Abstract
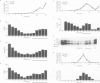
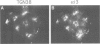
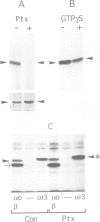
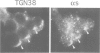
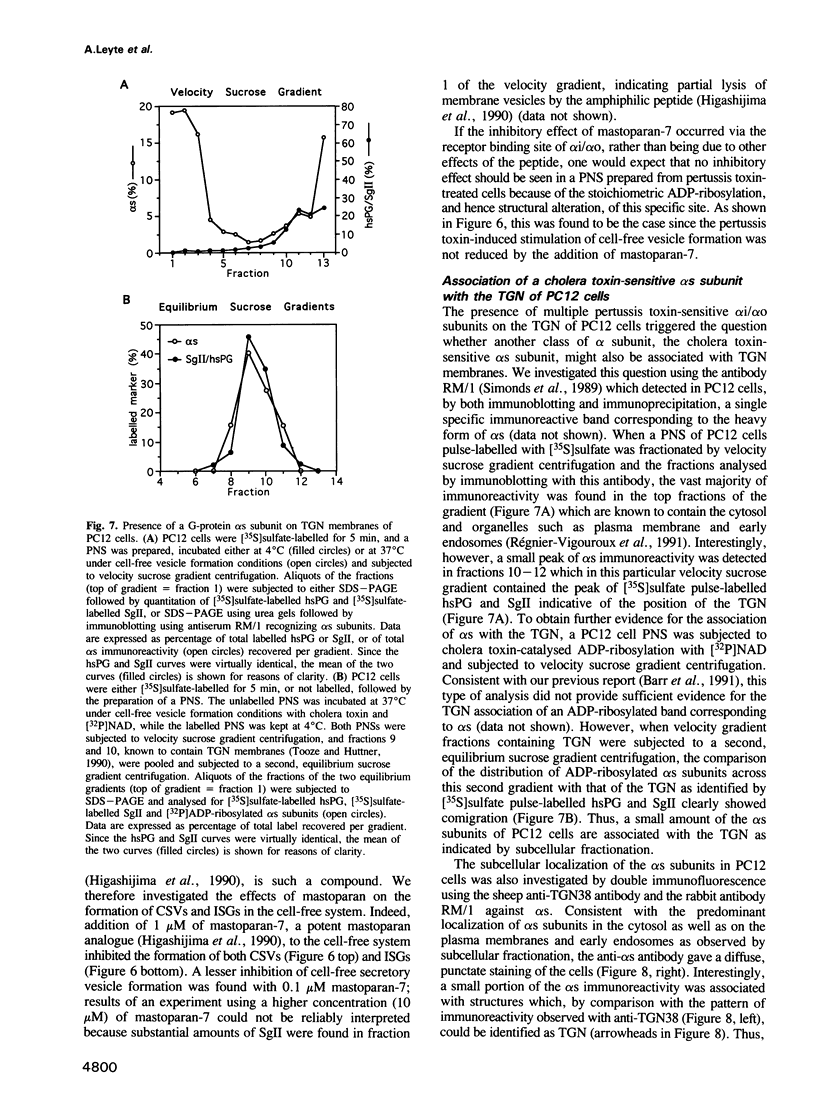
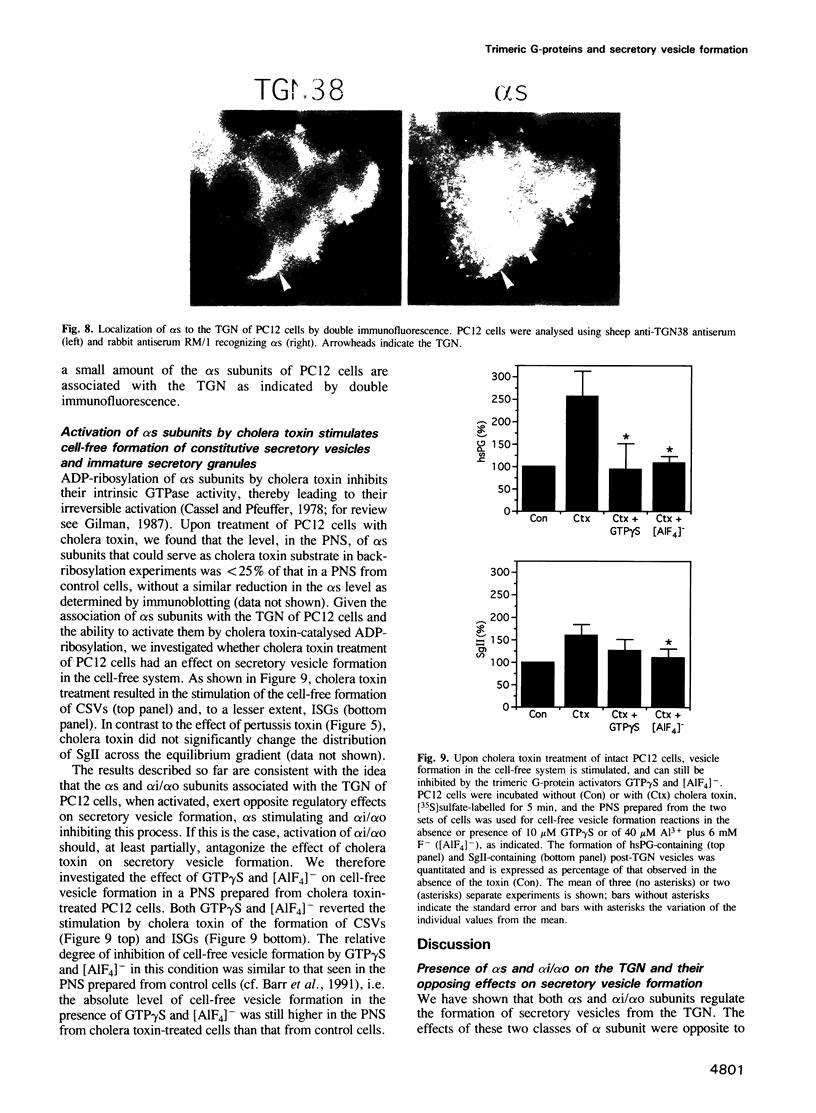
The role of heterotrimeric G-proteins on the formation of constitutive secretory vesicles (CSVs) and immature secretory granules (ISGs) from the trans-Golgi network (TGN) of PC12 cells was investigated. Using immunofluorescence and subcellular fractionation in conjunction with immunoblotting or ADP-ribosylation by either pertussis toxin or cholera toxin, TGN membranes were found to contain not only several alpha i/alpha o G-protein subunits including apparently alpha i3, but also alpha s. Pertussis toxin treatment of cells, which resulted in the stoichiometric ADP-ribosylation of alpha i/alpha o, a modification known to prevent their coupling to receptors, led to the stimulation of cell-free CSV and ISG formation, suggesting the presence of a guanine nucleotide exchange factor for alpha i/alpha o on the TGN. Mastoparan-7, a peptide known to mimic an activated receptor and to stimulate nucleotide exchange on alpha i/alpha o, inhibited cell-free vesicle formation, an effect abolished by pertussis toxin. In contrast, activation of alpha s by cholera toxin treatment of cells resulted in a stimulation of cell-free CSV and ISG formation. This stimulation could be reversed when the alpha subunits not activated by cholera toxin, i.e. alpha i/alpha o, were activated by GTP gamma S and [AIF4]-. Our results show that both inhibitory and stimulatory trimeric G-proteins on the TGN participate in the regulation of secretory vesicle formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausiello D. A., Stow J. L., Cantiello H. F., de Almeida J. B., Benos D. J. Purified epithelial Na+ channel complex contains the pertussis toxin-sensitive G alpha i-3 protein. J Biol Chem. 1992 Mar 5;267(7):4759–4765. [PubMed] [Google Scholar]
- Balch W. E. From G minor to G major. Curr Biol. 1992 Mar;2(3):157–160. doi: 10.1016/0960-9822(92)90276-g. [DOI] [PubMed] [Google Scholar]
- Barr F. A., Leyte A., Huttner W. B. Trimeric G proteins and vesicle formation. Trends Cell Biol. 1992 Apr;2(4):91–94. doi: 10.1016/0962-8924(92)90001-4. [DOI] [PubMed] [Google Scholar]
- Barr F. A., Leyte A., Mollner S., Pfeuffer T., Tooze S. A., Huttner W. B. Trimeric G-proteins of the trans-Golgi network are involved in the formation of constitutive secretory vesicles and immature secretory granules. FEBS Lett. 1991 Dec 9;294(3):239–243. doi: 10.1016/0014-5793(91)81438-e. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. Trimeric G proteins in Golgi transport. Trends Biochem Sci. 1992 Mar;17(3):87–88. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M. Aluminofluoride and beryllofluoride complexes: a new phosphate analogs in enzymology. Trends Biochem Sci. 1990 Jan;15(1):6–10. doi: 10.1016/0968-0004(90)90117-t. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Kahn R. A., Lippincott-Schwartz J., Klausner R. D. Binding of ARF and beta-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991 Nov 22;254(5035):1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- Ercolani L., Stow J. L., Boyle J. F., Holtzman E. J., Lin H., Grove J. R., Ausiello D. A. Membrane localization of the pertussis toxin-sensitive G-protein subunits alpha i-2 and alpha i-3 and expression of a metallothionein-alpha i-2 fusion gene in LLC-PK1 cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4635–4639. doi: 10.1073/pnas.87.12.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierschik P. ADP-ribosylation of signal-transducing guanine nucleotide-binding proteins by pertussis toxin. Curr Top Microbiol Immunol. 1992;175:69–96. doi: 10.1007/978-3-642-76966-5_4. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Jakobs K. H. Two distinct Gi-proteins mediate formyl peptide receptor signal transduction in human leukemia (HL-60) cells. J Biol Chem. 1989 Dec 25;264(36):21470–21473. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985 Sep;101(3):949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima T., Burnier J., Ross E. M. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J Biol Chem. 1990 Aug 25;265(24):14176–14186. [PubMed] [Google Scholar]
- Ktistakis N. T., Linder M. E., Roth M. G. Action of brefeldin A blocked by activation of a pertussis-toxin-sensitive G protein. Nature. 1992 Mar 26;356(6367):344–346. doi: 10.1038/356344a0. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Huttner W. B. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosylprotein sulfotransferase. J Biol Chem. 1983 Sep 25;258(18):11326–11334. [PubMed] [Google Scholar]
- Luzio J. P., Brake B., Banting G., Howell K. E., Braghetta P., Stanley K. K. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38). Biochem J. 1990 Aug 15;270(1):97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Katada T., Murayama Y., Ui M., Ogata E., Nishimoto I. A simple structure encodes G protein-activating function of the IGF-II/mannose 6-phosphate receptor. Cell. 1990 Aug 24;62(4):709–717. doi: 10.1016/0092-8674(90)90116-v. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R. GTP-binding proteins in intracellular transport. Trends Cell Biol. 1992 Feb;2(2):41–46. doi: 10.1016/0962-8924(92)90161-f. [DOI] [PubMed] [Google Scholar]
- Rexach M. F., Schekman R. W. Distinct biochemical requirements for the budding, targeting, and fusion of ER-derived transport vesicles. J Cell Biol. 1991 Jul;114(2):219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Neto F., Mattera R., Grenet D., Sekura R. D., Birnbaumer L., Field J. B. Adenosine diphosphate ribosylation of G proteins by pertussis and cholera toxin in isolated membranes. Different requirements for and effects of guanine nucleotides and Mg2+. Mol Endocrinol. 1987 Jul;1(7):472–481. doi: 10.1210/mend-1-7-472. [DOI] [PubMed] [Google Scholar]
- Rosa P., Mantovani S., Rosboch R., Huttner W. B. Monensin and brefeldin A differentially affect the phosphorylation and sulfation of secretory proteins. J Biol Chem. 1992 Jun 15;267(17):12227–12232. [PubMed] [Google Scholar]
- Rosa P., Weiss U., Pepperkok R., Ansorge W., Niehrs C., Stelzer E. H., Huttner W. B. An antibody against secretogranin I (chromogranin B) is packaged into secretory granules. J Cell Biol. 1989 Jul;109(1):17–34. doi: 10.1083/jcb.109.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I., Spiegel A. M., Malech H. L. Subcellular localization of Gi alpha in human neutrophils. J Biol Chem. 1988 Aug 5;263(22):10958–10964. [PubMed] [Google Scholar]
- Régnier-Vigouroux A., Tooze S. A., Huttner W. B. Newly synthesized synaptophysin is transported to synaptic-like microvesicles via constitutive secretory vesicles and the plasma membrane. EMBO J. 1991 Dec;10(12):3589–3601. doi: 10.1002/j.1460-2075.1991.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Schekman R. Protein localization and membrane traffic in yeast. Annu Rev Cell Biol. 1985;1:115–143. doi: 10.1146/annurev.cb.01.110185.000555. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Willingham M. C., Botstein D., Kahn R. A. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., de Almeida J. B., Narula N., Holtzman E. J., Ercolani L., Ausiello D. A. A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol. 1991 Sep;114(6):1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Sudo Y., Linder M. E., Fishman M. C. An intracellular guanine nucleotide release protein for G0. GAP-43 stimulates isolated alpha subunits by a novel mechanism. J Biol Chem. 1991 Nov 25;266(33):22465–22471. [PubMed] [Google Scholar]
- Taylor C. W. The role of G proteins in transmembrane signalling. Biochem J. 1990 Nov 15;272(1):1–13. doi: 10.1042/bj2720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T. C., Kahn R. A., Melançon P. Two distinct members of the ADP-ribosylation factor family of GTP-binding proteins regulate cell-free intra-Golgi transport. Cell. 1992 Jul 10;70(1):69–79. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free formation of immature secretory granules and constitutive secretory vesicles from trans-Golgi network. Methods Enzymol. 1992;219:81–93. doi: 10.1016/0076-6879(92)19012-u. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Weiss U., Huttner W. B. Requirement for GTP hydrolysis in the formation of secretory vesicles. Nature. 1990 Sep 13;347(6289):207–208. doi: 10.1038/347207a0. [DOI] [PubMed] [Google Scholar]
- Toutant M., Aunis D., Bockaert J., Homburger V., Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987 May 11;215(2):339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- Watson E. L., DiJulio D., Kauffman D., Iversen J., Robinovitch M. R., Izutsu K. T. Evidence for G proteins in rat parotid plasma membranes and secretory granule membranes. Biochem J. 1992 Jul 15;285(Pt 2):441–449. doi: 10.1042/bj2850441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C., Wuestehube L. J., Lila T., Schekman R. Sec12p-dependent membrane binding of the small GTP-binding protein Sar1p promotes formation of transport vesicles from the ER. J Cell Biol. 1991 Aug;114(4):663–670. doi: 10.1083/jcb.114.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]