Abstract
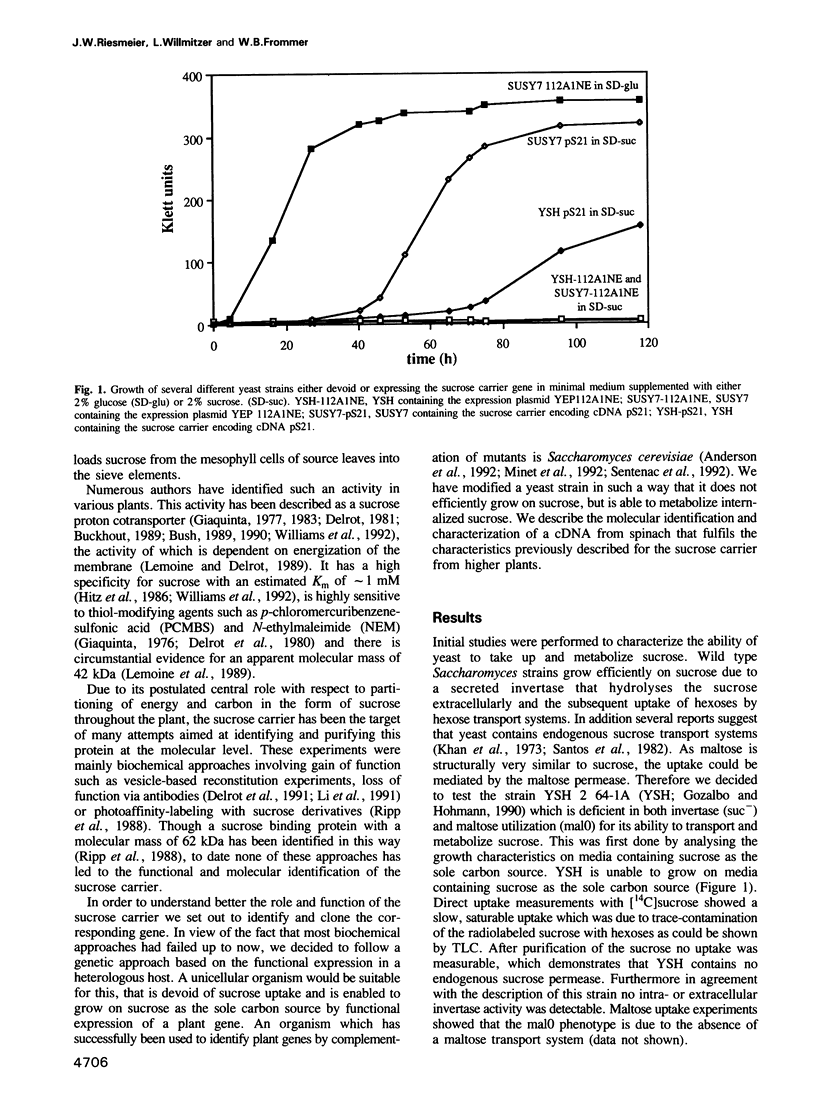
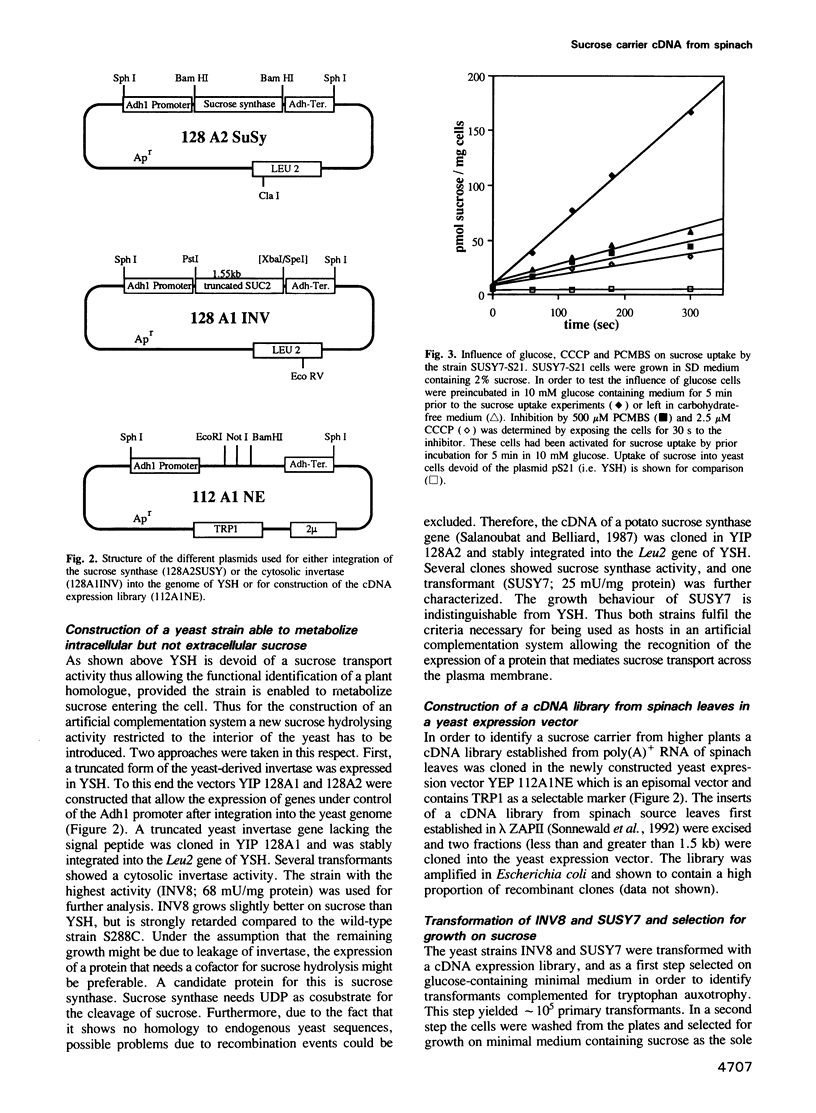
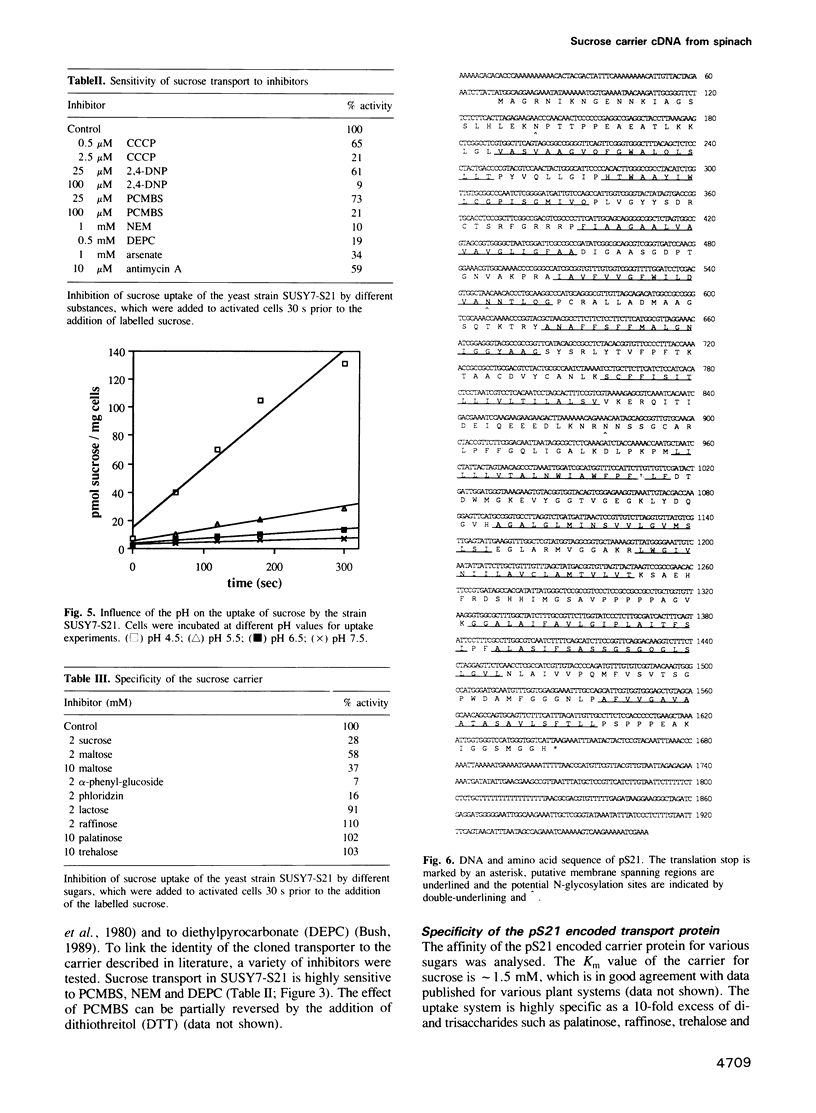
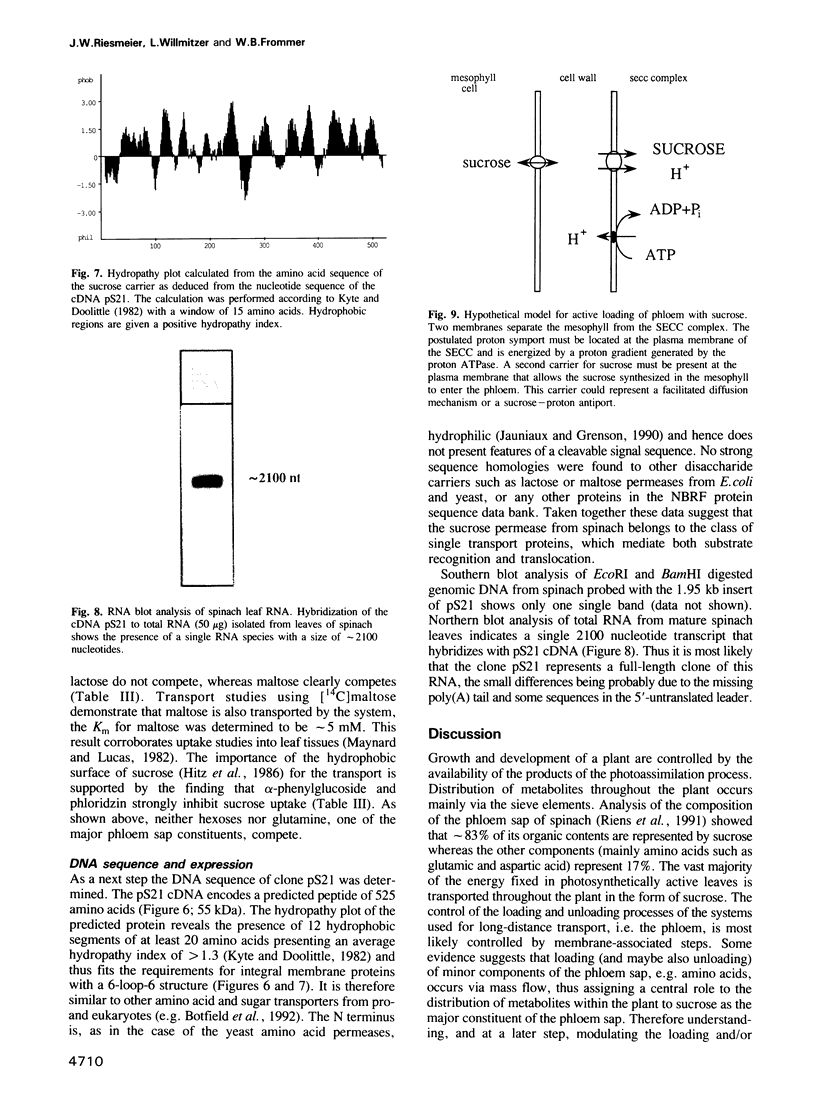
Active loading of the phloem with sucrose in leaves is an essential part of the process of supplying non-photosynthetic tissues with carbon and energy. The transport is protein mediated and coupled to proton-symport, but so far no sucrose carrier gene has been identified. Using an engineered Saccharomyces cerevisiae strain, a cDNA from spinach encoding a sucrose carrier was identified by functional expression. Yeast strains that allow the phenotypic recognition of a sucrose carrier activity were constructed by expressing a cytoplasmic invertase from yeast, or the potato sucrose synthase gene, in a strain unable to transport or grow on sucrose due to a deletion in the SUC2 gene. A spinach cDNA expression library established from the poly(A)+ RNA from source leaves of spinach and cloned in a yeast expression vector yielded transformed yeast clones which were able to grow on media containing sucrose as the sole carbon source. This ability was strictly linked to the presence of the spinach cDNA clone pS21. Analysis of the sucrose uptake process in yeast strains transformed with this plasmid show a pH-dependent uptake of sucrose with a Km of 1.5 mM, which can be inhibited by maltose, alpha-phenylglucoside, carbonyl cyanide m-chlorophenylhydrazone and p-chloromercuribenzenesulfonic acid. These data are in accordance with measurements using both leaf discs and plasma membrane vesicles from leaves of higher plants. DNA sequence analysis of the pS21 clone reveals the presence of an open reading frame encoding a protein with a molecular mass of 55 kDa. The predicted protein contains several hydrophobic regions which could be assigned to 12 membrane-spanning regions.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. A., Huprikar S. S., Kochian L. V., Lucas W. J., Gaber R. F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botfield M. C., Naguchi K., Tsuchiya T., Wilson T. H. Membrane topology of the melibiose carrier of Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):1818–1822. [PubMed] [Google Scholar]
- Buckley M. F., Goding J. W. Preparation of bacteriophage lambda DNA using the TL-100 ultracentrifuge. Anal Biochem. 1988 Nov 15;175(1):281–283. doi: 10.1016/0003-2697(88)90389-2. [DOI] [PubMed] [Google Scholar]
- Bush D. R. Electrogenicity, pH-Dependence, and Stoichiometry of the Proton-Sucrose Symport. Plant Physiol. 1990 Aug;93(4):1590–1596. doi: 10.1104/pp.93.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R. Proton-Coupled Sucrose Transport in Plasmalemma Vesicles Isolated from Sugar Beet (Beta vulgaris L. cv Great Western) Leaves. Plant Physiol. 1989 Apr;89(4):1318–1323. doi: 10.1104/pp.89.4.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P. Sugar transport in normal and mutant yeast cells. Methods Enzymol. 1989;174:617–622. doi: 10.1016/0076-6879(89)74040-4. [DOI] [PubMed] [Google Scholar]
- Delrot S. Proton Fluxes Associated with Sugar Uptake in Vicia faba Leaf Tissues. Plant Physiol. 1981 Sep;68(3):706–711. doi: 10.1104/pp.68.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen R. J., Strasser A. W., Höner C. B., Hollenberg C. P. An efficient transformation procedure enabling long-term storage of competent cells of various yeast genera. Yeast. 1991 Oct;7(7):691–692. doi: 10.1002/yea.320070704. [DOI] [PubMed] [Google Scholar]
- Flügge U. I., Fischer K., Gross A., Sebald W., Lottspeich F., Eckerskorn C. The triose phosphate-3-phosphoglycerate-phosphate translocator from spinach chloroplasts: nucleotide sequence of a full-length cDNA clone and import of the in vitro synthesized precursor protein into chloroplasts. EMBO J. 1989 Jan;8(1):39–46. doi: 10.1002/j.1460-2075.1989.tb03346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Gozalbo D., Hohmann S. Nonsense suppressors partially revert the decrease of the mRNA level of a nonsense mutant allele in yeast. Curr Genet. 1990 Jan;17(1):77–79. doi: 10.1007/BF00313252. [DOI] [PubMed] [Google Scholar]
- Hitz W. D., Card P. J., Ripp K. G. Substrate recognition by a sucrose transporting protein. J Biol Chem. 1986 Sep 15;261(26):11986–11991. [PubMed] [Google Scholar]
- Jauniaux J. C., Grenson M. GAP1, the general amino acid permease gene of Saccharomyces cerevisiae. Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur J Biochem. 1990 May 31;190(1):39–44. doi: 10.1111/j.1432-1033.1990.tb15542.x. [DOI] [PubMed] [Google Scholar]
- Khan N. A., Zimmermann F. K., Eaton N. R. Genetic and biochemical evidence of sucrose fermentation by maltase in yeast. Mol Gen Genet. 1973;123(1):43–50. doi: 10.1007/BF00282987. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Li Z. S., Gallet O., Gaillard C., Lemoine R., Delrot S. Reconstitution of active sucrose transport in plant proteoliposomes. FEBS Lett. 1991 Jul 29;286(1-2):117–120. doi: 10.1016/0014-5793(91)80954-2. [DOI] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. Sucrose and Glucose Uptake into Beta vulgaris Leaf Tissues : A Case for General (Apoplastic) Retrieval Systems. Plant Physiol. 1982 Nov;70(5):1436–1443. doi: 10.1104/pp.70.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet M., Dufour M. E., Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992 May;2(3):417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- Riens B., Lohaus G., Heineke D., Heldt H. W. Amino Acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the Phloem sap of spinach leaves. Plant Physiol. 1991 Sep;97(1):227–233. doi: 10.1104/pp.97.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripp K. G., Viitanen P. V., Hitz W. D., Franceschi V. R. Identification of membrane protein associated with sucrose transport into cells of developing soybean cotyledons. Plant Physiol. 1988 Dec;88(4):1435–1445. doi: 10.1104/pp.88.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanoubat M., Belliard G. Molecular cloning and sequencing of sucrose synthase cDNA from potato (Solanum tuberosum L.): preliminary characterization of sucrose synthase mRNA distribution. Gene. 1987;60(1):47–56. doi: 10.1016/0378-1119(87)90212-5. [DOI] [PubMed] [Google Scholar]
- Santos E., Rodriguez L., Elorza M. V., Sentandreu R. Uptake of sucrose by Saccharomyces cerevisiae. Arch Biochem Biophys. 1982 Jul;216(2):652–660. doi: 10.1016/0003-9861(82)90255-7. [DOI] [PubMed] [Google Scholar]
- Sauer N., Friedländer K., Gräml-Wicke U. Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J. 1990 Oct;9(10):3045–3050. doi: 10.1002/j.1460-2075.1990.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H., Bonneaud N., Minet M., Lacroute F., Salmon J. M., Gaymard F., Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992 May 1;256(5057):663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Sonnewald U., Brauer M., von Schaewen A., Stitt M., Willmitzer L. Transgenic tobacco plants expressing yeast-derived invertase in either the cytosol, vacuole or apoplast: a powerful tool for studying sucrose metabolism and sink/source interactions. Plant J. 1991 Jul;1(1):95–106. doi: 10.1111/j.1365-313x.1991.00095.x. [DOI] [PubMed] [Google Scholar]
- Vernet T., Dignard D., Thomas D. Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52(2-3):225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- von Schaewen A., Stitt M., Schmidt R., Sonnewald U., Willmitzer L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990 Oct;9(10):3033–3044. doi: 10.1002/j.1460-2075.1990.tb07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]