Abstract
Objective(s):
Diabetes mellitus causes impaired memory and cognitive functions. The hippocampus plays a key role in memory and learning. Curcumin attenuates diabetic nephropathy in vivo. Curcumin has shown a neurogenic effect and cognition-enhancing potential in aged rats. The aim of this study is to evaluate the possible protective role of curcumin on the histological and serological changes of the hippocampus in diabetic rats.
Materials and Methods:
Forty albino rats were divided into four groups, ten rats each. Group 1 control rats, group 2 rats received curcumin orally (200 mg/kg/day for six weeks), group 3 rats were injected intraperitoneally with streptozotocin (STZ) (100 mg/kg, single dose), group 4 received a single injection of STZ and received curcumin orally for six weeks. Paraffin sections of hippocampus were prepared and stained with hematoxylin and eosin stain, and immnunohistochemical staining for GFAP and caspase-3. Morphometrical and statistical analyses were performed. Glycemic status and parameters of oxidative stress was measured.
Results:
Examination of hippocampus of diabetic rats showed disorganization of small pyramidal cells in CA1, many cellular losses in the pyramidal cells of CA3, many degenerated granule cells in the dentate gyrus. GFAP positive astrocyte and caspase-3 positive neuron counts were significantly increased. There were significant serum glucose elevation and significant lowered levels of oxidative stress parameters as compared to control rats. Curcumin administration improved the structural and serological alterations of the hippocampus with significant reduction in serum glucose level.
Conclusion:
Curcumin ameliorates the deterious effect of diabetes on the hippocampus through its antioxidant, antiapoptotic and anti-inflammatory efficacies.
Keywords: Curcumin, Diabetes, Hippocampus, Neuroprotective, Rats
Introduction
Diabetes mellitus is a metabolic disorder characte-rized by the presence of high blood sugar (hyper-glycaemia) resulting from either deficient levels of the hormone insulin or from defective insulin action or both (1).
The rapidly rising prevalence of diabetes mellitus is highlighted all over the world. It was 2.8% in 2000 and will rise up to 4.4% in 2030. The total number of people suffering from diabetes was 171 million in 2000 and may rise as many as 366 million in 2030 (2).
Uncontrolled hyperglycemia can cause severe damage to almost all of the body’s major organs including vasculopathy, nephropathy, neuropathy and retinopathy (1). Peripheral neuropathy was known as the only complication involving the nervous system. In the last decade, it has become clear that central nervous system is also involved in both human and animal (3).
The central nervous system alterations include abnormal expression of hypothalamic neuropeptides, astrogliosis in the cerebellum, dysfunction of the glutamate transporter and neuronal degeneration (4).
Cognitive and behavioral impairments are being the most reported central nervous system dysfunction (5). Human subjects with type 1 or type 2 diabetes mellitus exhibited lower cognition compared to non–diabetic subjects (6). Impaired cognitive functions were reported in rodent models of diabetes. Rats with streptozotocin (STZ) induced diabetes showed impaired learning ability (7).
The hippocampal formation is a complex part of the limbic system. It is formed of the hippocampus proper, the dentate gyrus and the subiculum. It plays a key role in cognitive functions like memory and learning (8).
Oxidative stress plays an important role in the pathogenesis of diabetic complications. The most important free radicals are reactive oxygen species (ROS) and reactive nitrogen species. ROS include free radicals such as superoxide and hydroxyl (9).
A body of evidence is stating that apoptosis is implicated in several diabetic complications. These include neuronal cells apoptosis in diabetic neuropathy, myocardial apoptosis in cardiac patho-genesis and mesangial cell apoptosis in diabetic nephropathy (10).
Diabetes is a great problem to public health. Effective management of diabetes is a pressing goal of the research worldwide. Current treatments, like hypoglycemic drugs or insulin, causes many adverse effects and faces significant challenges as insulin resistance or hypoglycaemic attacks. The recent trend is to use medicinal natural products which may offer the same efficacy without so many side effects (11).
One of these natural compounds is curcumin which has considered a promising natural thera-peutic agent. It is the most active component found in turmeric (Curcuma longa Linn.), a popular Indian spice. Curcumin, a nontoxic polyphenol, has been shown to contain antioxidant, antiapoptotic, anti-infection, hypocholesterolemic and anti-inflamma-tory activities (12).
Curcumin attenuated podocyte apoptosis caused by high glucose in vitro and diabetic nephropathy in vivo (13). Curcumin had shown a neurogenic effect and cognition-enhancing potential in aged rats (14). Curcumin ameliorated the harmful effects of glutamate neurotoxicity on the hippocampus (15).
So, the aim of the present study was to evaluate the possible protective role of curcumin on the histological and serological changes of the hippocampus in diabetic rat.
Materials and Methods
Experimental animals
Forty healthy male rats of the Sprague Dawely strain aged four to six months were locally bred at the animal house of Research Center and Bilharzial Research Unit, Faculty of Medicine, Ain Shams University. The rats were housed in stainless steel cages, five rats per cage. The rats were exposed to dark/light cycle and allowed for free access to diet and water with suitable environmental conditions and good ventilation and at a temperature of 25 °C.
Induction of diabetes
After 12 hr of fasting; the animals of model groups received a single 100 mg/kg intraperitoneal injection of STZ dissolved in 0.1 mol/l cold citrate, pH 4.5. The animals were allowed to drink 5% glucose solution to overcome STZ-induced hypoglycemia. After 72 hr, animals with blood glucose levels greater than 200 mg/dl were considered diabetic (16).
Curcumin administration
Curcumin, available in the form of a powder, was administrated at a dose of 200 mg/kg/day dissolved in 1 ml/kg corn oil by daily gavage. Corn oil, available in the form of an oily solution, was used as a solvent for curcumin (Sigma Chemical Co., Cairo, Egypt).
Experimental design
The rats were divided into four groups, ten rats each.
Group 1: control group: rats received single intraperitoneal injection of citrate buffer alone.
Group 2: non diabetic received curcumin: rats received curcumin orally in a dose of 200 mg/kg/day for six weeks (17).
Group 3: diabetic group: rats were injected intraperitoneally with a single dose of 100 mg/kg of STZ (16).
Group 4: diabetic received curcumin: rats received a single intraperitoneal injection of STZ (100 mg/kg) then received curcumin orally in a dose of 200 mg/kg/day for six weeks.
Sampling blood: samples were attained from animals’ tail vein, and then centrifuged for 15 min. The separated serum was used for the detection of glucose and insulin.
At the end of the experiment the animals were anesthetized and decapitated. The skulls were opened and the brains were collected immediately and carefully. Coronal sections were obtained and left immersed in the 10% formalin to continue fixation two more days. Then, the specimens were processed for preparation of paraffin blocks. Paraffin sections (5 μm) were prepared and stained with hematoxylin and eosin stain (18).
Immunohistochemical study for glial fibrillary acidic protein (GFAP) and caspase-3
Sections were taken on positive slides and immunostained using avidin-biotin technique. Paraffin sections (5 μm) were deparaffinized in xylene, rehydrated and pretreated with 0.01% hydrogen peroxide (H2O2) for blocking endogenous peroxidise activity and unmasking of the antigenic site was carried out by transmitting sections into 0.01 M citrate buffer (pH 6) for 10 min and in ethanol for 10 min. Microwave-assisted antigen retrieval was then performed for 20 min. Sections were incubated overnight at 4 °C with the diluted primary antibody at dilution 1/100 monoclonal mouse antibodies for GFAP and caspase-3. Sections were incubated with the avidin-biotin complex (ABC) reagent for 60 min then incubated in peroxidase substrate solution for 6-10 min. Finally, haematoxylin was used as a counter stain, dehydration in absolute alcohol, clearing and mounting were done. Immunoreactivity was visualized as dark brown cytoplasmic staining for GFAP and caspase-3 (19).
Quantitative morphometric study: Ten fields per section were taken from high-power (400x) using Olympus digital camera installed on Olympus microscope. The number of immunopositive cells for GFAP and caspase-3 was counted in sections of each animal of the different studied groups, using ImageJ software program and averaged per field for each animal.
Biochemical analysis
Catalase (CAT) assay
Catalase (CAT) activity was assayed in tissue homogenates of hippocampus using a kit provided by Abcam according to manufacturer’s instructions, based on the elimination of H2O2 from a solution containing 30 mmol/l H2O2 in 10 mmol/l potassium phosphate buffer (pH 7) at 240 nm. Results were explained as mmol of H2O2 consumed/min/mg protein (20).
Glutathione peroxidase (GPx) assay
Glutathione peroxidase (GPx) activity in homo-genates of hippocampus tissue of rats was measured by a Glutathione Peroxidase Assay Kit provided by Abcam according to manufacturer’s instructions. The results were expressed as units/mg protein (21).
Superoxide dismutase (SOD) assay
Superoxide dismutase activity in homogenates of hippocampus tissue of rats was measured using a Superoxide Dismutase Activity Colorimetric Assay Kit provided by Abcam, according to manufacturer’s instructions. Assay method was based on a competitive inhibition assay using xanthine–xanthine oxidase system to reduce nitroblue tetrazolium. Results were explained as U/mg protein (22).
Reduced glutathione (GSH) assay
Reduced glutathione (GSH) was assayed using the method of Ellman, by treating 0.5 ml of the supernatant with 0.5 ml of Ellman’s reagent consisted of (19.8 mg of 5,5-dithiobisnitrobenzoic acid in 100 ml of 0.1% sodium nitrate) and 3 ml of phosphate buffer (0.2 M, pH 8). This method depends on reduction of Ellman’s reagent by SH group of (GSH) to form 5, 5- dithio-2-nitrobenzoic acid in phosphate buffer. The produced yellow color was measured spectrophotometrically at 412 nm, using a biochemical analyzer manufactured by (ERMA INC. Tokyo, JAPAN, model: AE-600N) (23).
Statistical analysis
The values were represented as mean±standard deviation (±SD). The data were analyzed by one-way ANOVA with post-hoc test for multiple comparisons between groups using SPSS software (SPSS Inc., Chicago, Illinois, USA).
Results
Histological results
Hematoxylin and eosin stained sections
Light microscopic examination of H&E-stained paraffin sections of the rats of control group (group 1) showed C-shaped hippocampus. It is divided into four regions CA1, CA2, CA3 and CA4 and dentate gyrus. Areas in between compact zones of cells comprise the molecular layer. The core of the dentate gyrus is forming the hilus (Figure 1).
Figure 1.
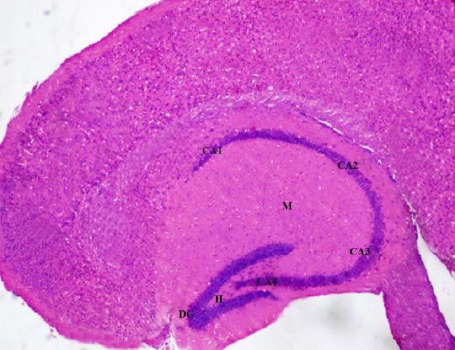
A photomicrograph of a coronal section of the hippocampus of an adult control rat showing C-shaped hippocampus with its four regions (CA1, CA2, CA3 and CA4), the dentate gyrus (DG). The molecular layer (M) is between compact zones of cells and the hilus (H). H & E; X 100
Cornu Ammonis CA1 is formed of 5–6 compact layers of small pyramidal cells arranged with characteristic palisade appearance. The pyramidal cells showed vesicular nuclei, prominent nucleoli and pale basophilic cytoplasm (Figure 2). CA3 is formed of zone of large pyramidal cells (Figure 3). The molecular layer consists of interacting axons and dendrites, glial cells, and capillaries (Figures 2, 3).
Figure 2.
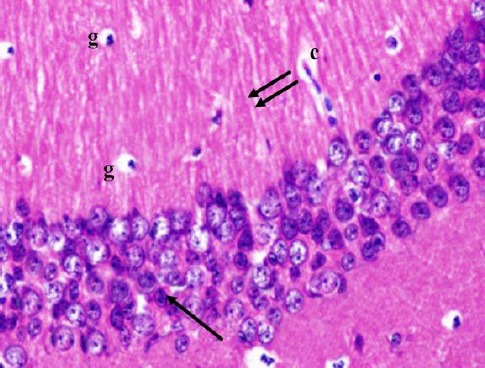
A photomicrograph of a coronal section of the hippocampus of an adult control rat showing small pyramidal cells of CA1 region (arrow) and molecular layer with the well apparent processes of pyramidal cells (double arrows), glial cells (g) and capillaries (c). H & E; X 400
Figure 3.
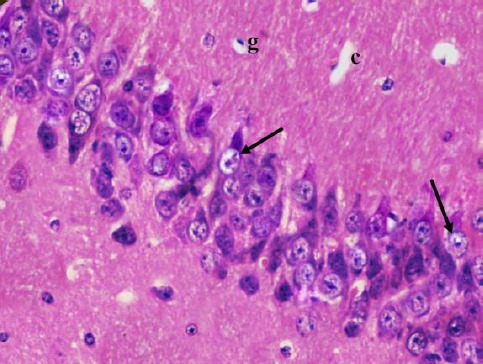
A photomicrograph of a coronal section of the hippocampus of an adult control rat showing layers of large pyramidal cells in CA3 region (arrows) and molecular layer with many glial cells (g) and capillaries (c). H & E; X 400
The dentate gyrus is formed of compact layers of small granule cells. The dentate hilar region contains axons, dendrites, and interneurons (Figure 4).
Figure 4.
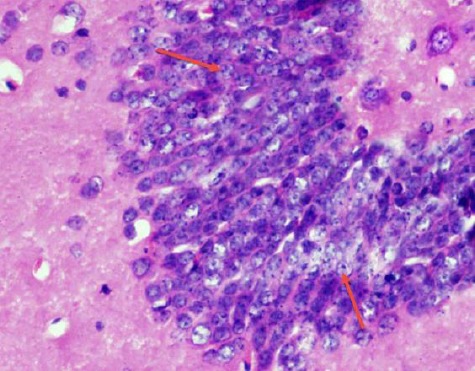
A photomicrograph of a coronal section of the hippocampus of an adult control rat showing dentate gyrus formed of layers of compact granular cells (red arrows). H & E; X 400
The non-diabetic received curcumin (group 2) showed the same histological appearance like control (group I).
Examination of the diabetic group (group 3) revealed disorganization and dispersion of pyramidal cells in CA1 region, most of them having eosinophilic cytoplasm and pyknotic nucleus. Some having hallo around them (Figure 5). The pyramidal cells of CA3 region were disorganized with many areas of cell loss. The cells appeared contracted. Many degenerated with eosinophilic cytoplasm were observed. Their nuclei were shrunken dark pyknotic or karyolitic or hyperchromatic (Figure 6). The molecular layer showed increased glial cells and widened congested capillaries (Figures 5, 6).
Figure 5.
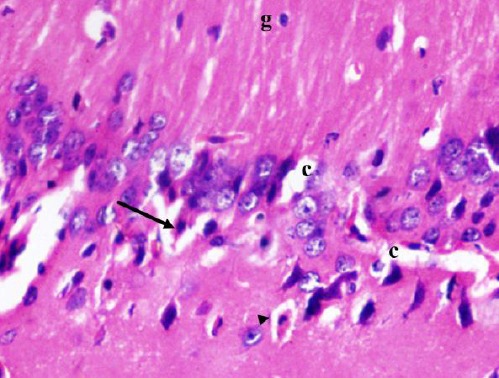
A photomicrograph of a coronal section of the hippocampus of an adult diabetic rat showing dispersed degenerated pyramidal cells with eosinophilic cytoplasm and pyknotic nucleus (arrow) with some having halo around them (arrow head). Notice widened congested capillaries (c) and glial cells (g). H & E; X 400
Figure 6.
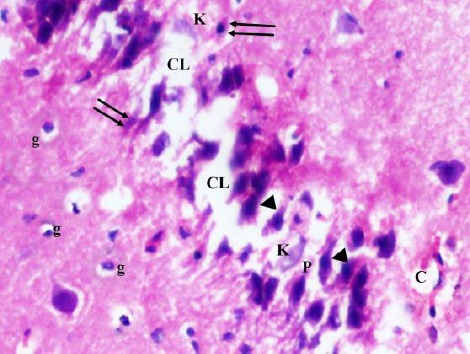
A photomicrograph of a coronal section of the hippocampus of an adult diabetic rat showing disorganization and areas of cell loss (CL) of contracted pyramidal cells (P) of CA3 region; some kariolitic nuclei (K), pyknotic nuclei (double arrows) and hyperchromatic nuclei (arrow head). Notice the dilated capillaries (C) and increased glial cells (g) in molecular layer. H & E; X 400
The dentate gyrus showed marked disorgani zation. Many degenerated granule cells with vacuolated cytoplasm and pyknotic nucleus were also detected. The dentate hilus showed many glial cells (Figure 7). Areas of hemorrhage and areas of cell loss were detected in the dentate gyrus (Figure 8).
Figure 7.
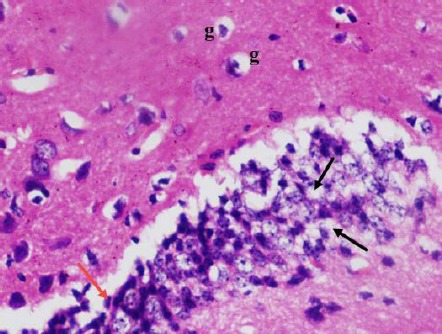
A photomicrograph of a coronal section of the hippocampus of an adult diabetic rat showed many degenerated granule cells in the dentate gyrus with vacuolated cytoplasm (black arrows) and pyknotic nucleus (red arrow). Notice the dentate hilus with increased glial cells (g). H & E; X 400
Figure 8.
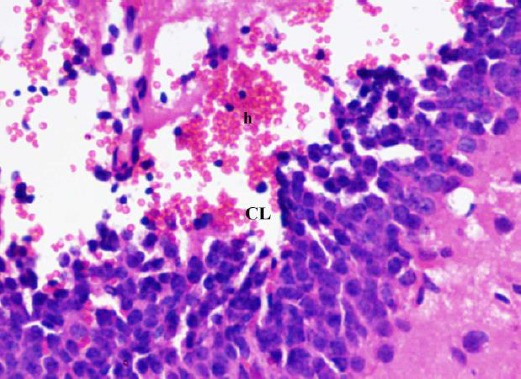
A photomicrograph of a coronal section of the hippocampus of an adult diabetic rat showed wide area of hemorrhage (h) in the dentate gyrus. Notice area of cell loss (CL). H & E; X 400
H&E stained paraffin sections of the diabetic received curcumin group (group 4) showed preservation of most of pyramidal cells of CA1 region. Most of them showed vesicular nuclei. Few nuclei were pyknotic (Figure 9). Large pyramidal cells of CA3 region were preserved. Most of them had centrally located vesicular nuclei and apparent nucleoli (Figure 10).
Figure 9.
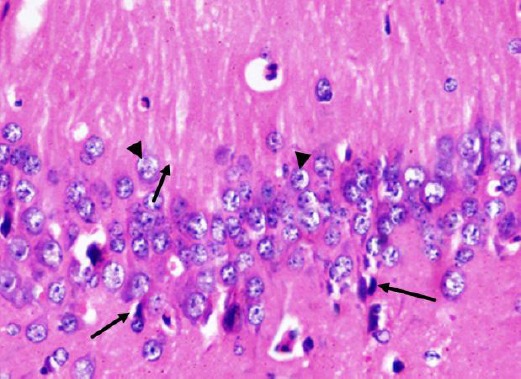
A photomicrograph of a coronal section of the hippocampus of an adult rat of the diabetic received curcumin group showing preservation of most of pyramidal cells of CA1 region (arrow head). Notice few cells with pyknotic nuclei (arrows). H & E; X 400
Figure 10.
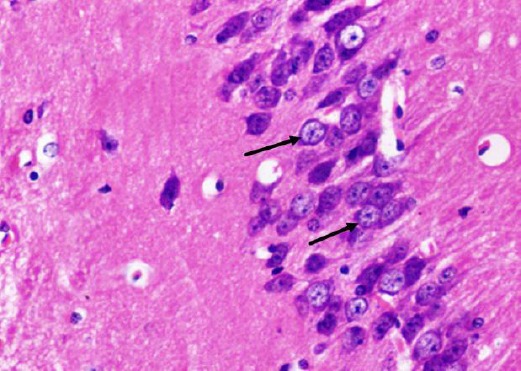
A photomicrograph of a coronal section of the hippocampus of an adult rat of the diabetic treated with curcumin group showing preservation of large pyramidal cells of CA3 region (arrows). H & E; X 400
The dentate gyrus was preserved. The granule cells were compactly arranged with rounded pale vesicular nuclei the dentate gyrus. The dentate hilus showed nearly normal neurons (Figure 11).
Figure 11.
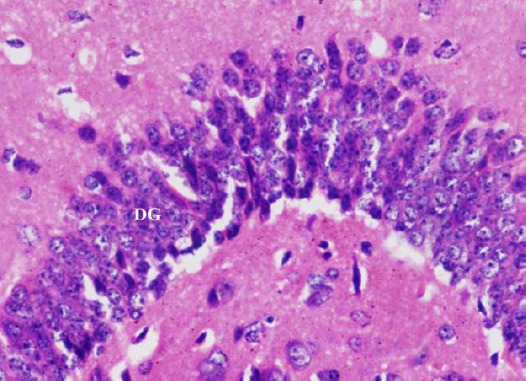
A photomicrograph of a coronal section of the hippocampus of an adult rat of the diabetic received curcumin group showing preservation of the dentate gyrus (DG). H & E; X 400
Immunohistochemical stains
GFAP immunostaining
The control group showed GFAP positive reaction in cytoplasm and in the few processes of scattered small astrocytes of molecular layer (Figure 12). The diabetic group showed many intensely stained GFAP-positive astrocytes with increased size of their cell body and increased number and length of their processes in the molecular layer and pyramidal layer (Figure 13). The diabetic received curcumin group showed relatively fewer astrocytes with thin processes in the molecular layer (Figure 14).
Figure 12.
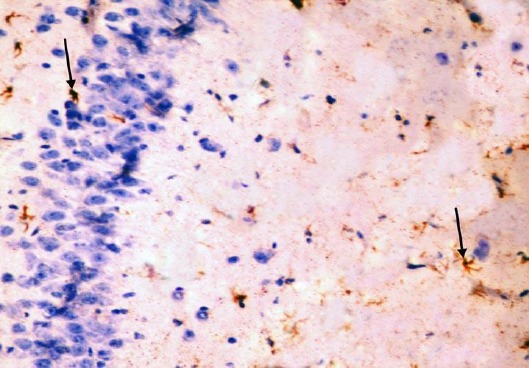
Immunohistochemical staining for the demonstration of GFAP in the hippocampus of an adult control rat showing GFAP-positive staining in cytoplasm and processes of scattered astrocytes; they appear small, with few processes (arrows). GFAP, × 400
Figure 13.
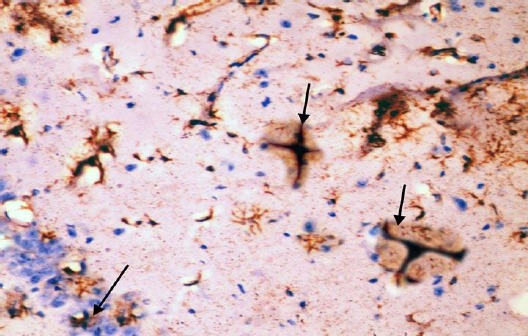
Immunohistochemical staining for the demonstration of GFAP in the hippocampus of an adult diabetic rat showing many GFAP-positive astrocytes with thick, long and intensely stained processes in the molecular layer and pyramidal layer (arrows). GFAP, ×400
Figure 14.
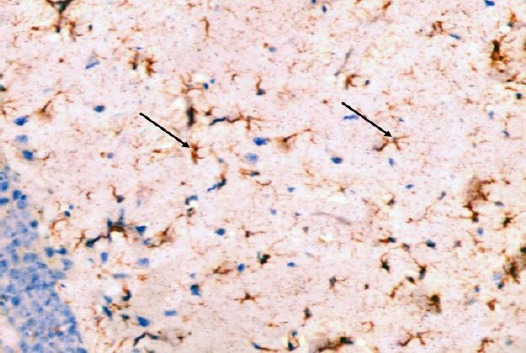
Immunohistochemical staining for the demonstration of GFAP in the hippocampus of an adult rat of the diabetic received curcumin group showing relatively fewer astrocytes with thin processes in the molecular layers (arrows). GFAP, × 400
Caspase-3 immunostaining
The control group showed negative immuno-staining reaction for caspase-3 in the pyramidal cells (Figure 15). The diabetic group showed marked cytoplasmic expression of the caspase-3 in most of the pyramidal cells (Figure 16). The diabetic received curcumin group showed minimal brownish immuno-reaction for caspase-3 in some pyramidal cells less than those of diabetic group (Figure 17).
Figure 15.
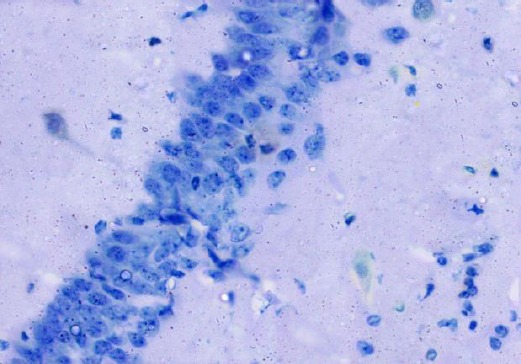
Immunohistochemical staining for the demonstration of caspase-3 in the hippocampus of an adult control rat showing negative immunoreaction in pyramidal cells. Caspase-3, × 400
Figure 16.
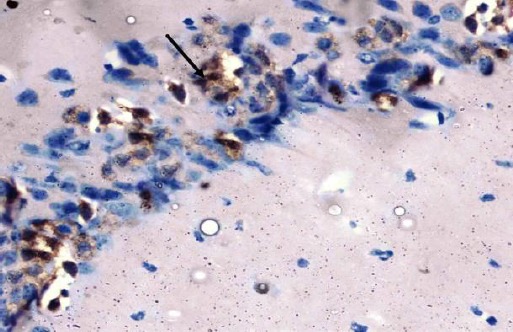
Immunohistochemical staining for the demonstration of caspase-3 in the hippocampus of an adult diabetic rat showing marked cytoplasmic expression of the caspase-3 in most of the pyramidal cells (arrow). Caspase-3, × 400
Figure 17.
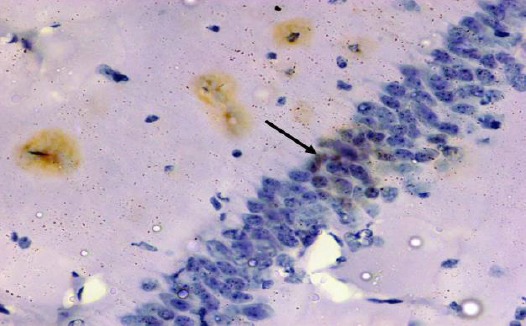
Immunohistochemical staining for the demonstration of caspase-3 in the hippocampus an adult rat of the diabetic received curcumin group showing weak immunoreactivity of caspase-3 in the cytoplasm of the pyramidal cells with brown cytoplasmic deposits (arrow). Caspase-3, × 400
Quantitative immunohistochemical assessment and statistical analysis
As revealed in (Table 1, Histogram 1) there was a highly statistically significant increase (P<0.001) in the number of GFAP positive astrocytes and caspase-3 positive neurons of the diabetic group as compared to control group. However, the diabetic received curcumin group revealed a dramatic decrease in the number of GFAP positive astrocytes and caspase-3 positive neurons as compared to the diabetic group (P<0.001).
Table 1.
Effect of curcumin on of GFAP positive astrocytes number and caspase-3 positive neurons in all studied groups
| Number of GFAP positive astrocytes/ feild | Number of caspase-3 positive neurons/ feild | Significance of GFAP positive astrocytes number and caspase-3 positive neurons | |
|---|---|---|---|
| Control group | 11.75 ±1.46 | 1.26 ± 0.5 | |
| non diabetic received curcumin | 10.62 ± 1.46 | 1.07 ± 0.6 | |
| diabetic group | 35.5*± 1.83 | 10.61*± 1.2 | Versus any of the other studied groups (P<0.001) |
| diabetic received curcumin | 16.1**± 1.73 | 3.72**± 0.8 | Versus control group (P<0.01) |
Data expressed as mean ± standard deviation.
highly statistically significant difference;
statistically significant difference
Histogram 1.
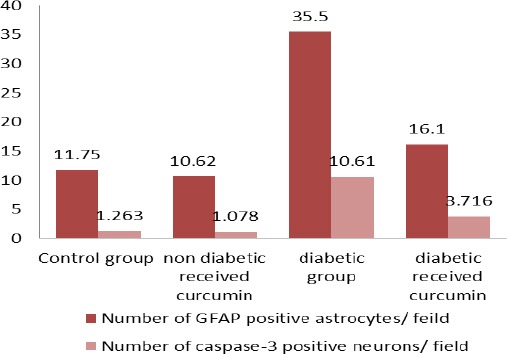
mean of the GFAP positive astrocytes number and caspase-3 positive neurons in studied groups
Biochemical results
Effect of curcumin on blood glycemic status
Table 2 shows the mean levels of fasting blood glucose, post prandial blood glucose, insulin and HOMA-IR for all studied groups. The diabetic rats had significantly (P<0.01) elevated blood glucose concentrations compared with non-diabetic rats. Feeding rats with curcumin had significantly (P<0.05) reduced blood glucose concentrations and HOMA-IR, however, significantly elevated mean insulin levels in comparison to control. Also, the treatment with curcumin significantly improved the glycemic status in diabetic rats when compared with untreated diabetic rats.
Table 2.
Effect of curcumin on glycemic status in all studied groups
| Parameters | Groups | |||
|---|---|---|---|---|
| Group 1 (control) | Group 2 (non-diabetic received curcumin) | Group 3 (diabetic group) | Group 4 (diabetic received curcumin) | |
| FBG (mg/dl) | 68.40±23.3 | 64.7±19.5 | 214±74.2a, b | 69.65+/-19.86 c |
| Postprandial BG (mg/dl) | 103.2±18.45 | 98±15.42 | 230±82.1a, b | 92.16±20.1 c |
| Insulin (ng/ml) | 2.7±-0.8 | 2.9±-0.7 | 0.73±0.54 a, b | 2.5±0.45 c |
| HOMA-IR | 1.46±0.36 | 1.23±0.52 | 4.68±1.5a, b | 1.34±0.470 c |
Data are expressed as mean ± SD, FBG: fasting blood glucose, Postprandial BG : 2-hours blood glucose after oral glucose loading (3 g/kg. b. w.), HOMA-IR: homeostasis model assessment of insulin resistance.
Significantly different from control group,
significantly different from non-diabetic group received curcumin,
significantly different from diabetic group
Effect of curcumin on parameters of oxidative stress
Table 3 shows significantly (P<0.05) lower levels of GSH, SOD, CAT and GPx in diabetic rats when compared with the control rats. Treatment with curcumin significantly (P<0.05) increased their levels.
Table 3.
Effect of curcumin on oxidative stress parameters in all studied groups
| Groups | Group 1 (control) | Group 2 (non-diabetic received curcumin) | Group 3 (diabetic group) | Group 4 (diabetic received curcumin) |
|---|---|---|---|---|
| GPx (μgGSHconsumed/ min/ mg protein) | 25.36 ± 2.3 | 27.24 ± 1.97 | 12.35 ± 2.14 a, b | 22.45 ± 1.75 c |
| GSH (μg/mg protein) | 9.32±2.8 | 10.6±3.4 | 3.19±0.76 a, b | 8.6±3.9 c |
| SOD (units/mg protein) | 4.8 ± 0.38 | 5.32 ± 0.47 | 2.23 ± 0.31 a, b | 4.6 ± 0.51 c |
| CAT (μmoles of H2O2) | 15.60 ± 0.62 | 16.52 ± 1.19 | 6.31 ± 0.73 a, b | 14.30 ± 0.46 c |
Data are expressed as mean±SD.
significantly different from control group,
significantly different from non-diabetic group received curcumin,
significantly different from diabetic group
Discussion
Diabetes mellitus is associated with lower cognitive performance and increased risk for dementia. The incidence of cognitive function was found to be declined by 40% in people with diabetes. It was reported that there is an overall 50-100% increase in the incidence of dementia in diabetic subjects (24).
Examination of diabetic hippocampus sections revealed that diabetes caused marked histological alterations. Many degenerative changes were seen in the pyramidal cells. Disorganization of pyramidal cells detected in CA1 region with absence of characteristic palisade arrangement. Many areas of cell loss leaving empty spaces were detected in CA3 region. The dentate gyrus showed marked disorganization, vacuolation and hemorrhage and decreased granule cells. Similar findings were found by other researchers (25). However, Zhou et al. (26) stated that STZ-induced diabetic rats showed neuronal cell death at the CA3 and dentate gyrus regions of the hippocampus.
Moreover; Pamidi and Satheesha Nayak, (27) reported that untreated diabetes mellitus can induce the damage in the hippocampus within two weeks of diabetic period or earlier and prolonged untreated diabetes leads to severe hippocampal neurodegeneration.
The current study revealed marked cytoplasmic expression for the caspase-3, an indicator of apoptosis, in most of the pyramidal cells. There was a highly statistically significant increase in caspase-3 positive neuron count of the diabetic group as compared to other studied groups.
Vincent et al. (28) reported increased caspase-3 activity indicating neuronal and Schwann cells death in uncontrolled hyperglycemia.
Zhao et al. (29) proved that diabetes increase bax and caspase-3 expression leading to apoptosis of the pyramidal neurons in STZ induced diabetic rats.
Hyperglycemia elevates ROS in hippocampal neuronal cells resulted in decrease in ATP activity and increase in caspases-3 and 9 leading to caspase-dependent apoptosis (30).
Several possible mechanisms have been explain-ed regarding diabetes induced neurodegeneration. Diabetes mellitus and the accompanying hyperglyce-mia is a chronic endogenous stressor that is accompani-
ed with increased oxidative stress in brain, particularly the hippocampus, by accelerating free radical generation. These radicals contribute to increased neuronal degeneration by inducing oxidation to proteins, DNA alterations and lipids oxidation in cell membranes (31). The present study indicated statistical high significant reduction in the levels of the parameters of oxidative stress in the diabetic rats.
Another mechanism is the activated polyol pathway during hyperglycaemia consuming NADPH which is the essential cofactor for regenerating reduced glutathione. Depletion of glutathione lowers the threshold for intracellular oxidative damage (32). This mechanism also explains the significantly (P<0.05) lower levels of reduced GSH in the diabetic rats of the current study.
Also, another important mechanism expected to lead to neuronal loss in the diabetic rats of this study could be due to lower insulin and HOMA-IR which has a neuroprotective anti-apoptotic effect (33).
In the present study, GFAP positive astrocytes were significantly increased in the diabetic group as compared to control group supported by the findings of Baydas et al (34). Similar findings were found by Saravia et al. (35) who stated that GFAP positive astrocytes increased 3-fold in the hippocampus of the diabetic mice compared to the control group.
In contrast, other studies have shown a decrease in the GFAP immunoreactivity in the astrocytes of diabetic tissues. GFAP immunoreactivity was reduc-ed in the olfactory bulb of 8-week STZ-diabetic animals (36). A decrease in the number of GFAP-immunoreactive astrocytes was also detected in the gray matter of the spinal cords of STZ-induced diabetic rats (37). These conflicting reports could result from discrepancy in immunohistochemical methods, diabetic models, doses of STZ, and antibody sources used by different researchers (38, 39).
Primary function of glial cells is to surround neurons and hold them in place, to nurture the neurons, to insulate one neuron from another and to destroy and remove dead neurons, a process essential to neural plasticity and stability. However, damaged neurons may activate glia, which may then kill neurons (40).
Treatment with curcumin significantly improved the glycemic status in diabetic rats. The results of our study showed significant decrease in both fasting and postprandial blood glucose levels as well as HOMA-IR after treatment with curcumin. Mahesh et al. (41), also reported a significant decrease in blood glucose levels in STZ-induced diabetes model after treatment with curcumin.
In the present study, the diabetic group received curcumin showed preserved histological structure of the hippocampus and the dentate gyrus. There was high significant reduction in the number of caspase-3 positive neurons as compared to the diabetic group. These finding may be explained on the basis that curcumin reduced the alterations in lysosomal enzymes activities, thus minimizing the injury due to diabetes (42). In line with the current results, it was reported that treatment of diabetic rats with curcumin cause reduction of pancreatic caspase-3 content (43) and decrease of cardiac caspase-3 immunoreactivity expression (44).
Curcumin induces proliferation and migration of neural progenitor cells and enhances neural plasticity and repair in vitro and in vivo in the adult hippocampus (45).
The present study showed decrease in GFAP immunohistochemical staining and in the number of GFAP positive astrocytes of the diabetic group received curcumin compared with the diabetic group.
Curcumin caused significant decrease in GFAP immunochemistry staining in retinal Müller glia in diabetic rat (46).
Tissue concentration of antioxidant parameters showed significant increase in the diabetic group received curcumin and that may suggest a significant protecting role of curcumin against harmful effects of diabetes. Results of Borra et al. (47) showed a significant protective effect of curcumin that may be due to modulating lipid peroxidation and augmenting antioxidant defense system.
El-Bahr, (48) attributed the curcumin induced anti-diabetic effect to the fact that it maintains normal glucose metabolism via amelioration of the diabetes induced oxidative stress via increasing the activities of antioxidant enzymes including superoxide dismutase, glutathione peroxidase and increasing the reduced glutathione concentration.
Conclusion
The results of the current work suggest that curcumin has a neuroprotective beneficial effect as it could protect rat hippocampus from STZ induced altered histological and serological changes owing to its antioxidant, anti-inflammatory, and antiapoptotic effects. Therefore, diabetic patients and high risk people for diabetes are recommended to consume daily doses of curcumin in their meals.
Acknowledgment
The authors wish to thank Dr Amgad Gaber Elsaid (Lecturer of Anatomy and Embryology, Faculty of Medicine, Ain Shams University, Egypt) for his efforts in conducting and reviewing this paper.
References
- 1.American Diabetes Association (2012) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Selvarajah D, Wilkinson ID, Davies J, Gandhi R, Tesfaye S. Central nervous system involvement in diabetic neuropathy. Curr Diab Rep. 2011;11:310–322. doi: 10.1007/s11892-011-0205-z. [DOI] [PubMed] [Google Scholar]
- 4.Nagayach A, Patro N, Patro I. Experimentally induced diabetes causes glial activation, glutamate toxicity and cellular damage leading to changes in motor function. Front Cell Neurosci. 2014;8:355. doi: 10.3389/fncel.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saravia F, Revsin Y, Lux-Lantos V, Beauquis J, Homo-Delarche F, De Nicola AF. Oestradiol restores cell proliferation in dentate gyrus and subventricular zone of streptozotocin-diabetic mice. J Neuroendo-crinol. 2004;16:704–710. doi: 10.1111/j.1365-2826.2004.01223.x. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood CE, Winocur G. High–fat diets, insulin resistance and declining cognitive function. Neurobiol Aging. 2005;26:42–45. doi: 10.1016/j.neurobiolaging.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 8.Kiernan JA. BARR’S The Human Nervous System: An anatomical viewpoint. 9th ed. Lippincott Williams & Wilkins; 2009. pp. 158–171. [Google Scholar]
- 9.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graves DT, Liu R, Oates TW. Diabetes-enhanced inflammation and apoptosis: impact on periodontal pathosis. Periodontol. 2007;45:128–137. doi: 10.1111/j.1600-0757.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 11.Ugwuja EI, Nwibo AN, Ugwu NC, Aloke C. Effect of aqueous extract of spices mixture containing curry, garlic and ginger on plasma glucose and lipid in alloxan-induced diabetic rats. Pak J Nutr. 2010;9:1131–1135. [Google Scholar]
- 12.Perez-Torres I, Ruiz-Ramirez A, Banos G, El-Hafidi M. “Hibiscus sabdariffa Linnaeus (Malvaceae), curcumin and resveratrol as alternative medicinal agents against metabolic syndrome”. Cardiovasc Hematol Agents Med Chem. 2013;11:25–37. doi: 10.2174/1871525711311010006. [DOI] [PubMed] [Google Scholar]
- 13.Sun LN, Liu XC, Chen XJ, Guan GJ, Liu G. Curcumin attenuates high glucose-induced podocyte apoptosis by regulating functional connections between caveolin-1 phosphorylation and ROS. bold>Acta Pharmacol Sin. 2016;37:645–655. doi: 10.1038/aps.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, et al. Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One. 2012;7:e31211. doi: 10.1371/journal.pone.0031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Li J, Li S, Li Y, Wang X, Liu B, et al. Curcumin attenuates glutamate neurotoxicity in the hippo-campus by suppression of ER stress associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol Appl Pharmacol. 2015;286:53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Niu Y, Liang S, Wang X. Abnormal change in body weight and non-fasting blood glucose levels of mouse strain C57BL/6J in generating type 2 diabetes model. Zool Res. 2007;28:507–510. [Google Scholar]
- 17.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol. 2008;46:1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 18.Bancroft JD, Gamble M. “Theory and Practice of Histological Techniques”. 5th ed. New York: Churchill Livingstone; 2002. pp. 593–620. [Google Scholar]
- 19.Zarnescu O, Brehar FM, Chivu M, Ciurea AV. Immunohistochemical localization of caspase-3, caspase-9 and Bax in U87 glioblastoma xenografts. J Mol Histol. 2008;39:561–569. doi: 10.1007/s10735-008-9196-8. [DOI] [PubMed] [Google Scholar]
- 20.Aebi H. “Catalase in vitro” Methods in enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Geraghty P, Hardigan AA, Wallace AM, Mirochnitchenko O, Thankachen J, Arellanos L, et al. The glutathione peroxidase 1-protein tyrosine phosphatase 1B-protein phosphatase 2A axis. A key determinant of airway inflammation and alveolar destruction. Am J Respir Cell Mol Biol. 2013;49:721–730. doi: 10.1165/rcmb.2013-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Oberley LW, Li Y. “A simple method for clinical assay of superoxide dismutase,”. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 23.Ellman M. A spectrophotometric method for determination of reduced glutathione in tissues. Anal Biochem. 1959;74:214–226. doi: 10.1016/0003-2697(76)90326-2. 27. Aebi HE. Catalase. In: Bergmeyer HU, ed. Methods of enzymatic analysis. New York: Academic Press; 1983.p.3:276-86. [DOI] [PubMed] [Google Scholar]
- 24.Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: Links to Cognition and Depression. Neurosci Biobehav Rev. 2013;37:1346–1362. doi: 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin SN, Younan SM, Youssef MF, Rashed LA, Mohamady I. A histological and functional study on hippocampal formation of normal and diabetic rats. F1000Res. 2013;2:151–173. doi: 10.12688/f1000research.2-151.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Wang L, Ling S, Zhang X. Expression changes of growth-associated protein-43 (GAP-43) and mitogen-activated protein kinase phosphatase-1 (MKP-1) and in hippocampus of streptozotocin-induced diabetic cognitive impairment rats. Exp Neurol. 2007;206:201–208. doi: 10.1016/j.expneurol.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Pamidi N, Satheesha Nayak BN. Effect of streptozotocin induced diabetes on rat hippocampus. Bratisl Lek Listy. 2012;113:583–588. doi: 10.4149/bll_2012_130. [DOI] [PubMed] [Google Scholar]
- 28.Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao CH, Liu HQ, Cao R, Ji AL, Zhang L, Wang F, et al. Effects of dietary fish oil on learning function and apoptosis of hippocampal pyramidal neurons in streptozotocin-diabetic rats. Brain Res. 2012;1457:33–43. doi: 10.1016/j.brainres.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 30.Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–363. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- 31.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a ‘causal’ antioxidant therapy. Diabetes Care. 2003;26:1589–1596. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- 32.Klein JP, Waxman SG. The brain in diabetes: molecular changes in neurons and their implications for end-organ damage. Lancet Neurol. 2003;2:548–554. doi: 10.1016/s1474-4422(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 33.Li ZG, Zhang W, Grunberger G, Sima AA. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002;946:221–231. doi: 10.1016/s0006-8993(02)02887-1. [DOI] [PubMed] [Google Scholar]
- 34.Baydas G, Reiter RJ, Yasar A, Tuzcu M, Akdemir I, Nedzvetskii VS. Melatonin reduces glial reactivity in the hippocampus, cortex, and cerebellum of streptozotocin-induced diabetic rats. Free Radic Biol Med. 2003;35:797–804. doi: 10.1016/s0891-5849(03)00408-8. [DOI] [PubMed] [Google Scholar]
- 35.Saravia FE, Revsin Y, Gonzalez Deniselle MC, Gonzalez SL, Roig P, Lima A, et al. Increased astrocyte reactivity in the hippocampus of murine models of type 1 diabetes: the nonobese diabetic (NOD) and streptozotocin-treated mice. Brain Res. 2002;957:345–353. doi: 10.1016/s0006-8993(02)03675-2. [DOI] [PubMed] [Google Scholar]
- 36.Dennis JC, Coleman ES, Swyers SE, Moody SW, Wright JC, Judd R, et al. Changes in mitotic rate and GFAP expression in the primary olfactory axis of streptozotocin-induced diabetic rats. J Neurocytol. 2005;34(1-2):3–10. doi: 10.1007/s11068-005-5044-x. [DOI] [PubMed] [Google Scholar]
- 37.Afsari ZH, Renno WM, Abd-El-Basset E. Alteration of glial fibrillary acidic proteins immunoreactivity in astrocytes of the spinal cord diabetic rats. Anat Rec. 2008;291:390–399. doi: 10.1002/ar.20678. [DOI] [PubMed] [Google Scholar]
- 38.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Invest Ophthalmol Vis Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- 39.Coleman E, Judd R, Hoe L, Dennis J, Posner P. Effects of diabetes mellitus on astrocyte GFAP and glutamate transporters in the CNS. Glia. 2004;48:166–178. doi: 10.1002/glia.20068. [DOI] [PubMed] [Google Scholar]
- 40.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 41.Mahesh T, Sri Balasubashini MM, Menon VP. Photo-irradiated curcumin supplementation in streptozotocin-induced diabetic rats: effect on lipid peroxidation. Therapie. 2010;59:639–644. doi: 10.2515/therapie:2004110. [DOI] [PubMed] [Google Scholar]
- 42.Chougala MB, Bhaskar JJ, Rajan MG, Salimath PV. Effect of curcumin and quercetin on lysosomal enzyme activities in streptozotocin-induced diabetic rats. Clin Nutr. 2012;31:749–755. doi: 10.1016/j.clnu.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Kamel R, Hashim A, Ali S. Palliative effect of curcumin ON STZ-induced diabetes in rats. Int J Pharm Sci. 2014;1491:558–563. [Google Scholar]
- 44.Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D, et al. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One. 2012;7:e52013. doi: 10.1371/journal.pone.0052013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;2008:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuo ZF, Zhang Q, Liu XZ. Protective effects of curcumin on retinal Müller cell in early diabetic rats. Int J Ophthalmol. 2013;6:422–424. doi: 10.3980/j.issn.2222-3959.2013.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borra SK, Gurumurthy P, Mahendra M, Jayamathi KM, Cherian CN, Ram C. “Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models,”. J Med Plants Res. 2013;7:2680–2690. [Google Scholar]
- 48.El-Bahr SM. Curcumin regulates gene expression of insulin like growth factor, B-cell CLL/lymphoma 2 and antioxidant enzymes in streptozotocin induced diabetic rats. BMC Complement Altern Med. 2013;13:368. doi: 10.1186/1472-6882-13-368. [DOI] [PMC free article] [PubMed] [Google Scholar]


