Abstract
Background
Patients with diabetes have a two to fourfold increased risk for development of and death from cardiovascular disease [CVD]. The current oral hypoglycaemic agents result in limited reduction in this cardiovascular risk. Sodium glucose linked co-transporter type 2 [SGLT2] inhibitors are a relatively new class of antidiabetic agent that have been shown to have potential cardiovascular benefits. In support of this, the EMPA-REG trial showed a striking 38% and 35% reduction in cardiovascular mortality and heart failure [HF] hospitalisation respectively. The exact mechanism (s) responsible for these effects remain (s) unclear. One potential mechanism is regression of Left ventricular hypertrophy (LVH).
Methods
The DAPA-LVH trial is a prospective, double-blind, randomised, placebo-controlled ‘proof of concept’ single-centre study that has been ongoing since January 2017. It is designed specifically to assess whether the SGLT2 inhibitor dapagliflozin regresses left ventricular [LV] mass in patients with diabetes and left ventricular hypertrophy [LVH]. We are utilising cardiac and abdominal magnetic resonance imaging [MRI] and ambulatory blood pressure monitoring to quantify the cardiovascular and systemic effects of dapagliflozin 10 mg once daily against standard care over a 1 year observation period. The primary endpoint is to detect the changes in LV mass. The secondary outcomes are to assess the changes in, LV volumes, blood pressure, weight, visceral and subcutaneous fat.
Discussion
This trial will be able to determine if SGLT2 inhibitor therapy reduces LV mass in patient with diabetes and LVH thereby strengthening their position as oral hypoglycaemic agents with cardioprotective benefits.
Trial registration
Clinical Trials.gov: NCT02956811. Registered November 2016.
Keywords: Diabetes, SGLT2 inhibitor, Left ventricular hypertrophy, Mechanistic trial, Cardiac MRI
Background
Patients with type 2 diabetes mellitus [T2DM] have double the risk of cardiovascular death [CVD] compared with patients without T2DM [1, 2]. Hyperglycaemia itself contributes to both the pathogenesis of atherosclerosis and heart failure [3]. While intensive glucose control reduces the risk of microvascular complications it appears to be insufficient to reduce cardiovascular [CV] events [4]. Three large randomized controlled trials [RCTs] ADVANCE, ACCORD and VADT failed to demonstrate any significant effect on macrovascular events of more intensive glycaemic control in patients with longstanding T2DM when compared with standard medical care [5–7].
The EMPA-REG OUTCOME trial was a landmark trial as it demonstrated for the first time that a glucose lowering agent could reduce CV events [8]. It was a multicentre, randomised, double blind, placebo-controlled trial performed in 7020 patients with T2DM at high cardiovascular risk comparing the SGLT2 inhibitor empagliflozin to placebo. In the empagliflozin group there were significantly lower rates of death from cardiovascular causes and heart failure hospitalisations, by 38 and 35% respectively. The exact mechanisms responsible for these effects remains unclear.
Left ventricular hypertrophy
One potential mechanism is regression of left ventricular hypertrophy [LVH]. Left ventricular hypertrophy is thought to be present in up to 70% of patients with T2DM [9] It is a strong independent predictor of cardiovascular deaths and events and is even worse than triple vessel coronary disease [10, 11]. The reason why LVH is so adverse is because it predates so many different cardiovascular events i.e. LVH is intrinsically arrhythmogenic and causes sudden death, it impedes left ventricular [LV] filling and leads to diastolic heart failure, it reduces coronary perfusion reserve and causes ischaemia and it causes left atrial enlargement leading to atrial fibrillation [AF], and cardio-embolic strokes [12].
How does one cause regression of LVH? Controlling blood pressure [BP] and using a drug that blocks the renin-angiotensin system [RAS] are the standard approaches to the management of LVH but this approach is only partially effective since 44% of all patients with T2DM are normotensive patients with LVH [9]. Thus normotensive LVH is very common [9, 13]. Indeed, BP only contributes 25% to the variability in LV mass seen in a population [14]. Despite a “normal” BP, normotensive LVH is just as risky as is hypertensive LVH [15]. Nevertheless, we do know that regressing LVH irrespective of BP changes is an effective way to reduce the incidence of all major cardiovascular [CV] events including specifically sudden deaths, heart failure hospitalisations, new onset AF and strokes [16–23]. The LIFE trial provides conclusive proof that in diabetes, LVH regression per se reduces future cardiovascular events [by 24%], reduces CV deaths [by 37%] and reduces total deaths [by 41%] irrespective of BP [24].
Since controlling BP and using an angiotensin enzyme inhibitor or angiotensin receptor blocker is only partially effective at regressing LVH, we now need additional ways of regressing LVH. Insulin resistance is another mediator of LVH. The literature is awash with observational studies linking insulin resistance to LVH. The large studies are mostly positive which includes the Framingham Study, the Whitehall trial, the Strong Heart trial and the Women’s Health Initiative trial while HyperGEN is the one large negative trial [25–29]. Therefore, it is likely that glycaemia contributes to LVH. However, there is little evidence to date that glycaemic control alone affects the risk of CV events and thus key ancillary properties of each anti-glycaemic drug will be necessary to deliver the CV benefits we so badly need in diabetes [4, 30, 31]. A separate albeit related factor associated with LVH is also obesity [25, 32, 33].
Sodium glucose linked co-transporter2 [SGLT2] inhibitors and their potential to regress LVH
In this study, we hypothesize that SGLT 2 inhibitors may be able to lead to LVH regression. Firstly, SGLT2 inhibitors employ a novel mechanism to lower blood glucose by enhancing urinary glucose excretion by competitively blocking the sodium glucose linked co-transporters in the proximal renal tubules, thus preventing the reabsorption of filtered glucose and sodium, resulting in glycosuria and natriuresis [34, 35]. This is in contrast with other antidiabetic medications which focus on restoring β-cell activity, insulin sensitivity and tissue glucose uptake to reduce plasma glucose levels. Accordingly, SGLT2 inhibitors are expected to maintain their potency as beta cell function declines with disease progression. Secondly, the glycosuric effects of SGLT2 inhibitors result in around 240-400 kcal/day loss through the urinary tract [36]. This calorific loss results in an average weight loss of around 2-3 kg that could help lead to LVH regression [37]. Finally, the natriuretic effect and subsequent osmotic diuresis could also be significant in patients with cardiovascular disease. This diuretic effect should reduce preload on the heart. The SGLT2 inhibitors lower blood pressure by 7-10 mmHg, reduce arterial stiffness and afterload [38–40].
In summary, SGLT2 inhibitors may improve cardiac structure because they appear to reduce the four main causes of LVH: glycaemia/insulin resistance, weight, preload and afterload [blood pressure] [41]. Our hypothesis is that dapagliflozin will regress LVH in normotensive patients with T2DM. If so, this could be a large part of explaining why such drugs reduce CV events in the EMPA-REG OUTCOME trial.
The issue of how SGLT2 Inhibitors reduces CV events in diabetes is a hot topic following EMPAREG OUTCOME. A large ongoing trial [DECLARE – TIMI 58] is also in progress assessing the effect of Dapagliflozin on CV events. If DECLARE-TIMI 58 shows clearly that dapagliflozin reduces CV events, then our trial if positive will have revealed a possible contributing mechanism to the reduced CV events i.e. LVH regression.
Methods
Study design
The DAPA-LVH trial is a prospective, double-blind, randomised, placebo-controlled ‘proof of concept’ single-centre study conducted in NHS Tayside, Scotland designed to evaluate the efficacy of 12 months of the SGLT2 inhibitor dapagliflozin compared to placebo on left ventricular hypertrophy [LVH] in 64 normotensive participants with diabetes identified to have LVH. A recruitment window of 1.5 years from December 2016 has been set. Participants will be enrolled in this trial for a period of 12–13 months, [Fig. 1]. Therefore, the overall trial end date will be May 2019.
Fig. 1.
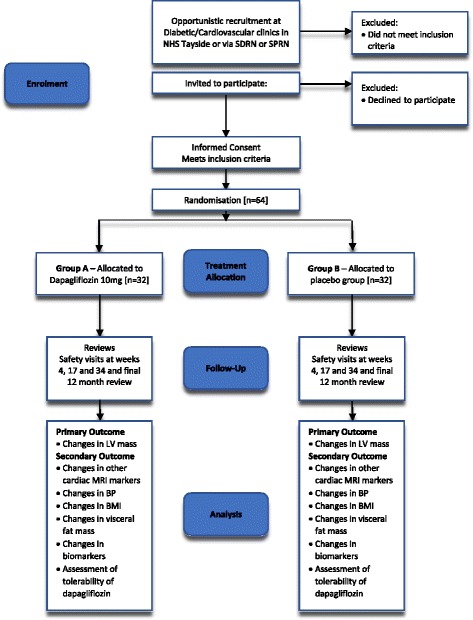
Study design flowchart. SDRN Scottish Diabetes Research Network; SPRN Scottish Primary Research Network; MRI Magnetic Resonance Imaging; BP Blood Pressure; BMI: Body Mass Index
At the screening visit an initial medical history and clinical examination will be performed following informed consent. Participants will have an electrocardiogram performed and bloods taken for safety analysis. Vital signs including blood pressure will be recorded to confirm eligibility prior to enrolment. Blood pressure will be taken using an Omron M10-IT blood pressure monitor and eligible patients will have an office blood pressure of 145/90 mmHg averaged over three readings. Patients who require optimisation of their blood pressure will do so but will have to be stable on their current antihypertensive medications for 3 months prior to enrolment. Patients with borderline office blood pressure will undergo ambulatory blood pressure measurement (AMBP) This will be performed using a Spacelab 90,217 ambulatory blood pressure monitor. Inclusion will be possible with a 24 h mean blood pressure < 140/85 mmHg. Participants will also be screened for echocardiographic evidence of left ventricular hypertrophy [LVH] by the standard American Society of Echocardiology [ASE] criteria. This will be performed using a Philips Epiq 7 machine by a fully trained operator. Eligible participants identified to have LVH on echocardiography will be recruited. The full inclusion criteria are as listed below.
Recruited patients will return for a cardiac magnetic resonance imaging [CMRI] at the Clinical Research Centre, Ninewells Hospital, Dundee, within 3 weeks of the planned baseline [randomisation] visit. At the randomisation visit participants will have vital signs, body mass index, waist circumference and waist to hip ratio recorded. Participants will also be asked to undergo 24 h ambulatory BP monitoring using a Spacelab 90,217 ambulatory monitor. Examinations with greater than 50% successful readings will be deemed an acceptable exam. Bloods for safety analysis and research purposes [BNP, FIRI and Uric acid] will also be taken. During the visit, participants will also be randomly be assigned to either dapagliflozin 10 mg or matching placebo. The first dose will be administered during this visit and participants will be educated on the symptoms of both hypoglycaemia and diabetic ketoacidosis and given written instructions of how to manage it if either event occurs.
To reduce the likelihood of hypoglycaemia in participants taking insulin, participants who are already on insulin at time of recruitment will have their total daily dose of insulin reduced by 10% on the day they are randomised. Further dose titration will be done by the study team or GP based on the participant’s symptoms, home and laboratory-based blood sugar levels. Down-titration of therapy will be done in a stepwise manner starting with insulin. Other anti-diabetic agents will only be down-titrated once insulin has been discontinued.
In order to make the two groups comparable, a target HbA1c of ≤ 53 mmol/mol will be set for all participants. New onset diabetic patients will not be included in this study as SGLT2 inhibitors are currently only licensed as second line therapy. We will therefore be comparing a dapagliflozin [mostly as a second drug after metformin] based group against a conventionally treated group but without a SLGT2 inhibitor.
This will ensure that any difference in LV mass between groups is because dapagliflozin and all its ancillary cardiac properties and not because the two groups differed in glycaemic control.
With regards to BP, the main criteria will be that the baseline office BP is < 145/90 mmHg. However, the investigator will have clinical discretion to change anti-hypertensive drugs during the trial for safety reasons, under two circumstances. Firstly, if the systolic BP rises to above 140 mmHg on 2 consecutive visits during the trial then the participant can be started on extra anti-hypertensive drugs to re-achieve a systolic BP of < 145 mmHg. Secondly, if the participant suffers from dizziness and/or their systolic BP has fallen either by ≥ 25 mmHg or to an absolute level of ≤ 110 mmHg, then the attending physician can reduce or stop one of their antihypertensive drugs. These criteria serve two functions: firstly, to copy normal clinical practice and secondly to maintain participant safety.
Participants will return for three visits throughout the year to have safety and research bloods taken and to have vital signs, BMI, waist to hip ratio and waist circumference recorded. They will also be assessed for adverse events and to alter diabetic/antihypertensive therapy [if applicable].
The biomarker samples will be centrifuged and decanted into an aliquot which will be stored at -80 °C. Uric acid will be analysed with an elisa method using SIGMA-ALDRICH assay, UK. BNP will be measured by a MULTI-ARRAY system. The kit is from MESO SCALE DISCOVERY, USA. FIRI will be analysed with an elisa method using an ALPCO assay UK.
At the end of the 1 year study period, participants will return for repeated assessment of vital signs, BMI, waist circumference and waist to hip ratio, ambulatory blood pressure, echocardiography and CMRI. These values will be compared with their baseline tests to determine if any significant change has occurred with each of the two arms of the study populations. [See Table 1 for an overview of all visits scheduled for the trial].
Table 1.
Overview of all the study visits in the DAPALVH trial
| Visit | 1 Screening | 2 Baselinea | 3 follow-upa | 4 Follow-upa | 5 Follow-upa | 6 Last visita |
|---|---|---|---|---|---|---|
| Timeline - weeks | 0 to − 4 | 0 Within 4 weeks of screening visit | 4b [+/− 1 week] | 17 [+/− 4 weeks] | 34 [+/− 4 weeks] | 52 [+/− 4 weeks] |
| Informed Consent | X | |||||
| Medical History | X | |||||
| Demographics | X | |||||
| Concomitant Medications | X | X | X | X | X | X |
| Physical Examination | X | |||||
| Height & weight | X | |||||
| BP & P | X | X | X | X | X | X |
| Temperature | X | |||||
| ECG | X | |||||
| Echoc | X | X | ||||
| Safety Bloodsd | X | X | X | X | X | X |
| Inclusion/Exclusion | X | |||||
| Pregnancy Testing if applicablee | X | X | X | X | X | X |
| Research Blood Sample f | X | X | X | X | X | |
| Genetic blood sample | X | |||||
| 24 h BP | X | X | ||||
| Cardiac & abdominal MRIg | X | X | ||||
| Waist & hip measurement | X | X | X | X | X | |
| Adjustment of diabetes medication | X | X | X | X | ||
| Adjustment of anti-hypertensive medication | X | X | X | X | ||
| Record Adverse Events | X | X | X | X | X | |
| Randomisation | X | |||||
| Dispense Trial Drugs | Xh | X | X | X | ||
| Return trial drugs | X | X | X | X |
aParticipants will be fasted for these visits
bAt least 3 weeks after commencing study medication
cIf a participant has had a clinical echo done within the previous 6 months the results from this will be used for assessing eligibility, otherwise an echo will be performed at the screening visit
dU&E, FBC, LFT, cholesterol, HDL-cholesterol
eSee section 7.2
fHbA1c, FIRI, BNP, glucose, uric acid. A sample to be held for future research will be taken at the last visit
gThe MRI scan may be performed ± 3 weeks from the baseline visit and may therefore require a separate visit
hIf the participant as not yet had their MRI scan they will be asked not to start their study medication until they have had their scan. Their Visit 3 will be delayed until the patient has had at least 3 weeks of study medication
Study population
Diabetic patients with LVH will be identified for this study and all potential participants who meet the following criteria will be eligible for the trial:
Inclusion criteria:
Diagnosed with type 2 diabetes mellitus based on the current American Diabetes Association guidelines.
Aged 18–80 years
Body Mass Index ≥ 23 kg/m2
HbA1c 48-85 mmol/mol [last known result within in the previous 6 months]
BP < 145/90 mmHg. Office BP at screening visit will be used however if this is above the inclusion criteria then the 24 h recording at screening visit will be used to confirm that in the opinion of the PI the BP is adequately controlled.
Echocardiographic LV hypertrophy (defined as either an LV mass index of >115 g/m2 for men and > 95 g/m2 for women indexed to body surface area or > 48 g/m2.7 or 44 g/m2.7 when indexed to height2.7)
Women of childbearing potential* [WoCBP] must agree to take precautions to avoid pregnancy throughout the trial and for 4 weeks after intake of the last dose
Exclusion criteria:
Any condition that in the opinion of the investigator may render the participant unable to complete the trial including non CV disease [e.g. active malignancy].
Participants with type 1 diabetes mellitus
Participants who have previously had an episode of diabetic ketoacidosis.
Serum Potassium or Sodium results outwith the normal range
Diagnosis of clinical heart failure
History of human immunodeficiency virus
LV systolic dysfunction [LVEF < 45%] [last known result within in the previous 6 months]
eGFR < 60 ml/min/1.73m2 [last known result within in the previous month] assessed using an abbreviated Modification of Diet in Renal Disease (MDRD) equation and indexed to 1.73m2.
Known liver function tests > 3 times upper limit of normal [based on last measures and documented laboratory measurement in the previous 6 months]
Body weight > 150Kg [unable to fit into a MRI scanner]
Contraindications to MRI [e.g. claustrophobia, metal implants, penetrative eye injury or exposure to metal fragments in eye requiring medical attention]
Past or current treatment with any SGLT2 inhibitor
Allergy to any SGLT2 inhibitor or lactose or galactose intolerance
Current treatment with loop diuretic
Currently receiving long term [> 30 consecutive days] treatment with an oral steroid
Pregnant or breast feeding participants
Involvement in the planning and/or conduct of the trial [applies to Astra Zeneca or representative staff and/or staff at the trial site].
Participation in another interventional study [other than observational trials and registries] within 30 days before visit 1.
Individuals considered at risk for poor protocol or medication compliance
Randomisation and treatment allocation
After successful screening for eligibility and safety, participants will be randomised to either dapagliflozin 10 mg or matching placebo [identical tablet containing lactose] in a double blind fashion. The double blind medication [dapagliflozin or placebo] will be prepared and packaged by AstraZeneca and labelled by our onsite clinical trials pharmaceutical pharmacy. Randomisation will be carried out via our Tayside Randomisation System [TRuST], a Good Clinical Practice [GCP] compliant web-based system run by the Tayside Clinical Trials Unit [TCTU], to preserve allocation concealment. This will securely backup both the randomisation seed and the randomisation allocation and have it available in the onsite 24 h emergency unblinding facility.
Once randomised, the participant will continue to take the trial medication once daily for 1 year, if tolerated. Compliance will be checked and documented, by the dispensing pharmacy, using tablet counts at each visit. If non-compliant, they will be encouraged to become compliant. If study drug needs to be stopped due to intolerance or adverse events, they will remain in the study in order to do an “intention to treat” analysis.
Study outcomes
Primary objective and outcome
The primary outcome is to determine if dapagliflozin reduces left ventricular [LV] mass in patients with type 2 diabetes and LV hypertrophy when compared to placebo.
Secondary outcomes
To determine if dapagliflozin has any effect on LV diastolic function.
To determine if as expected dapagliflozin reduces blood pressure [BP]
To assess the effect of dapagliflozin on left ventricular diastolic function and global longitudinal strain
To determine if as expected dapagliflozin reduces body weight
To determine if dapagliflozin reduces visceral fat mass
To determine if as expected dapagliflozin reduces HbA1C.
To determine the effect of dapagliflozin on B-type natriuretic peptide [BNP], Uric acid and Fasting Insulin Resistance Index [FIRI]
To assess the tolerability of dapagliflozin in this patient group
Sample size and power calculations
For the primary outcome of LV mass regression using cardiac MRI, we have powered this study for an absolute change in LV mass based on previous studies that we have conducted in our unit. In our recently published study of LVH regression using allopurinol in participants with ischaemic heart disease [41], we found that allopurinol significantly reduced LV mass by − 5·2 ± 5·8 g compared to placebo − 1·3 ± 4·5 g [p < 0·007]. In per-cent terms, this degree of LVH regression is the same as seen between the two arms of the echo sub-study of the LIFE study where CV events were also different between groups. For an 80% power at a 5% significance level [α = 0·05], to detect a similar change in LV mass, we will require 29 subjects per group. Both our previous studies have shown a 10% dropout rate. Therefore, accounting for this, we will require a total of 64 participants [32 per group]. The 10% dropout rate is standard for such studies and includes those who died and those who withdraw consent.
Cardiac MRI protocol
Baseline and repeat Cardiac magnetic resonance imaging [CMRI] examinations at baseline visit [+/− 3 weeks] and after the final 12 month [+/− 4 weeks] visit will be performed on a 3 T Magnetrom Trio scanner [Siemens, Erlangen, Germany] using body array and spine matrix radiofrequency coils.. Short axis images from the atrio-ventricular ring to the LV apex will be acquired using a 2D ECG-gated breath hold segmented SSFP cine sequence with retrospective gating. Quantitative measurement of LV mass, ejection fraction (EF), end-diastolic volume (EDV), end-systolic volume (ESV) and stroke volume (SV) will be derived by region of interest contours placed around endocardial and epicardial LV borders at end systole and end diastole.
Transmitral flow and the isovolumetric relaxation time will be assessed using through plane phase contrast images with electrocardiographic synchronisation.
At the same time of the CMRI visceral and subcutaneous abdominal fat mass will be assessed. For measurement of subcutaneous adipose tissue (SCAT) and visceral adipose tissue (VAT) two successive axial 3D DIXON volume interpolated breath hold examination sequences will be acquired. These sequences will cover an anatomical area from the diaphragm to the pelvic floor, with a slice thickness of 3 mm and up to 88 slices (dependent on patient size) collected within a single 3D block.
For image analysis the ‘fat only’ DIXON MR images will be segmented using Analyze (Mayo Clinic) software, and the SCAT and VAT compartments are defined using a signal intensity threshold method with manual correction where required. Epicardial fat structures and fat associated with the vertebrae will both be omitted from the final calculated volumes.
From a single MRI slice at the L2-L3 intervertebral level. This single observer will analyse all the scans. Analysis will be performed offline [Argus Software, Siemens] by a single blinded observer.
The reproducibility of all parameters using MRI will be derived by this observer. A test-retest intra-observer co-efficient of variation of 2.0% is usual in this department’s past MRI studies. Should the scanner become unavailable for a prolonged period of time during the study an alternative scanner will be used. MRI methods will be adapted as appropriate to ensure optimal study results can be obtained.
Discussion
SGLT2 inhibitors including dapagliflozin improve systemic glucose metabolism, lower blood pressure and lower body weight, thus they ameliorate the metabolic and haemodynamic risk factors heavily implicated in causing LVH. In this study, we propose that the SGLT2 inhibitors may be particularly suitable at regressing LVH. This effect might ultimately explain the reduced CV events seen so far in one large outcome trial with these drugs [Fig. 2].
Fig. 2.

DAPA LVH trial hypothesis. The above figure explains the hypothesis of the DAPALVH trial where reduction in preload and afterload, weight and insulin resistance will all contribute to regression of left ventricular hypertrophy. This will be measured by cardiac MRI. BNP: B Type Natriuretic Peptide; BP: Blood Pressure; EDV: End Diastolic Volume; FIRI: Fasting Insulin Resistance Index; HBA1C: Glycosylated Haemoglobin; IVRT: Isovolumetric Relaxation Time; LA: Left Atrial; LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction; LVH: Left Ventricular Hypertrophy; MRI: Magnetic Resonance Imaging
The primary haemodynamic effect of SGLT2 inhibitors is an osmotic diuresis. Patients treated with dapagliflozin produce approximately 375mls of extra urine per day [36]. Several trials have shown that SGLT2 inhibitors lead to a reduction in systolic BP in a range of 3-5 mmHg and ~ 2-3 mmHg in diastolic BP [37]. This will be further assessed in our trial with ambulatory blood pressure recordings at randomisation and upon completion of the trial. The reason for the observed BP reduction with SGLT2 inhibition is not completely understood but is likely secondary to several different things including the modest diuretic effect, mild natriuresis and weight reduction [39, 42]. Data from a mechanistic trial has also demonstrated that empagliflozin reduced arterial stiffness in patients with type 1 diabetes mellitus [43]. These effects on intravascular volume and blood pressure will result in reduced preload and afterload respectively, thereby facilitating a reduction in intra-cardiac pressure and thereby an improvement in cardiac structure [24, 44]. Indeed following EMPA-REG Outcome trial there has been a lot of interest in the effects of SGLT2 inhibition on cardiac structure with a number of ongoing trials looking into the effects in patients with both diastolic and systolic heart failure in addition to cardiovascular outcomes [Tables 2, 3, and 4].
Table 2.
Ongoing trials assessing the use of SGLT2 inhibitors in patients with systolic heart failure
| SGLT2 Inhibitor | Trial Name; Clinical Trial Identifier | Primary Outcome measure | Patient Populationa | Final Results |
|---|---|---|---|---|
| Empagliflozin | Empagliflozin Impact on Haemodynamics in Patients with Diabetes and Heart Failure [EMBRACE-HF]. [54] NCT03030222 |
Change in pulmonary artery diastolic pressure |
N = 60 10 mg vs placebo Either LVEF 40% or > 40% NHYA II-IV HbA1c ≥ 6.5% and ≤ 11% GFR > 30 ml/min |
June 2018 |
| Empagliflozin | SGLT2 Inhibition in Diabetic Patients With Heart Failure with Reduced Ejection Fraction [55] NCT02862067 |
SGLT2 inhibition effects on cardiorespiratory fitness |
N = 31 10 mg/25 mg standard care LVEF ≤ 50% [in maximum tolerated HF therapy HbA1c 7–10% Age ≥ 18 years GFR > 45 ml/min |
June 2018 |
| Empagliflozin | EMPagliflozin outcomE tRial in Patients with chrOnic heaRt Failure with Reduced Ejection Fraction [EMPEROR-Reduced] [56] NCT03057977 |
Time to first event of adjudicated CV death or adjudicated hospitalisation for HF in patients with HF with reduced ejection fraction |
N = 2850 LVEF ≥ 36to ≤ 40%: NTproBNP ≥ 2500 pg/ml LVEF ≥ 31% to ≤ 35%: NT-proBNP ≥ 1000 pg/ml If LVEF ≤ 30% NT-proBNP ≥ 600 pg/ml Age > 18 years GFR > 20 ml/min |
June 2020 |
| Dapagliflozin | Dapagliflozin Effect on Symptoms and Biomarkers in Diabetes Patients with Heart Failure [DEFINE-HF] [57] NCT02653482 |
Differences in the average reduction of NTproBNP Proportion of patient that achieve a ≥ 5pts increase in heart failure disease specific quality of life score or a ≥ 20% decrease in NTproBNP |
N = 250 10 mg vs placebo LVEF ≤ 40/NHYA II-III HbA1c 6.5–11.0% Age 19–119 years GFR > 45 ml/min BNP ≥ 125 pg/ml and/or NTproBNP ≥ 600 pg/ml |
May 2017 |
| Dapagliflozin | Study to Evaluate the Effect of dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure With Reduced Ejection Fraction [Dapa-HF]. [58] NCT03036124 | Time to first occurrence of the composite: CV death or hospitalisation for HF or urgent HF visit. |
N = 4500 5/10 mg vs placebo LVEF ≤ 40/NHYA II-IV Age 18 to 130 years GFR > 30 ml/min NTproBNP ≥ 600 pg/ml |
December 2019 |
| Dapagliflozin | Safety and Effectiveness of SGLT2 inhibitors in Patients with Heart Failure and Diabetes [REFORM] [59] NCT02397421 |
Change in LV end systolic volume or LV end diastolic volume as determined by CMRI |
N = 56 10 mg vs placebo. LVEF < 50%/NYHA I-II HbA1c > 6% Age 18 to 75 years GFR > 45 ml/min |
August 2017 |
| Canagliflozin | A Randomised Active-Control Double-Blinded Study to Evaluate the Treatment of Diabetes in Patients with Systolic Heart Failure. [60] NCT02920918 |
Change from baseline aerobic exercise capacity Change from baseline ventilator efficiency |
N = 88 LVEF ≤ 40%/NYHAII-III HbA1C 6.5%–10% Age ≥ 18 years GFR >50 ml/min |
November 2018 |
aEnrolment details correct at the time of writing as per ClinicalTrials.gov
BNP B Type Natriuretic Peptide, CMRI Cardiac Magnetic Resonance Imaging, ESKD End Stage Kidney Disease, GFR Glomerular Filtration Rate, HbA1 C Glycosylated Haemoglobin, HF Heart Failure, LV Left Ventricular, LVEF Left Ventricular Ejection Fraction, NTproBNP N Terminal pro brain natriuretic peptide, NYHA New York Heart Association, SGLT2 Sodium Glucose Linked Co-Transporter2
Table 3.
Ongoing Trials assessing the use of SGLT2 in patients with left ventricular hypertrophy or heart failure with preserved ejection fraction
| SGLT 2 Inhibitor | Trial Name; Clinical Trial Identifier | Primary Outcome Measure | Patient Populationa | Final Results |
|---|---|---|---|---|
| Empagliflozin | Effects of Empagliflozin on Left Diastolic Function Compared to Usual Care in Type 2 Diabetics [EmDia]. [61] NCT02932436 | Difference in E/E’ ratio measured by echocardiography |
N = 264 10 mg vs placebo Age 18–84 years HbA1C ≥ 7–10% on diabetic therapy or ≥ 7–9% diet controlled GFR > 60 ml/min |
October 2017 |
| Empagliflozin | SGLT2 Inhibition and Left Ventricular Mass [EMPATROPHY] [62]; NCT 02728453 |
Change in ventricular mass assessed using CMRI |
N = 60 25 mg vs 2-4 mg Glimepiride LVEF ≥ 45% Age ≥ 40 and < 80 years Office BP ≤ 150/95 mmHg HbA1C 6.5–9% GFR > 60 ml/min |
April 2018 |
| Empagliflozin | Effects of Empagliflozin on Cardiac Structure in Patients with Type 2 Diabetes [EMPA-HEART] [63] NCT02998970 |
Left Ventricular Mass changes measured by CMRI at 24 weeks |
N = 90 10 mg vs placebo LVEF > 30% Age ≥ 40 and ≤ 80 years HbA1C 6.5- ≤ 10% GFR > 60 ml/min |
June 2017 |
| Dapagliflozin | Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Type 2 Diabetes or Pre-diabetes, and PRESERVED Ejection Fraction; [64] NCT030302235 |
Changes from baseline in NTproBNP |
N = 320 10 mg vs placebo LVEF ≥ 45% Age 19 to < 119 years HbA1C ≥ 5.7 - < 11% GFR < 30 ml/min |
March 2019 |
| Dapagliflozin | Does Dapagliflozin Regress Left Ventricular Hypertrophy in Patients with Type 2 Diabetes; [65] NCT02956911 |
Left Ventricular Mass changes measured by CMRI at 52 weeks |
N = 64 10 mg vs placebo LVEF ≥ 45% Age ≥ 18 and ≤ 75 years HbA1C ≥ 7 - < 10% GFR > 60 ml/min |
May 2019 |
aEnrolment details correct at the time of writing as per ClinicalTrials.gov
CMRI Cardiac Magnetic Resonance Imaging, GFR Glomerular Filtration Rate, HbA1 C Glycosylated Haemoglobin, HF Heart Failure, LV Left Ventricular, LVEF Left Ventricular Ejection Fraction, NTproBNP N Terminal pro brain natriuretic peptide, NYHA New York Heart Association, SGLT2 Sodium Glucose Linked Co-Transporter2
Table 4.
Ongoing Cardiovascular Outcome Trials with SGLT2 inhibitors
| SGLT2 Inhibitor | Trial Name; Clinical Trial Identifier | Primary Outcome Measure | Patient Populationa | Final Results |
|---|---|---|---|---|
| Dapagliflozin | Dapagliflozin Effect on Cardiovascular Events. [DECLARE TIMI 58]; [66] NCT 01730534 | CV Death, non-fatal MI, non-fatal ischaemic stroke |
N = 17,276 10 mg vs placebo HbA1C range not specified Age ≥ 40 years High CV risk |
2019 [Estimated] |
| Canagliflozin | Canagliflozin Cardiovascular Assessment Study. CANVAS]; [67] NCT 01032629 |
CV Death, non-fatal MI, non-fatal ishaemic stroke |
N = 4422 100 mg/300 mg vs placebo HbA1C 7–10.5% Age ≥ 30 years High CV risk |
June 2017 |
| Canagliflozin | Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy [CREDENCE] [68]; NCT 02065791 |
Time to first occurrence of an event in the primary composite of endpoint of ESKD, doubling of serum creatinine, renal or CV death. |
N = 4200 100 mg vs placebo HbA1c 6.5–12% Age > 30 years GFR ≥ 30 to < 90 ml/min |
June 2019 |
aEnrolment details correct at the time of writing as per ClinicalTrials.gov
ESKD End Stage Kidney Disease, GFR Glomerular Filtration Rate, HbA1 C Glycosylated Haemoglobin, HF Heart Failure, LV Left Ventricular, LVEF Left Ventricular Ejection Fraction, NYHA New York Heart Association, SGLT2 Sodium Glucose Linked Co-Transporter2
Dapagliflozin is known to produce clinically meaningful reductions in HbA1c. [45] Studies have also shown that treatment with SGLT2 inhibitors improves insulin sensitivity as measured by peripheral glucose uptake [46, 47]. One such study showed that insulin mediated tissue glucose disposal increased by around 18% with only 2 weeks of dapagliflozin therapy [47]. Insulin resistance and hyperinsulinaemia have been associated with increased atherosclerosis risk and left ventricular hypertrophy [25–29, 48].
Other metabolic effects of the SGLT2 inhibitors include weight loss. With selective SGLT2 inhibition urinary glucose is increased resulting in a negative energy balance and subsequent weight loss [36]. A 24 week study comparing dapagliflozin to placebo showed a 2.5–3.5 kg weight reduction as a result of the calorific loss produced by glycosuria [49]. This is a finding throughout the SGLT2 class [37]. Of potential greater interest is how they change visceral fat mass as this is associated with an increased risk of T2DM and increased risk of CVD and overall mortality [50]. Indeed all the three currently available SGLT2 inhibitors when compared to glimepiride in dedicated body composition studies have shown that the majority of weight loss associated with SGLT2 inhibition was due to a reduction in visceral fat or subcutaneous fat [45, 51, 52]. Accordingly, we have chosen to also measure visceral and subcutaneous fat mass as a secondary outcome of the DAPA-LVH.
Given these metabolic and haemodynamic effects our hypothesis is that we will see a reduction in left ventricular mass. Indeed, pre-clinical work has shown that SGLT2 inhibitors are capable of reducing LV mass in a rat model with progressive HF [53]. We have therefore selected CMRI measurements of LV mass as our primary outcome measures for the DAPALVH Trial. By ensuring the trial is adequately powered we will determine if treatment with an SGLT2 inhibitor is able to reduce LV mass in diabetic patients with LVH.
The EMPA-REG Outcomes trial revealed a reduction in cardiovascular death and HF hospitalisations with the use of empagliflozin in patients with T2DM. However, it is unknown if these effects are seen throughout the SGLT2 inhibitor class. Other cardiovascular outcome trials such as DECLARE-TIMI 58 for dapagliflozin and CANVAS for canagliflozin will reveal whether the cardioprotective effects of SGLT2- inhibitor therapy is seen across the drug class. As described above this study will provide insights into the mechanism of the positive cardiovascular effects conferred by SGLT2 inhibitor therapy and may also help decide the course of future research – should LVH be a favoured target?
Limitations
Firstly, this is a relatively small, single centre trial. The use of CMRI though has allowed the power of the trial to be preserved despite the small number of participants. However, given the small numbers some differences observed may still be the result of chance. Secondly, diabetes is a dynamic disease as a patient’s glycaemia control may fluctuate and this may necessitate dose adjustments of anti-diabetic medications during the trial which may confound the outcome. However, every measure will be taken to ensure blinding of the investigators is maintained and uniformity in the dose adjustments made.
Conclusion
Historically much attention has focused on the prevention and treatment of the microvascular complications of diabetes. CVD however is still the main co-morbid condition and primary contributor to mortality in patients with diabetes. Besides metformin therapeutic options to optimise glycaemic control which reduce cardiovascular risk are limited. Empagliflozin an SGLT2 inhibitor has been shown to produce significant reductions in cardiovascular mortality and hospitalisation with heart failure [8]. We propose that SGLT2 inhibitors may cause regression of LVH due to their ability to reduce preload/afterload, weight and insulin resistance which may account for their positive cardiovascular effects. Upcoming major trials will establish if the effect seen with empagliflozin is an SGLT2 class effect. If so, the results of this study if positive will help us understand the mechanisms of the cardioprotective effects of SGLT2 inhibitors and if positive further establish this group of medications as anti-diabetic agents with the added value of protecting the heart.
Acknowledgements
Not applicable.
Funding
This study is funded by Astra Zeneca.
Availability of data and materials
Not applicable.
Abbreviations
- ABMP
Ambulatory blood pressure measurement
- AF
Atrial fibrillation
- ASE
American society for echocardiography
- BMI
Body mass index
- BNP
B type natriuretic peptide
- BP
Blood pressure
- CMRI
Cardiac magnetic resonance
- CVD
cardiovascular death
- EDV
End diastolic volume
- eGFR
Estimated glomerular filtration rate
- ESV
End systolic volume
- FBC
Full blood count
- FIRI
Fasting insulin resistance index
- GCP
Good clinical practice
- HbA1C
Glycosylated haemoglobin
- HDL
High density lipoprotein
- LFT
Liver function tests
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- LVH
Left ventricular hypertrophy
- MDRD
Modification of diet in renal disease
- MRI
Magnetic resonance imaging
- NTproBNP
N-terminal pro brain natriuretic peptide
- RAS
Renin angiotensin system
- SCAT
Scottish diabetes research network
- SDRN
Subcutaneous adipose tissue
- SGLT2
Sodium glucose co-transporter 2
- SPCRN
Scottish primary care research network
- SV
Stroke volume
- T2DM
type2 diabetes mellitus
- TCTU
Tayside clinical trials unit
- TRuST
Tayside randomisation system
- U&Es
Urea and electrolytes
- VAT
Visceral adipose tissue
- WoCBP
Woman of child bearing potential
Authors’ contributions
AB participated in the design of the study, data collection and drafting the manuscript. ADS conceived the study, participated in its design and helped draft the manuscript. CLL and RM participated in the design of the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study has been granted ethics approval by the East of Scotland Research Ethics Service [16/ES/0131].
Consent for publication
This article does not contain any individual person’s data thus consent for publication is not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexander J.M. Brown, Email: a.y.brown@dundee.ac.uk
Chim Lang, Email: c.c.lang@dundee.ac.uk.
Rory McCrimmon, Email: r.mccrimmon@dundee.ac.uk.
Allan Struthers, Email: a.d.struthers@dundee.ac.uk.
References
- 1.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, et al. Association of Cardiometabolic Multimorbidity with Mortality. JAMA. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizamtsidi M, Paschou SA, Grapsa J, Vryonidou A. Diabetic cardiomyopathy: a clinical entity or a cluster of molecular heart changes? Eur J Clin Investig. 2016;46(11):947–953. doi: 10.1111/eci.12673. [DOI] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes [UKPDS 35]: prospective observational study. BMJ Clinical Research Ed. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, et al. Action to control cardiovascular risk in diabetes [ACCORD] trial: design and methods. Am J Cardiol. 2007;99(12a):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2016;374(11):1094. doi: 10.1056/NEJMc1600140. [DOI] [PubMed] [Google Scholar]
- 9.Dawson A, Morris AD, Struthers AD. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia. 2005;48(10):1971–1979. doi: 10.1007/s00125-005-1896-y. [DOI] [PubMed] [Google Scholar]
- 10.Cooper RS, Simmons BE, Castaner A, Santhanam V, Ghali J, Mar M. Left ventricular hypertrophy is associated with worse survival independent of ventricular function and number of coronary arteries severely narrowed. Am J Cardiol. 1990;65(7):441–445. doi: 10.1016/0002-9149(90)90807-D. [DOI] [PubMed] [Google Scholar]
- 11.Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA. 1995;273(20):1592–1597. doi: 10.1001/jama.1995.03520440046035. [DOI] [PubMed] [Google Scholar]
- 12.Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009;52(2):153–167. doi: 10.1016/j.pcad.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, Carugo S, Grassi G, Lanzarotti A, Schiavina R, Cesana G, et al. Prevalence of left ventricular hypertrophy in hypertensive patients without and with blood pressure control: data from the PAMELA population. Pressioni Arteriose Monitorate E Loro Associazioni. Hypertension. 2002;39(3):744–749. doi: 10.1161/hy0302.104669. [DOI] [PubMed] [Google Scholar]
- 14.Fraser R. Studying genes and the development of cardiac hypertrophy: convenient intermediate phenotypes in man. J Hypertens. 2003;21(5):873–874. doi: 10.1097/00004872-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140(6):848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- 16.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97(1):48–54. doi: 10.1161/01.CIR.97.1.48. [DOI] [PubMed] [Google Scholar]
- 17.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, de Simone G, Devereux RB, et al. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta-analysis. Am J Hypertens. 2003;16(11 Pt 1):895–899. doi: 10.1016/S0895-7061(03)01018-5. [DOI] [PubMed] [Google Scholar]
- 18.Verdecchia P, Angeli F, Gattobigio R, Sardone M, Pede S, Reboldi GP. Regression of left ventricular hypertrophy and prevention of stroke in hypertensive subjects. Am J Hypertens. 2006;19(5):493–499. doi: 10.1016/j.amjhyper.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292(19):2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 20.Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296(10):1242–1248. doi: 10.1001/jama.296.10.1242. [DOI] [PubMed] [Google Scholar]
- 21.Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, et al. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med. 2007;147(5):311–319. doi: 10.7326/0003-4819-147-5-200709040-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wachtell K, Okin PM, Olsen MH, Dahlof B, Devereux RB, Ibsen H, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE study. Circulation. 2007;116(7):700–705. doi: 10.1161/CIRCULATIONAHA.106.666594. [DOI] [PubMed] [Google Scholar]
- 23.Koren MJ, Ulin RJ, Koren AT, Laragh JH, Devereux RB. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens. 2002;15(12):1021–1028. doi: 10.1016/S0895-7061(02)03061-3. [DOI] [PubMed] [Google Scholar]
- 24.Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan intervention for endpoint reduction in hypertension study [LIFE]: a randomised trial against atenolol. Lancet [London, England] 2002;359(9311):1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 25.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham heart study. Circulation. 2003;107(3):448–454. doi: 10.1161/01.CIR.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi N, McKeigue PM, Marmot MG, Nihoyannopoulos P. A comparison of left ventricular abnormalities associated with glucose intolerance in African Caribbeans and Europeans in the UK. Heart. 2001;85(6):643–648. doi: 10.1136/heart.85.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberman A, Prineas RJ, Larson JC, LaCroix A, Lasser NL. Prevalence and determinants of electrocardiographic left ventricular hypertrophy among a multiethnic population of postmenopausal women [the Women's Health Initiative] Am J Cardiol. 2006;97(4):512–519. doi: 10.1016/j.amjcard.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 28.Ilercil A, Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, et al. Associations of insulin levels with left ventricular structure and function in American Indians: the strong heart study. Diabetes. 2002;51(5):1543–1547. doi: 10.2337/diabetes.51.5.1543. [DOI] [PubMed] [Google Scholar]
- 29.Devereux RB, de Simone G, Palmieri V, Oberman A, Hopkins P, Kitzman DW, et al. Relation of insulin to left ventricular geometry and function in African American and white hypertensive adults: the HyperGEN study. Am J Hypertens. 2002;15(12):1029–1035. doi: 10.1016/S0895-7061(02)03080-7. [DOI] [PubMed] [Google Scholar]
- 30.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 31.UK Prospective Diabetes Study [UKPDS] Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes [UKPDS 33] Lancet [London, England] 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 32.de Simone G, Devereux RB, Palmieri V, Roman MJ, Celentano A, Welty TK, et al. Relation of insulin resistance to markers of preclinical cardiovascular disease: the strong heart study. Nutr Metab Cardiovasc Dis. 2003;13(3):140–147. doi: 10.1016/S0939-4753(03)80173-4. [DOI] [PubMed] [Google Scholar]
- 33.Dorresteijn JA, Visseren FL, Spiering W. Mechanisms linking obesity to hypertension. Obes Rev. 2012;13(1):17–26. doi: 10.1111/j.1467-789X.2011.00914.x. [DOI] [PubMed] [Google Scholar]
- 34.Shubrook JH, Bokaie BB, Adkins SE. Empagliflozin in the treatment of type 2 diabetes: evidence to date. Drug Des Devel Ther. 2015;9:5793–5803. doi: 10.2147/DDDT.S69926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalra S. Sodium glucose co-transporter-2 [SGLT2] inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5(2):355–366. doi: 10.1007/s13300-014-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335–1380. doi: 10.2147/DDDT.S50773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 38.Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 [SGLT2] inhibitors. J Am Soc Hypertens. 2014;8(5):330–339. doi: 10.1016/j.jash.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Majewski C, Bakris GL. Blood pressure reduction: an added benefit of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care. 2015;38(3):429–430. doi: 10.2337/dc14-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rekhraj S, Gandy SJ, Szwejkowski BR, Nadir MA, Noman A, Houston JG, et al. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol. 2013;61(9):926–932. doi: 10.1016/j.jacc.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 42.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):262–275. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devereux RB, Roman MJ. Left ventricular hypertrophy in hypertension: stimuli, patterns, and consequences. Hypertens Res. 1999;22(1):1–9. doi: 10.1291/hypres.22.1. [DOI] [PubMed] [Google Scholar]
- 45.Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16(2):159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 46.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(2):509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, et al. Insulin sensitivity and atherosclerosis. The insulin resistance atherosclerosis study [IRAS] investigators. Circulation. 1996;93(10):1809–1817. doi: 10.1161/01.CIR.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 49.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 50.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 51.Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 52.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin [CANTATA-SU]: 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet [London, England] 2013;382(9896):941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 53.Abassi Z, Leor J, Landa N, Younis F, Hollander K, Mayoux E, et al. Os 05-04 Empagliflozin exerts cardio- and Nephro-protective effects in Cohen-ROSENTHAL diabetic hypertensive rats. J Hypertens. 2016;34(Suppl 1 - ISH 2016 Abstract Book):e58–ee9. doi: 10.1097/01.hjh.0000500004.26187.fe. [DOI] [Google Scholar]
- 54.Empagliflozin Impact on Hemodynamics in Patients With Diabetes and Heart Failure - Tabular View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/record/NCT03030222?term=empagliflozin&rank=1.
- 55.SGLT2 Inhibition in Diabetes and Heart Failure - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT02862067?term=sglt+heart+failure&rank=1.
- 56.EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction [EMPEROR-Reduced] - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT03057977?term=empagliflozin&rank=41.
- 57.Dapagliflozin Effect on Symptoms and Biomarkers in Diabetes Patients With Heart Failure - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT02653482?term=sglt+heart+failure&rank=4.
- 58.Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT03036124?term=DAPAGLIFLOZIN&rank=18.
- 59.Safety and Effectiveness of SGLT-2 Inhibitors in Patients With Heart Failure and Diabetes - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT02397421?term=sglt+heart+failure&rank=2.
- 60.Treatment of Diabetes in Patients With Systolic Heart Failure - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT02920918?term=sglt+heart+failure&rank=8.
- 61.Effects of Empagliflozin on Left Ventricular Diastolic Function Compared to Usual Care in Type 2 Diabetics - Tabular View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/record/NCT02932436?term=empagliflozin&rank=4.
- 62.SGLT2 Inhibition and Left Ventricular Mass - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT02728453?term=SGLT+HEART+FAILURE&rank=4.
- 63.Effects of Empagliflozin on Cardiac Structure in Patients With Type 2 Diabetes - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT02998970?term=empagliflozin&rank=8.
- 64.Dapagliflozin in Type 2 Diabetes or Pre-diabetes, and PRESERVED Ejection Fraction Heart Failure - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT03030235?term=SGLT+HEART+FAILURE&rank=7.
- 65.Does Dapagliflozin Regress Left Ventricular Hypertrophy In Patients With Type 2 Diabetes? - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT02956811. [DOI] [PMC free article] [PubMed]
- 66.Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT01730534?term=DECLARE&rank=2.
- 67.CANVAS - CANagliflozin cardioVascular Assessment Study - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT01032629?term=CANVAS&rank=1.
- 68.Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy - Full Text View - ClinicalTrials.gov 2017 [Available from: https://clinicaltrials.gov/ct2/show/NCT02065791?term=CREDENCE&rank=2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


