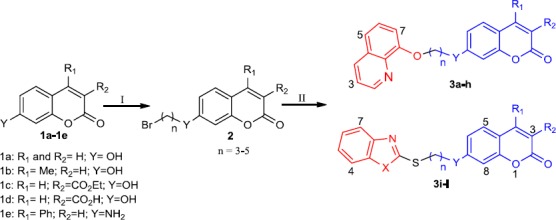
Synthesis of target compounds 3a-l. Reagent and condition: (I) Br(CH2)nBr (n= 3-5), K2CO3, acetone, reflux; (II) DMF, K2CO3, appropriate benzoheterocycle, 24 hr, 70 ºC
Preparation of compound 3a-l
A mixture of benzoheterocycle (0.5 mmol), anhydrous K2CO3 (0.5 mmol), and the corresponding coumarin derivate 2a-l (0.5 mmol) in dry N, N-dimethylformamide (8 ml) was stirred at 70 °C for 8 hr. After completion of the reaction (monitored by TLC), the mixture was cooled to the room temperature and ice water was then added to the mixture and stirred for 30 min. The obtained solid was finally filtered and dried to obtain 3a-l.
Docking study
Ligand-protein docking was conducted using Autodock Vina (1.1.2) (26) and the binding poses were retrieved. For this purpose, the 3D structure of the AChE was obtained from Protein Data Bank (PDB) at http://www.rcsb.org/pdb/home/home.do. The crystal structure of AChE (PDB ID: 1eve) in complex with donepezil was chosen. None-protein atoms were removed in the protein preparation process. The appropriate pdbqt format of the receptor was then prepared using Autodock Tools (1.5.6) (27). To prepare ligands, the 2D chemical structure of ligands was sketched using MarvinSketch 5.8.3, 2012, ChemAxon (http://www.chemaxon.com) and then converted to 3D format by Openbabel (ver 2.3.1) (28). Finally, the required pdbqt format of ligands was prepared using an Autodock Tools python script, prepare_ligand4. py. The grid box with the size of 15×15×15 Å was determined and the center of the box was fixed on the center of co-crystalized ligand. After docking, the best pose was selected for further analysis. The graphics are depicted using Chimera 1.10 software (29).
Pharmacology
The capacity of the target compounds against AChE and BuChE was determined by Ellman’s method (30). The stock solution of the tested compounds was dissolved in DMSO 1% and diluted in 3 ml phosphate buffer (0.1 mol/l, pH 8.0), 100 μl of 5,5’-dithio-bis(2-nitrobenzoic acid), 100 μl of 2.5 IU/ml of acetylcholinesterase or butyrylcholinesterase. Assays were measured at 412 nm for 6 min at 25 °C by using a UV Unico Double Beam spectrophotometer. The IC50 values were determined graphically from log concen-tration vs. % of inhibition curves. All experiments were performed in three different experiments.
Results
Chemistry
All synthesized derivatives were characterized by spectroscopic methods such as 1H NMR, IR, and CHN.
7-(4-(Quinolin-8-yloxy)butoxy)-2H-chromen-2-one (3a).
White solid; Yield (55%) mp 131-133 ºC; IR (KBr, cm-1) ῡ: 3050 (N-H), 2951(C-H), 1717 (C=O), 1615 (C=N), 1552 (C=C). 1H NMR (DMSO-d6, 500 MHz) δ: 8.86 (d, 1H, J= 4.0Hz, H2 quinoline), 8.29 (d, 1H, J= 8.5 Hz, H4 quinoline), 7.97 (d, 1H, J= 10.0 Hz, H4 coumarin), 7.60 (d, 1H, J = 8.5 Hz, H5 coumarin), 7.53-7.48 (m, 3H, H5,6,7 quinoline), 7.19-7.20 (m, 1H, H3 quinoline), 7.01 (s, 1H, H8 coumarin), 6.96 (d, 1H, J = 8.5 Hz, H6 coumarin), 6.27 (d, 1H, J = 10.0 Hz, H3 coumarin), 4.25 (s, 4H, CH2-O), 2.02 (s, 4H, CH2). 13C NMR (DMSO-d6, 125 MHz) δ: 161.8, 160.1, 155.3, 154.4, 148.8, 144.2, 139.7, 135.6, 129.3, 128.9, 126.7, 121.6, 119.4, 112.6, 112.2, 112.1, 109.3, 101.1, 68.1, 67.9, 25.5, 25.2. Anal. Calcd for: C22H19NO4 (361.39): C, 73.12; H, 5.30; N, 3.88. Found: C, 73.36; H, 5.66; N, 3.44.
7-((5-(Quinolin-8-yloxy)pentyl)oxy)-2H-chromen-2-one (3b)
White solid; Yield (94%) mp 107-109 ºC; IR (KBr, cm-1) ῡ: 2947 (C-H), 1726 (C=O), 1616 (C=N). 1H NMR (DMSO-d6, 500 MHz) δ: 8.85 (dd, 1H, J= 2.0 Hz, J= 4.0 Hz, H2 quinoline), 8.29 (dd, 1H, J= 2.0 Hz, J = 4.0 Hz, H4 quinoline), 7.96 (d, 1H, J= 9.5 Hz, H4 coumarin), 7.60 (d, 1H, J= 8.5 Hz, H5 coumarin), 7.50-7.53 (m, 1H, H5 quinoline), 7.49-7.46 (m, 2H, H6,7 quinoline), 7.18-7.20 (m, 1H, H3 quinoline), 6.97 (s, 1H, H8 coumarin), 6.93 (d, 1H, J= 8.5 Hz, H6 coumarin), 6.26 (d, 1H, J = 9.5 Hz, H3 coumarin), 4.2 (t, 2H, J = 6.5 Hz, CH2-O), 4.13 (t, 2H, J = 6.5 Hz, CH2-O), 1.94 (quint, 2H, J = 6.5 Hz, CH2), 1.87 (quint, 2H, J = 6.5 Hz, CH2), 1.68-1.71 (m, 2H, CH2). 13C NMR (DMSO-d6, 125 MHz) δ: 161.8, 160.1, 155.3, 154.5, 148.7, 144.2, 139.7, 135.6, 129.3, 128.9, 126.7, 121.6, 119.3, 112.6, 112.2, 112.1, 109.3, 101.1, 68.2, 68.1, 28.3, 28.1, 22.2. Anal. Calcd for: C21H17NO4 (375.42): C, 72.61; H, 4.93; N, 4.03. Found: C, 72.31; H, 4.33; N, 4.28.
4-Methyl-7-(3-(quinolin-8-yloxy)propoxy)-2H-chromen-2-one (3c)
Red solid; Yield (72%); mp 112-114 ºC; IR (KBr, cm-1) ῡ: 3407 (N-H), 2949 (C-H), 1715 (C=O), 1616 (C=N). 1H NMR (DMSO-d6, 500 MHz) δ: 8.86 (d, 1H, J = 2.5 Hz, H2 quinoline), 8.29 (d, 1H, J = 8.0 Hz, H4 quinoline), 7.65 (d, 1H, J=8.5 Hz, H5 coumarin), 7.53 (m, 1H, H5 quinoline), 7.50-7.47 (m, 2H, H6,7 coumarin), 7.23 (t, 1H, J = 2.5Hz, H3 quinoline), 7.00 (s,1H, H8), 6.98 (d, 1H, J = 2.5Hz, H6 quinoline), 6.18 (s, 1H, H3 coumarin), 4.35-4.37 (m, 4H, CH2-O), 2.37 (s, 3H, CH3), 2.34-2.36 (m, 2H, CH2). 13C NMR (DMSO-d6, 125 MHz) δ:161.5, 159.9, 154.6, 154.2, 153.1, 148.8, 139.7, 135.6, 128.9, 126.6, 126.3, 121.6, 119.6, 113.0, 112.2, 111.0, 109.6, 101.2, 65.1, 64.9, 28.4, 17.9. Anal. Calcd for: C22H19NO4 (361.39): C, 73.12; H, 5.30; N, 3.88. Found: C, 73.36; H, 5.56; N, 3.96.
4-Methyl-7-((5-(quinolin-8-yloxy)pentyl)oxy)-2H-chromen-2-one (3d)
White solid; Yield (50%); mp 100-102 ºC; IR (KBr, cm-1) ῡ: 2943-2871 (C-H), 1723 (C=O), 1612 (C=N), 1107 (C-O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.85 (d, 1H, J=3.0 Hz, H2 quinoline), 8.29 (dd, 1H, J = 8.0 and J=2.0 Hz, H4 quinoline), 7.65 (d, 1H, J=9.0 Hz, H5 coumarin), 7.50-7.53 (m, 1H, H5 quinoline), 7.49-7.47 (m, 2H, H6,7 quinoline), 7.18-7.20 (m, 1H, H3 quinoline), 6.96 (s, 1H, H8 coumarin), 6.94 (d, 1H, J=2.5 Hz, H6 coumarin), 6.18 (s, 1H, H3 coumarin), 4.20 (t, 2H, J=6.5 Hz, CH2-O), 4.13 (t, 2H, J=6.5 Hz, CH2-O), 2.38 (s, 3H, CH3), 1.94 (quint, 2H, J=7.0 Hz, CH2), 1.87 (quint, 2H, J=8.0 Hz, CH2), 1.69 (m, 2H, CH2). 13C NMR (DMSO-d6, 125 MHz) δ: 161.6, 160.0, 154.6, 154.4, 153.2, 148.7, 139.7, 135.6, 128.9, 126.6, 126.2, 121.6, 119.3, 112.9, 112.3, 110.9, 109.3, 101.1, 68.1, 28.3, 28.1, 22.2, 17.9. Anal. Calcd for: C24H23NO4 (375.42): C, 74.02; H, 5.95; N, 3.60. Found: C, 74.15; H, 5.68; N, 3.85.
Ethyl 2-oxo-7-(3-(quinolin-8-yloxy)propoxy)-2H-chromene-3-carboxylate(3e).
Red solid; Yield (95%); mp 63-65 ºC; IR (KBr, cm-1) ῡ: 2963-2878 (C-H), 1747 (C=O), 1610 (C=N), 1552 (C=C). 1H NMR (DMSO-d6, 500 MHz) δ: 8.87 (bs, 1H, H2 quinoline), 8.68 (s,1H, H4 coumarin), 8.29 (d, 1H, J = 7.0 Hz, H4 quinoline), 7.81(d, 1H, J = 8.0 Hz, H5 coumarin), 7.51-7.50 (m, 3H, H5,6,7 quinoline), 7.23 (bs, 1H, H3 quinoline), 7.07-7.03 (m, 2H, H6,8 coumarin), 4.40-4.27 (m, 2H, CH2CH3 and 4H, CH2-O), 2.36 (s, 2H, CH2), 1.30 (t, 3H, J = 7.0 Hz CH3). 13C NMR (DMSO-d6, 125 MHz) δ:163.9, 162.7, 156.8, 156.1, 154.2, 148.9, 139.7, 135.6, 131.5, 128.9, 126.6, 121.7, 119.7, 113.4, 113.3, 111.3, 109.6, 100.7, 65.5, 64.8, 60.8, 28.4, 14.0. Anal.Calcd for: C24H21NO6 (419.43): C, 68.73; H, 5.05; N, 3.34. Found: C, 68.42; H, 5.28; N, 3.25.
Ethyl 2-oxo-7-((5-(quinolin-8-yloxy)pentyl)oxy)-2H-chromene-3-carboxylate (3f).
Red solid; Yield (94%); mp 66-68 ºC; IR (KBr cm-1) ῡ: 2941-2877 (C-H), 1764 (C=O), 1609 (C=N). 1H NMR (DMSO-d6, 500 MHz) δ: 8.84 (s, 1H, H2 quinoline), 8.68 (s,1H, H4 coumarin), 8.27 (d, 1H, J = 7.0 Hz, H4 quinoline), 7.80 (d, 1H, J = 8.0 Hz, H5 coumarin), 7.50-7.47 (m, 3H, H5,7,6 quinoline), 7.18 (bs,1H, H3 quinoline), 6.99 (m, 2H; H6,8 coumarin), 4.28-4.18 (m, 2H, CH2CH3 and 4H, CH2-O), 1.93-1.88 (m, 4H, CH2), 1.68 (s, 2H, CH2), 1.30 (s, 3H, CH3). 13C NMR (DMSO-d6, 125 MHz) δ: 164.1, 162.7, 156.8, 156.1, 154.5, 148.9, 148.7, 139.7, 135.5, 131.5, 128.9, 126.6, 121.6, 119.3, 113.4, 113.1, 111.2, 109.3, 100.6, 68.5, 68.1, 60.7, 28.3, 28.0, 22.2, 14.0. Anal.Calcd for: C26H25NO6 (477.41): C, 69.79; H, 5.63; N, 3.13. Found: C, 69.58; H, 5.95; N, 4.31.
2-Oxo-7-(3-(quinolin-8-yloxy)propoxy)-2H-chromene-3-carboxylic acid (3g)
Light brown solid; Yield (95%) mp 63 ºC; IR (KBr, cm-1) ῡ: 3438 (O-H), 2930 (C-H), 1760 (C=O), 1623 (C=N). 1H NMR (DMSO-d6, 500 MHz) δ: 9.14 (bs, 1H, H2 quinoline), 9.01 (d, 1H, J = 7.0 Hz H4 quinoline), 8.68 (s, 1H, H4 coumarin), 8.01 (bs, 1H, H5 coumarin), 7.80-7.78 (m, 3H, H5,6,7 quinoline), 7.59 (d, 1H, J = 7.0Hz, H3 quinoline), 7.04-7.00 (m, 2H, H6,8 coumarin), 4.50-4.47 (m, 4H, CH2-O), 2.39 (m, 2H, CH2). 13C NMR (DMSO-d6, 125 MHz) δ:164.1, 163.8, 157.1, 156.7, 149.9, 148.9, 145.9, 144.1, 131.5, 129.4, 122.7, 120.0, 113.8, 113.5, 112.7, 111.6, 100.8, 65.8, 65.5, 28.1, 14.0. Anal.Calcd for: C20H15NO6S (397.06): C, 60.45; H, 3.80; N, 3.52. Found: C, 60.71; H, 3.63; N, 3.77.
4-Phenyl-7-((3-(quinolin-8-yloxy)propyl)amino)-2H-chromen-2-one (3h)
Cream solid; Yield (48%) mp 74-76 ºC; IR (KBr, cm-1) ῡ: 3339 (NH), 2925-2871(C-H), 1713 (C=O), 1106 (C-O). 1H NMR (DMSO-d6, 500 MHz) δ: 8.91 (bs, 1H, H2 quinoline), 8.30 (d, 1H, J = 8.0Hz, H4 quinoline), 7.53-7.47 (m, 6H, 5Hphenyl and 1H, H5 coumarin), 7.21-7.00 (m,3H, H5,6,3 quinoline), 6.66 (d, 1H, J = 9.0 Hz, H6 coumarin), 6.58 (s, 1H, H8 coumarin), 5.90 (s,1H, H3 coumarin), 4.31 (bs, 2H, CH2-O), 3.41 (bs, 2H, CH2-NH), 2.16 (bs, 2H, CH2). 13CNMR (DMSO-d6, 125 MHz) δ: 160.4, 156.4, 155.6, 154.2, 152.5, 149.0, 139.7, 135.7, 135.6, 129.2, 128.9, 128.6, 128.1, 127.3, 126.7, 121.7, 119.5, 110.5, 109.4, 107.2, 107.0, 96.6, 66.5, 39.8, 28.0. Anal. Calcd for: C27H22N2O3 (422.48): C, 76.76; H, 5.25; N, 6.66. Found: C, 76.30; H, 4.92; N, 6.33.
7-((5-((1H-Benzo[d]imidazol-2-yl)thio) pentyl)oxy)-2H-chromen-2-one (3i)
White solid; Yield (84%) mp 107-109 °C; IR (KBr, cm-1) ῡ: 3173 (N-H), 2929-2880 (C-H), 1700 (C=O), 1618 (C=N). 1H NMR (CDCl3, 500 MHz) δ: 8.04 (d, 1H, J = 9.7 Hz, 1H, H4 coumarin), 7.61(d, 1H, J = 9.7 Hz, H7 benzimidazole), 7.50 (bs, 1H, H4 benzimidazole), 7.31 (d, 1H, J = 8.5 Hz, H5 coumarin), 7.17 (m, 2H, H5,6 benzimidazole), 6.77 (d, 1H, J = 8.5 Hz, H6 coumarin), 6.73 (s, 1H, H8cumarin), 6.23 (d, 1H, J = 9.7 Hz, H3 coumarin), 3.93 (t, 2H, J = 6.5 Hz, CH2-O), 3.35 (t, 2H, J = 6.5 Hz, CH2-S), 1.83 (quint, 2H, J = 7.4 Hz, CH2), 1.77 (quint, 2H, J = 7.4 Hz, CH2), 1.58 (quint, 2H, J=7.4 Hz, CH2). 13C NMR (CDCl3, 125 MHz) δ: 162.2, 161.4, 155.7, 150.4, 143.5, 128.7, 122.1, 114.0, 112.9, 112.8, 112.3, 101.3, 68.2, 32.2, 29.2, 28.3, 24.9. Anal. Calcd for: C21H21N3O2S (380.46): C, 66.47; H, 5.58; N, 11.07. Found: C, 66.71; H, 5.22; N, 10.89.
7-((5-(Benzo[d]oxazol-2-ylthio)pentyl)oxy)-2H-chromen-2-one (3j)
White solid; Yield (89%) mp 54-56 ºC; IR (KBr, cm-1) ῡ: 2931-2974 (C-H), 1711(C=O), 1623 (C=N). 1H NMR (CDCl3, 500 MHz) δ: 7.58-7.62 (m, 2H, H4 coumarin and H7 benzoxazole), 7.42 (d, 1H, J = 7.4 Hz, H4 benzoxazole), 7.34 (d, 1H, J = 8.5 Hz, H5 coumarin), 7.29-7.21 (m, 2H, H5,6 benzoxazole), 6.81 (d, 1H, J = 8.5Hz, H6 coumarin), 6.79 (s, 1H, H8 coumarin), 6.23 (d, 1H, J = 9.7 Hz, H3 coumarin), 4.03 (t, 2H, J = 6.5 Hz, CH2-O), 3.35 (t, 2H, J=6.5 Hz, CH2-S), 1.94 (quint, 2H, J = 7.5 Hz, CH2), 1.88 (quint, 2H, J = 7.5 Hz, CH2), 1.69 (quint, 2H, J = 7.5 Hz, CH2). 13C NMR (CDCl3, 125 MHz) δ: 176.8, 162.2, 155.9, 143.35, 128.6, 124.2, 123.8, 118.3, 112.9, 112.4, 109.8, 101.3, 68.1, 32.0, 28.9, 28.4, 25.0. Anal. Calcd for: C21H19NO4S (381.44): C, 66.12; H, 5.02; N, 3.67. Found: C, 66.32; H, 5.36; N, 3.54.
7-(3-(Benzo[d]oxazol-2-ylthio)propoxy)-4-methyl-2H-chromen-2-one (3k)
White solid; Yield (50%) mp 107-109 ºC; IR (KBr, cm-1) ῡ: 2952 (C-H), 1731 (C=O), 1615 (C=N). 1H NMR (CDCl3, 500 MHz) δ: 7.57 (d, 1H, J = 7.5 Hz, H7 benzoxazole), 7.47 (d, 1H, J = 7.5 Hz, H4 benzoxazole), 7.41 (d, 1H, J = 7.5 Hz, H5 coumarin), 7.28-7.22 (m, 2H, H5,6 benzoxazole), 6.85 (d, 1H, J = 7.5 Hz, H6 coumarin), 6.81(s, 1H, H8 coumarin), 6.13 (s, 1H, H3 coumarin), 4.20 (t, 2H, J = 6.0 Hz, CH2-O), 3.51 (t, 2H, J = 7.0 Hz, CH2-S), 2.39 (bs, 5H, 2H, CH2 and 3H, CH3). 13C NMR(CDCl3, 125 MHz) δ: 164.3, 161.6, 161.1, 155.2, 152.3, 151.8, 141.8, 125.5, 124.2, 123.9, 118.4, 113.7, 112.4, 112.0, 109.8, 101.5, 66.2, 28.8, 28.7, 18.6. Anal. Calcd for: C20H17NO4S (367.42): C, 65.38; H, 4.66; N, 3.81. Found C, 65.59; H, 4.03; N, 3.98.
7-((3-(Benzo[d]oxazol-2-ylthio)propyl)amino)-4-phenyl-2H-chromen-2-one (3l)
Cream solid; Yield (54%) mp 135-137 °C; IR (KBr, cm-1) ῡ: 3329 (NH), 2930 (C-H), 1704 (C=O). 1H NMR (CDCl3, 500 MHz) δ: 7.60 (d, 1H, J= 7.0 Hz, H7 benzoxazole), 7.48-7.42 (m, 3H, benzoxazol and 1H, H5 coumarin), 7.29-7.22 (m, 5H, phenyl), 6.56 (s, 1H, H3 coumarin), 6.50 (d, 1H, J = 8.0 Hz, H6 coumarin), 6.05 (s, 1H, H8 coumarin), 5.16 (s, 1H, NH), 3.39-3.43 (m, 2H, CH2-NH and 2H, CH2-S), 2.19-2.21 (m, 2H, CH2). 13CNMR (CDCl3, 125 MHz) δ: 161.8, 156.7, 156.1, 151.9, 151.4, 141.6, 136.1, 129.2, 128.6, 128.3, 128.0, 124.4, 124.0, 118.2, 110.2, 109.9, 109.4, 109.1, 98.2, 41.4, 29.2, 28.8. Anal. Calcd for: C25H20N2O3S (422.48): C, 70.07; H, 4.70; N, 6.54. Found: C, 70.31; H, 4.62; N, 6.27.
Enzymatic assay
All synthesized compounds 3a-l were evaluated against AChE and BuChE in comparison with commer-cial donepezil as standard drug. The activities were summarized in (Table 1) as IC50 values. Also, Kinetic study was used to determine the mechanism of enzyme inhibition by compound 3b. The relative velocity of the enzyme was determined on three increasing concentrations of acetylthio-choline (ATChI) (Figures 2, 3).
Table 1.
Inhibitory activity of the target compounds 3a-m against AChE and BuChE
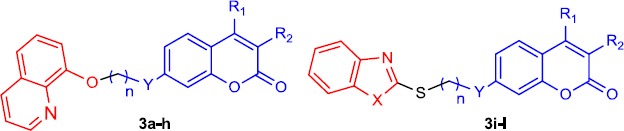 | |||||||
|---|---|---|---|---|---|---|---|
| Compounds | R1 | R2 | n | X | Y | AChE IC50 (μM) | BuChE IC50 (μM) |
| 3a | H | H | 4 | - | O | 48.00% at 35 μM | 28.30 |
| 3b | H | H | 5 | - | O | 8.80 | 26.50 |
| 3c | Me | H | 3 | - | O | 11.29 | 32.40 |
| 3d | Me | H | 5 | - | O | 13.96 | 34.60% at 35 μM |
| 3e | H | CO2 Et | 3 | - | O | 16.19 | 46.00% at 35 μM |
| 3f | H | CO2 Et | 5 | - | O | 8.94 | 29.16% at 35 μM |
| 3g | H | CO2H | 3 | - | O | 15.00% at 35 μM | 2.00% at 35 μM |
| 3h | Ph | H | 3 | - | NH | 30.20 | 37.00% at 35 μM |
| 3i | H | H | 5 | NH | O | 11.72 | 38.00% at 35 μM |
| 3j | H | H | 5 | O | O | 25.37 | 14.00% at 35 μM |
| 3k | Me | H | 3 | O | O | 44.00% at 35 μM | 11.00% at 35 μM |
| 3l | Ph | H | 3 | O | NH | 13.00 | 26.00% at 35 μM |
| Donepezil | 0.016 | 5.41 | |||||
Figure 2.
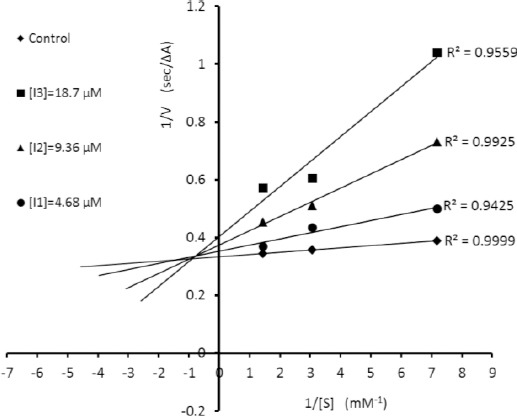
Lineweaver-Burk plot for the inhibition of AChE by 3b
Figure 3.
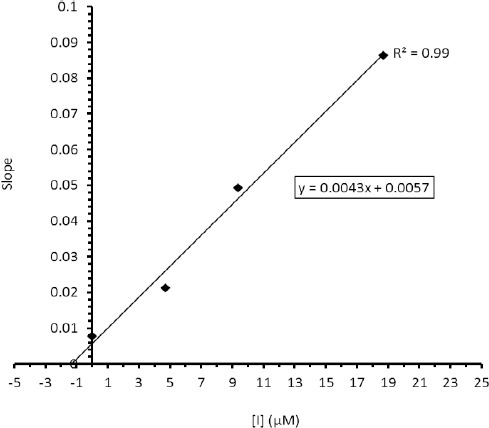
Lineweaver-Burk secondary plot for Ki calculation
Docking
The most active compound 3b was optimized and docked into the active site of the enzyme using Autodock Vina program to investigate binding mode of the ligand to AChE (Figure 4).
Figure 4.
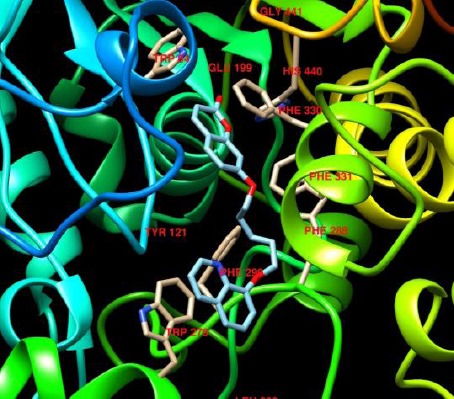
Schematic interaction of compound 3b with the active site of AChE
Discussion
Chemistry
Target compounds 3a-l were easily synthesized via the procedure outlined in (scheme 1). The coumarin derivatives 1a-e were initially prepared via Pechmann or Knoevenagel condensation accor-ding to the previously reported procedure (31-33). 7-Hydroxycoumarin 1a was commercially purchased.
Compound 1b was synthesized via Pechmann condensation between resorcinol and ethyl acetoacetate in the presence of sulfuric acid as a catalyst (31). Compound 1c was prepared through Knoevenagel condensation between diethyl malo-nate and 2,4-dihydroxybenzaldehyde catalyzed by a few drops of piperidine which was then hydrolyzed in an aqueous solution of sodium hydroxide to prepare acid analog 1d (32). Compound 1e was also prepared via reaction of ethyl (3-hydroxyphenyl) carbamate with phenyl acetoacetate (33). Different dibromoalkanes (n=3-5) were then employed as cross-linkers to bridge between coumarin ring and different benzoheterocycles. This substitution nucleophilic reaction was performed in the presence of acetone as solvent and K2CO3 as the base with an excess amount of appropriate dibromoalkanes (10 equivalents). Compound 2 was finally reacted with different benzoheterocycles in DMF as solvent and K2CO3 as the base at 70ºC to prepare target compounds 3a-l.
Ligand-protein docking simulation
As depicted in Figure 4, the coumarin ring of the compound is placed in the mid gorge of AChE active site in parallel with phenyl ring of Phe330 and makes a π-π stacking with this residue. The quinoline head is oriented toward the PAS. The flexibility of the linker allows the quinoline ring to form another π-π interaction with Trp279 in the PAS. The mentioned interactions are responsible for the affinity between the ligand and the enzyme. The aliphatic linker also participates in the binding of the ligand to the enzyme through hydrophobic interactions with side chains of amino acids in the active site of AChE.
Anti-cholinesterase activity
As indicated in Table 1, target compounds showed a diverse range of activity and different parameters affect their inhibitory. Compounds 3d and 3k containing 8-hydroxyquinoline linked to simple and ethyl 3-coumarincarboxylate, respective-ly, through a 5-carbon spacer, exhibited the most potent inhibitory activity against AChE (IC50=8.80 and 8.94 μM). The results revealed that the AChE inhibitory was closely dependent on the length of the alkylene chain and 5-carbon bond spacer is the proper length for derivatives without any substituent at 4-position of the coumarin ring. The comparison of compounds 3e and 3g showed that conversion of ester groups at 3-position of coumarin ring to the corresponding acid profoundly diminished the AChE inhibitory (IC50=16.19 μM vs. 15.00% inhibition at 35 μM). The results also revealed that the presence of substituent at 4-position of coumarin ring could affect the inhibitory activity. Compound 3b showed superior activity compared with compound 3d (IC50=8.80 vs. 13.96 μM). The comparison of unsubstituted coumarins with five carbons linker (n= 5) in terms of anti-AChE activity showed that the order of activity by considering the type of benzoheterocycle moiety was as follow: quinoline> benzimidazole > benzoxazole (3b, 3i, and 3j, respectively). But, the presence of bulky phenyl group at 4-position of coumarin ring changed the results and the anti-AChE activity of compound 3l was higher than compound 3h (IC50= 13.00 vs. 30.20 μM).
All of the target compounds showed significantly less activity against BuChE than those of AChE except for compound 3a. Compounds 3a-c were the most potent compounds for inhibition of BuChE with an IC50 value of less than 3250 μM. But, all other compounds show no proper activity against BuChE. Compound 3b containing unsubstituted coumarin attached to the 8-hydroxyquinoline via five carbons linker was the most superior compound for both enzymes.
Kinetic study of AChE inhibition
In order to depict the Lineweaver-Bruke plot, the enzyme velocity was measured in the presence of inhibitor 3b at following concentrations: 0.575, 0.115, and 0.23 μM. The Lineweaver-Burke plot was then schemed using the reciprocal of velocity (1/v) and substrate concentration (S) (Figure 2). A mixed type inhibition of AChE was established by compound 3b. The Lineweaver-Burk secondary plot (Figure 3) was also applied to determine the Ki value for 3b (1.32 μM).
Conclusion
In summary, we designed and synthesized a novel series of coumarin derivatives bearing quinoline, benzoxazole, or benzoimidazole to evaluate their anti-AChE/BuChE activity. Compound 3b with quinoline pendent group displayed the highest AChE and BuChE inhibitory activity (IC50= 8.80 and 26.50 μM, respectively). The docking study of the most potent compound 3b revealed that the target ligand can interact with the PAS of AChE preventing the formation of stable and toxic AChE-Aβ complex. These results make the prototype compound 3b a promising cholinesterase inhibitor for further developments.
Acknowledgment
The results presented in this paper were part of a student thesis. This research was supported financially by the grants from Tehran University of Medical Sciences, and Iran National Science Foundation (INSF), Tehran, Iran.
References
- 1.Tang H, Zhao HT, Zhong SM, Wang ZY, Chen ZF, Liang H. Novel oxoisoaporphine-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. Bioorg Med Chem. 2012;22:2257–2261. doi: 10.1016/j.bmcl.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 2.Querfurth HW, La Ferla FM. Mechanism of disease Alzheimer’ disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Tumiatti V, Minarini A, Bolognesi ML, Milelli A, Rosini M, Melchiorre C. Tacrine derivatives and alzheimer’s disease. Curr Med Chem. 2010;17:1825–1838. doi: 10.2174/092986710791111206. [DOI] [PubMed] [Google Scholar]
- 4.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol. 2003;2:539–547. doi: 10.1016/s1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 5.Pepeu G, Giovannini MG. Cholinesterase inhibitors and beyond. Curr Alzheimer Res. 2009;6:86–96. doi: 10.2174/156720509787602861. [DOI] [PubMed] [Google Scholar]
- 6.Mohammadi-Farani A, Abdi N, Moradi AR, Aliabadi AR. 2-(2-(4-Benzoylpiperazin-1-yl) ethyl)isoindoline-1,3-dione derivatives: Synthesis, docking and acetylcholinesterase inhibitory evaluation as anti-alzheimer agents. Iran J Basic Med Sci. 2017;20:59–66. doi: 10.22038/ijbms.2017.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacobini E. Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacol Res. 2004;50:433–440. doi: 10.1016/j.phrs.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 8.McGeer EG, McGeer PL. Clinically tested drugs for Alzheimer’s disease. Expert Opin Investig Drugs. 2003;12:1143. doi: 10.1517/13543784.12.7.1143. [DOI] [PubMed] [Google Scholar]
- 9.Osborn GG, Saunders AV. Current treatments for patients with Alzheimer disease. J Am Osteopath Assoc. 2010;110:16–26. [PubMed] [Google Scholar]
- 10.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Sun J, Fang L, Liu M, Peng S, Liao H, et al. Tacrine–ferulic acid–nitric oxide (NO) donor trihybrids as potent, multifunctional acetyl-and butyrylcholinesterase inhibitors. J Med Chem. 2012;55:4309–4321. doi: 10.1021/jm300106z. [DOI] [PubMed] [Google Scholar]
- 12.Shanks M, Kivipelto M, Bullock R, Lane R. Cholinesterase inhibition: is there evidence for disease-modifying effects? Curr Med Res Opin. 2009;25:2439–2446. doi: 10.1185/03007990903209332. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Lee US, Kim WJ, Moon SK. Inhibitory effect of esculetin on migration, invasion and matrix metalloproteinase-9 expression in TNF-α-induced vascular smooth muscle cells. Mol Med Rep. 2011;4:337. doi: 10.3892/mmr.2011.420. [DOI] [PubMed] [Google Scholar]
- 14.Huang XY, Shan ZJ, Zhai HL, Su L, Zhang XY. Study on the anticancer activity of coumarin derivatives by molecular modeling. Chem Biol Drug Des. 2011;78:651. doi: 10.1111/j.1747-0285.2011.01195.x. [DOI] [PubMed] [Google Scholar]
- 15.Kostova I, Bhatia S, Grigorov P, Balkansky S, Parmar VS, Prasad AK, Saso L. Coumarins as antioxidants. Curr Med Chem. 2001;18:3929. doi: 10.2174/092986711803414395. [DOI] [PubMed] [Google Scholar]
- 16.Piazzi L, Cavalli A, Colizzi F, Belluti F, Bartolini M, Mancini F, et al. Multi-target-directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorg Med Chem Lett. 2008;18:423. doi: 10.1016/j.bmcl.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 17.Schalk I, Ehret-Sabatier L, Le Feuvre Y, Bon S, Massoulie J, Goeldner M. 6-Coumarin diazonium salt: a specific affinity label of the Torpedo acetylcholinesterase peripheral site. Mol Pharmcol. 1995;48:1063. [PubMed] [Google Scholar]
- 18.Radic´ Z, Taylor P. The influence of peripheral site ligands on the reaction of symmetric and chiral organophosphates with wildtype and mutant acetylcholinesterases. Chem Biol Interact. 1999;119-120:111. doi: 10.1016/s0009-2797(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 19.Radic´ Z, Taylor P. Peripheral site ligands accelerate inhibition of acetylcholinesterase by neutral organophosphates. J Appl Toxicol. 2001;21:S13. doi: 10.1002/jat.790. [DOI] [PubMed] [Google Scholar]
- 20.Fallarero A, Oinonen P, Gupta S, Blom P, Galkin A, Mohan CG, Vuorela PM. Inhibition of acetylcholin-esterase by coumarins: The case of coumarin 106. Pharmacol Res. 2008;58:215. doi: 10.1016/j.phrs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Kontogiorgis CA, Xu Y, Hadjipavlou-Litina D, Luo Y. Coumarin derivatives protection against ROS production in cellular models of Aβtoxicities. Free Radic Res. 2007;41:1168. doi: 10.1080/10715760701447884. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Su T, Shan W, Luo Z, Sun Y, He F, Li X. Inhibition of cholinesterase activity and amyloid aggregation by berberine-phenyl-benzoheterocyclic and tacrine-phenyl-benzoheterocyclic hybrids. Bioorg Med Chem. 2012;20:3038–3048. doi: 10.1016/j.bmc.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Bachiller MI, Villarroya M, Perez C, Garcı AG, Gonzalez-Munoz GC, Rodrıguez-Franco MI, Conde S, Lopez MG. Novel tacrine-8-hydroxy-quinoline hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with neuroprotective, cholinergic, antioxidant, and copper complexing properties. J Med Chem. 2010;53:4927–4937. doi: 10.1021/jm100329q. [DOI] [PubMed] [Google Scholar]
- 24.Alipour M, Khoobi M, moradi M, Nadri H, Homayouni Moghadam F, Emami S, et al. Synthesis and anti-cholinesterase activity of new 7-hydroxycoumarin derivatives. Eur J Med Chem. 2014;82:536–544. doi: 10.1016/j.ejmech.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 25.Ghanei-Nasab S, Khoobi M, Hadizadeh F, Marjani A, Moradi A, Nadri H, et al. Synthesis and anticholinesterase activity of coumarin-3-carboxamides bearing tryptamine moiety. Eur J Med Chem. 2016;121:40–46. doi: 10.1016/j.ejmech.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Asadipour A, Alipour M, Jafari M, Khoobi M, Emami S, Nadri H, et al. Novel coumarin-3-carboxamides bearing N-benzylpiperidine moiety as potent acetylcholinesterase inhibitors. Eur J Med Chem. 2013;70:623–630. doi: 10.1016/j.ejmech.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Razavi SF, Khoobi M, Nadri H, Sakhteman A, Moradi A, Emami S, et al. Synthesis and evaluation of 4-substituted coumarins as novel acetylcholin-esterase inhibitors. Eur J Med Chem. 2013;64:252–259. doi: 10.1016/j.ejmech.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Alipour M, Khoobi M, Foroumadi Nadri H, Moradi A, Sakhteman A, et al. Novel coumarin derivatives bearing N-benzyl pyridinium moiety: Potent and dual binding site acetylcholinesterase inhibitors. Bioorg Med Chem. 2012;20:7214–7222. doi: 10.1016/j.bmc.2012.08.052. [DOI] [PubMed] [Google Scholar]
- 29.Trott O, Olson A. J autoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanner MF. Python: a programming language for software integration and development. J Mol Graph Model. 1999;17:57–61. [PubMed] [Google Scholar]
- 31.O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: An open chemical toolbox. J Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC. UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 33.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 34.Xie SS, Wang XB, Li JY, Yang L, Kong LY. Design, synthesis and evaluation of novel tacrine–coumarin hybrids as multifunctional cholinesterase inhibitors against Alzheimer’s disease. Eur J Med Chem. 2013;64:540–553. doi: 10.1016/j.ejmech.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Rajesh H, Vekariya HD. Patel recent advances in the synthesis of coumarin derivatives via knoevenagel condensation: a review. Synth Commun. 2014;0:1–33. [Google Scholar]
- 36.Kathuria A, Priya N, Chand K, Singh P, Gupta A, Jalal S, et al. Substrate specificity of acetoxy derivatives of coumarins and quinolones towards Calreticulin mediated transacetylation: Investigations on antiplatelet function. Bioorg Med Chem. 2012;20:1624–1638. doi: 10.1016/j.bmc.2011.11.016. [DOI] [PubMed] [Google Scholar]


