Abstract
Background
Ileus is one of the more common suspected diagnoses in everyday clinical practice. The term can refer either to mechanical or to functional ileus. Any physician who takes care of patients can be confronted with these entities; thus, all should be familiar with them and competent in their management.
Methods
Recommendations are summarized for the diagnostic evaluation and treatment of ileus of various causes on the basis of a selective literature review.
Results
The manifestations of ileus and its degree of severity generally depend on the site of blockage. The rule until recently was that a patient with suspected mechanical ileus should be taken to surgery within 12 hours; today, however, ileus—particularly of the small bowel—can often be successfully treated conservatively. Likewise, functional ileus only rarely requires surgery: supportive measures, depending on the etiology, usually suffice.
Conclusion
Proper treatment depends on the timely determination of the pathogenesis (mechanical versus functional) and on close interdisciplinary collaboration. A special challenge is posed by patients with peritoneal involvement with cancer who present with symptoms of ileus, in whom a clear distinction between mechanical and functional causation cannot always be drawn.
By definition, ileus is an occlusion or paralysis of the bowel preventing the forward passage of the intestinal contents, causing their accumulation proximal to the site of the blockage. A key distinction is drawn between mechanical and functional ileus.
In the pathophysiology of ileus, both types lead to the accumulation of fluids and gases at elevated intraluminal pressure, microcirculatory dysfunction of the bowel wall, and disruption of the mucosal barrier. This can, in turn, lead to fluid shifts, transmigration peritonitis, and hypovolemia.
Learning goals
This article is intended to enable the reader to:
Be familiar with the necessary diagnostic tests in suspected mechanical ileus
Know what clinical parameters should be closely monitored so that a patient with mechanical ileus can be spared an operation, if possible
List the types of functional ileus and know how they should be treated.
Mechanical ileus
Mechanical ileus necessitating surgery is a common complication after previous surgery; for example, its lifetime incidence after colectomy is 11% (1).
Possible causes include:
External compression (adhesions, hernia)
Changes in the bowel wall (tumor, inflammation/infection)
Blockage of the lumen (coprostasis, intussusception).
Definition.
Ileus is an occlusion or paralysis of the bowel preventing the forward passage of the intestinal contents, causing their accumulation proximal to the site of the blockage.
The passage of intestinal contents can be blocked either partially (subileus, incomplete ileus) or totally (complete ileus). Mechanical ileus affects the small bowel more often than the large bowel, in a ratio of 4:1 (2). Small-bowel ileus is usually due to adhesions from prior surgery (65%) or hernia (15%), while large-bowel ileus is usually due to cancer (70%) or to adhesions and stenoses after recurrent diverticulitis (up to 10%). Rarer causes of large-bowel ileus include sigmoid volvulus (5%) and hernia (2.5%) (2).
The clinical manifestations of ileus and their degree of severity depend to a large extent on the site of the blockage. Thus, the common manifestations of small-bowel ileus include nausea and vomiting, cramps, bloating, and retention of stool and flatus. The more proximally the pathological process is located, the more rapidly the patient becomes symptomatic with vomiting of undigested food. The retention of stool and flatus, although a classic manifestation of ileus, may not appear until several days later. In contrast to small-bowel ileus, which usually begins acutely with severe symptoms, large-bowel ileus often begins with mild symptoms (volvulus of sudden onset is an exception). Its main manifestations are bloating (80%), cramps (60%), and retention of stool and flatus (50%). The overt illness is often preceded by a long phase of altered bowel habits and worsening constipation (3).
Localization.
Mechanical ileus affects the small bowel more often than the large bowel, in a ratio of 4:1. Small-bowel ileus is usually due to adhesions, while large-bowel ileus is usually due to cancer.
The diagnostic evaluation of mechanical ileus
Physical examination
The physical examination may yield evidence of mechanical ileus. In particular, intensified bowel sounds are a classic finding in the early phase, while peritoneal signs are usually absent. This picture is nonspecific, and, particularly in the late phase, bowel damage can cause paralysis without any peristaltic activity. It follows that even an experienced surgeon cannot make the diagnosis with certainty in all cases, as was shown in a prospective study (4).
Laboratory tests
There is no specific laboratory test for the assessment of mechanical ileus with accompanying bowel ischemia (5, 6). Only the procalcitonin concentration seems to be a potentially useful mark. In a prospective study, values above 0.57 ng/mL predicted bowel ischemia with a probability of 83%, while values below 0.57 ng/mL ruled it out with a probability of 91% (6).
Clinical features.
The clinical manifestations of ileus and their degree of severity depend to a large extent on the site of the blockage. Thus, the common manifestations of small-bowel ileus include nausea and vomiting, cramps, and bloating.
The following tests should be performed for further evaluation:
Parameters of systemic infection
Electrolytes (hypokalemia may indicate functional ileus)
Renal function tests (these may suggest renal failure due to fluid shifts)
Cholestasis parameters, transaminases, and lipase (pancreatitis is a potential cause of functional ileus).
The work-up should also include the following:
Coagulation testing (a clotting defect can be a sign of liver failure)
Arterial blood-gas analysis (the pH and lactate values may be nonspecific evidence of organ hypoperfusion).
Abdominal ultrasonography
Ultrasonography in the emergency room is still a useful means of detecting free fluid or an incarcerated hernia. It plays a less important role in the evaluation of ileus, as its utility is limited by artefact from air in the distended abdomen (7).
Abdominal plain films and bowel contrast studies
An abdominal plain film in the standing or lateral position is inexpensive and readily obtained, but also relatively insensitive and nonspecific (8). A plain film is recommended as the first study for clinically stable patients who have no evidence of infection and whose symptoms are only mild. Thereafter, a gastrointestinal transit study can be obtained with oral administration of undiluted contrast medium. An important incidental property of contrast studies is the laxative effect of hypertonic iodinated contrast medium. A meta-analysis has shown that, because of this effect, bowel contrast studies can lessen the need for laparotomy with adhesiolysis, and thereby also shorten hospital stays (9).
Computed tomography of the abdomen
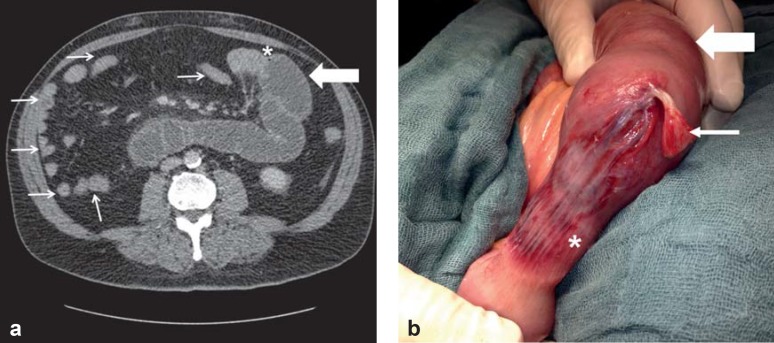
Abdominal computed tomography (CT) with oral and intravenous contrast medium is more than 90% sensitive and specific for the diagnosis of mechanical ileus (Figure 1a) and is thus the gold standard (9). It enables assessment of the degree of severity (complete versus incomplete ileus), precise localization (caliber difference), and determination of the cause (incarcerated hernia, tumor, inflammatory changes), along with the detection of potential complications (ischemia, perforation).
Figure 1: Abdominal computed tomography (CT) and intraoperative findings
CT of a patient with mechanical small-bowel ileus, showing prestenotic dilatation of the small bowel (thick arrow), abrupt change of caliber (*), and a “hungry bowel” distal to the stenosis (thin arrows).
The corresponding intraoperative findings, with a dilated small bowel proximal to the area of previous stenosis (thick arrow); the adhesion (thin arrow), now divided, that caused the ileus; and the slowly recovering segment of small bowel (*), still hypoperfused because of the adhesion-related strangulation.
Diagnostic evaluation.
Abdominal CT is the gold standard for the diagnostic evaluation of mechanical ileus.
Further diagnostic tests
In rare cases of large-bowel ileus, colonoscopy is useful both as a diagnostic procedure (malignant versus benign stenosis) and as a mode of access to the bowel for so-called bridging treatment, in which a decompression tube can be introduced proximal to the blockage for decompression, or a stenosis can be stented, in order to provide temporary relief until definitive surgery is performed.
Magnetic resonance imaging still generally plays no role in the acute evaluation of ileus. Nonetheless, in young, clinically stable patients whose site of blockage is unclear, a so-called MR Sellink study can be performed to localize the problem and facilitate treatment planning (10). This magnetic resonance version of the Sellink double-contrast study (enteroclysma) enables the detection of inflammatory/infectious changes or stenoses, particularly of the small bowel.
The treatment of mechanical ileus
Initial treatment in the emergency room
Intravenous fluid administration should be started at once to replace volume deficits and correct any electrolyte or acid–base disturbances. Patients who are vomiting should undergo placement of a nasogastric tube for gastrointestinal decompression (11). Analgesic medication can be started immediately after the initial physical examination. In the past, it was often feared that the pharmacological suppression of pain might mask the clinical manifestations of an acute abdomen and impede diagnosis, but modern CT imaging has eliminated this concern. Vagolytic agents such as butylscopolamine have an antiperistaltic effect and should not be given to patients with partial ileus. If there is any clinical or laboratory evidence of infection (or even sepsis), antibiotics should be given early, as per the recommendations of the Surviving Sepsis Campaign (12).
After initial treatment and completion of the diagnostic evaluation, it must be determined whether the patient should be taken to surgery at once or conservative treatment can be tried. Recent retrospective studies of data on more than 100 000 patients have revealed an apparent advantage in having a surgical (rather than medical) team in charge of further treatment, as this resulted in lower morbidity and mortality, a shorter interval to surgery if needed, and shorter hospital stays (13, 14).
Ancillary tests.
In young, clinically stable patients whose site of blockage is unclear, a so-called MR Sellink study can be performed to localize the problem and facilitate treatment planning.
Trial of conservative treatment
A trial of conservative treatment is justified as long as there is no absolute indication for surgery (strangulation, ischemia, complete absence of transit of bowel contents) and there is no clinical evidence of an acute abdomen. For incomplete ileus, the success rate of purely supportive treatment is 80%, while the probability that bowel resection will be needed is under 5% (9, 15, 16). On the other hand, if complete ileus is treated conservatively, the probability that bowel resection will be needed is roughly 30% (5).
In addition to the supportive measures mentioned above (fluid replacement, nasogastric tube, nil per os or sips of clear fluids at most), the administration of 100 mL of water-soluble, iodinated contrast medium per nasogastric tube is recommended. Hypertonic ionic contrast medium is usually used, e.g., sodium amidotrizoate 100 mg/mL + meglumine amidotrizoate 660 mg/mL. A meta-analysis has shown that this lessens the need for surgery and shortens hospital stays by an average of 1.9 days. Moreover, if the contrast medium reaches the colon within 24 hours, this predicts successful conservative treatment with 96% sensitivity and 98% specificity (level Ia evidence) (9).
There is no definitive recommendation for the duration of conservative treatment; the historic dictum “Never let the sun rise or set on a case of bowel obstruction” is no longer universally applicable (17). Conservative treatment can even be continued for several days under close clinical and laboratory observation. It should be borne in mind, however, that a failed trial of conservative treatment for more than three days is associated with a greater need for bowel resection (12% versus 29%) and with higher morbidity and mortality (level IV evidence) (5, 17, 18).
Indications for surgery
The decision whether to operate is not always easy, even for experienced surgeons (4). The risk factors discussed by Schwenter et al. can serve as decisional aids: in a multivariate analysis, the authors identified six factors associated with an elevated risk of bowel strangulation (box 1) (19).
BOX 1. Risk factors,
according to Schwenter* (19)
Abdominal pain for 4 days or more
Peritoneal signs
C-reactive protein >75 mg/L
Leukocytes >10 500 µL
>500 mL free fluid
Reduced contrast enhancement of the bowel wall
*One point is given for each criterion that is met. A score of 3 or more is nearly 70% sensitive and over 90% specific for the danger of strangulation and is thus an indication for emergency surgery (level IIa evidence) (19).
Initial treatment.
Intravenous fluid administration should be started at once to replace volume deficits and correct any electrolyte or acid–base disturbances, and analgesics should be given.
While small-bowel ileus is usually due to adhesions (Figure 1b) and nearly three-quarters of cases can be treated conservatively, ileus of the colon is usually due to cancer and three-quarters of cases need urgent surgery (18, 20). When cancer is found at laparotomy, the surgeon is confronted with an often difficult choice between two possible ways to proceed:
Single procedure—The malignant stenosis is resected together with the proximal dilated segment with attention to clean oncological margins, and a primary anastomosis is created without colostomy. Arguments in favor of this concept include the low incidence (2–6%) of anastomosis failure, the elimination of the need for a second procedure to reverse the colostomy, and the high morbidity of emergency colostomy procedures (lower quality of life, skin irritation, malabsorption) (20). On the other hand, if the anastomosis fails, the morbidity and mortality rise considerably. In addition, the oncological outcome is poorer (21).
Treatment in a single procedure should always be considered, particularly if the patient is otherwise healthy, the proximal bowel segment is only mildly dilated, or the stenosis is located in the ascending colon or the right flexure (20).
An aid to decision-making.
The decision whether to operate is not always easy, even for experienced surgeons. The risk factors discussed by Schwenter et al. can serve as decisional aids.
Staged procedure—Treatment in two procedures involves, first, resection of the malignant lesion with attention to clean oncological margins and, at the same operation, creation of either a terminal (Hartmann) stoma or a direct anastomosis of the two bowel stumps with a protective double stoma. The stoma can later be internalized in a second procedure. Arguments in favor of a staged procedure include rapid recovery and the lower incidence of anastomosis failure. It should be borne in mind, however, that emergency ileostomy often leads to difficulties in the care of the stoma, as well as to severe malabsorption syndromes. Moreover, reversal of the stoma carries a high morbidity as well; in large-scale retrospective studies, the stoma was never reversed in as many as 60% of patients (20). The staged procedure has proven useful particularly for patients who are at especially high risk of anastomosis failure because of peritonitis, immunosuppression, malnutrition, etc.
A decompressive tube or a stent can be used for “bridging” before definitive surgery, particularly in patients with very distal stenoses (sigmoid colon and rectum). This obviates the need to operate in the face of manifest ileus and gives the bowel wall a chance to recover from its prestenotic dilatation (20). Which type of procedure to perform must be decided on an individual basis and depends on many factors (box 2).
BOX 2. Circumstances favoring colostomy.
Dilated prestenotic bowel segment
Elderly patient with other accompanying disease
Less experienced surgeon
Risk factors for anastomosis failure
Pre-existing incontinence
Perforation with peritonitis
Systemic sepsis
Metastatic cancer with low life expectancy
Current chemotherapy or immunosuppressive treatment
Rectal stenosis
One-stage surgery without colostomy.
This is the treatment of choice, particularly for young patients with no risk factors for anastomosis failure.
Functional ileus
Unlike mechanical ileus, functional ileus is not due to a process obstructing the lumen of the bowel and impeding the passage of its contents, but rather to reduced contraction of the smooth muscle of the bowel wall.
Paralytic/functional ileus has multiple causes:
Reflectory ileus—after abdominal or retroperitoneal surgery (e.g., spinal surgery), or induced by intra-abdominal or retroperitoneal lesions (tumor, hemorrhage, infection)
Drug-induced ileus—due to the consumption of opioids, neuroleptic drugs, etc.
Metabolic ileus—in patients with hypokalemia or diabetes mellitus
Vascular ileus—due to hypoperfusion of the bowel.
We will now discuss the three causes of functional ileus that are most common and most relevant in everyday clinical practice and consider the therapeutic options for each.
Postoperative ileus
Postoperative ileus is a frequent complication of surgery, particularly visceral surgery: its reported incidence after colorectal operations is 17.4% (22). Postoperative ileus is defined as the temporary cessation of coordinated bowel peristalsis after surgery, restricting the passage of bowel contents and rendering the patient unable to tolerate the oral intake of liquids or solid food (23). It is, in principle, a reversible disturbance. Its socioeconomic cost is high, as it prolongs hospital stays and thereby puts an additional financial burden on the U.S. health-care system amounting to some 1.5 billion dollars per year (22).
The pathophysiology of postoperative ileus is multifactorial. An important factor is activation by surgical trauma of the macrophages residing in the tunica muscularis externa of the bowel wall. These cells release cytokines that induce the activation of further pro-inflammatory cells and their migration to the site of injury. Next, other antiperistaltic cytokines (including interleukin-6 and TNF-alpha) are released, along with neuropeptides and nitric oxide. The full clinical picture of postoperative ileus ensues, with inflammation of the tunica muscularis externa of the entire gastrointestinal tract (24).
Clinical features and diagnostic evaluation
Functional ileus.
Unlike mechanical ileus, functional ileus is not due to a process obstructing the lumen of the bowel and impeding the passage of its contents, but rather to reduced contraction of the smooth muscle of the bowel wall.
Postoperative ileus usually manifests itself from the third to the fifth day after surgery, mainly with nausea, vomiting, retention of stool and flatus, and abdominal distention with sparse or absent bowel sounds. There are generally no major laboratory abnormalities (25). Postoperative (functional) ileus is very common and generally benign, yet it should always be recalled that bowel paralysis after surgery may be due to early postoperative mechanical ileus (torqueing, internal hernia) or septic ileus (abscess, peritonitis). If the diagnosis is in doubt, an abdominal CT should be obtained.
Treatment
Postoperative ileus.
Postoperative ileus is a frequent complication of surgery, particularly visceral surgery. It puts an additional financial burden on the U.S. health-care system amounting to some 1.5 billion dollars per year.
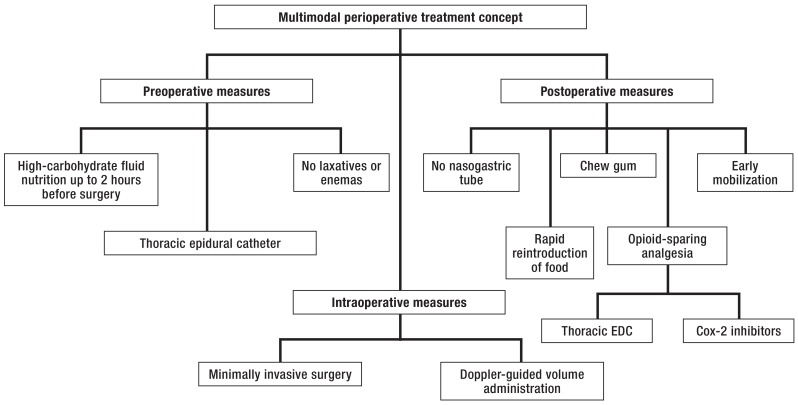
There is no single effective means of preventing or treating postoperative ileus. The Fast Track or ERAS (enhanced recovery after surgery) concept was introduced a decade ago by Kehlet (26, 27): this is a multimodal perioperative treatment concept consisting of a set of measures to lessen postoperative morbidity and shorten hospital stays (figure 2) (25).
Figure 2.
Multimodal perioperative fast-track concept [modified from Vilz (25)]; EDC, epidural catheter
Only three of the measures included in the ERAS concept have been shown in meta-analyses to shorten the duration of postoperative ileus (28, 29):
Minimally invasive surgery (reduced surgical trauma; level Ia evidence)
Postoperative analgesia with an epidural catheter (sympatholysis and reduced need for peristalsis-inhibiting opioids; level Ia evidence)
Postoperative gum-chewing to stimulate the cephalovagal reflex, which promotes peristalsis and inhibits inflammation (level Ia evidence) (30).
Pathophysiology of postoperative ileus.
The pathophysiology of postoperative ileus is multifactorial. An important factor is activation by surgical trauma of the macrophages residing in the tunica muscularis externa of the bowel wall.
These measures, however, are prophylactic, not therapeutic. Once postoperative ileus has become manifest, there is no evidence-based treatment. None of the routinely used prokinetic drugs (neostigmine, metoclopramide, erythromycin) or laxatives have been found in meta-analyses to shorten the duration of postoperative ileus (level Ia evidence) (31).
Opioid-induced constipation and ileus
Opioids are in widespread use for the treatment of chronic pain, in cancer patients as well. They are highly effective and can be given by multiple routes. The most common (15%) and most serious side effect of chronic opioid use is constipation, of which ileus is the most severe variant (32).
Clinical features and diagnostic evaluation
Prophylaxis of postoperative ileus by:
Minimally invasive surgery
Thoracic epidural catheter
Postoperative gum-chewing
Opioid-induced constipation can almost always be diagnosed from the history (initiation or change of opioid treatment) and the clinical examination, including digital rectal examination (fecal impaction). In patients with opioid-induced ileus, the laboratory parameters of infection should be checked, and any abnormal findings should be followed up with imaging studies.
Prevention and treatment
The prevention of opioid-induced constipation is a major concern. In particular, a high-fiber diet, adequate fluid intake, and physical exercise are beneficial. If constipation nonetheless arises, further help is available in the form of a therapeutic algorithm in the German S2k guideline on chronic constipation in adults (33).
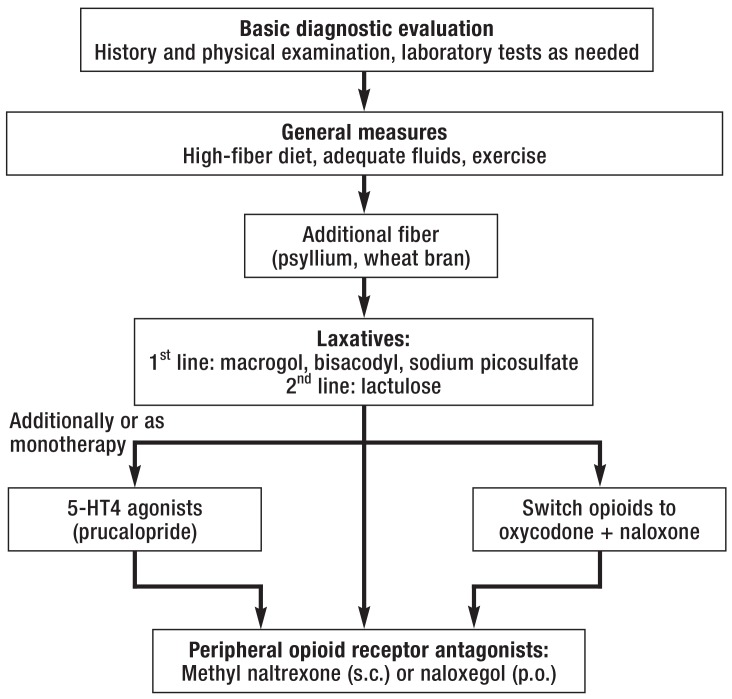
It may be necessary, particularly in cases of ileus or intractable constipation, to switch the opioid to a combined preparation (e.g., oxycodone + naloxone [level Ib evidence], naloxone being a peripheral opioid antagonist with a high first-pass effect) or to add on a peripheral opioid antagonist (subcutaneous methylnaltrexone or oral naloxegol [level Ib evidence]) (figure 3). Surgical treatment is needed only in exceptionally rare cases.
Figure 3.
Algorithm for the treatment of opioid-induced constipation or ileus (after [33]);
s.c., subcutaneously; p.o., per os
Intestinal pseudo-obstruction: (Ogilvie syndrome)
Acute colonic pseudo-obstruction (Ogilvie syndrome) is defined as colonic dilatation of the colon (usually the cecal pole and the ascending colon) without evidence of a mechanical stenosis. Its precise pathogenesis is unclear but is suspected to involve an imbalance in the neuronal input to the bowel so that the sympathetic influence predominates, causing atonic dilatation of the colon (34, 35). Ogilvie syndrome arises almost exclusively in critically ill hospitalized patients. It is particularly associated with the postoperative state (mainly after orthopedic procedures, with an incidence of 1%), severe infection, and neurologic disease (such as Parkinson’s disease) (36).
Clinical features and diagnostic evaluation
The main manifestation of this syndrome is progressive abdominal distention. Most patients (80%) complain of abdominal pain and of nausea and vomiting (60%), while many also have fecal retention or diarrhea (45%). Physical examination typically reveals abdominal tympanism and, usually, audible bowel sounds on auscultation. If peritoneal signs or fever are present, bowel ischemia and perforation must be ruled out (35).
Chronic opioid medication.
Measures should be taken to prevent constipation in any patient under chronic treatment with opioid drugs.
There are no characteristic laboratory abnormalities in acute pseudo-obstruction. Leukocytosis, if present, may be a sign of ischemia or perforation. Because Ogilvie syndrome is a diagnosis of exclusion, the initial evaluation should include an abdominal CT scan to rule out other conditions, particularly mechanical ileus or intestinal paralysis due to other intra-abdominal or retroperitoneal disease.
Treatment
Ogilvie syndrome.
Ogilvie syndrome arises almost exclusively in critically ill hospitalized patients. It is particularly associated with the postoperative state, severe infection, and neurologic disease.
The choice of further treatment (conservative versus surgical) largely depends on the radiologically measured diameter of the colon, on the presence or absence of signs of sepsis, and on whether bowel perforation seems imminent or has already occurred.
The treatment of Ogilvie syndrome.
The treatment depends on the diameter of the colon. The first measure is neostigmine administration; the second, colonoscopic decompression; the third, surgery.
Conservative treatment—This is possible only if there are no signs of sepsis and if (imminent or existing) perforation and ischemia have been ruled out. Supportive measures are given, including nil per os, a nasogastric tube, a decompressive rectal tube, correction of electrolyte disturbances, and discontinuation of constipating drugs. The patient must be closely observed both clinically and radiologically, with repeated abdominal plain films every 12 to 24 hours to monitor the diameter of the colon.
A meta-analysis confirmed the efficacy of neostigmine 2 mg i.v. in the treatment of Ogilvie syndrome: successful treatment (flatus, defecation, reduction of abdominal circumference) was documented 30 minutes after the injection in 90% of patients (p<0.001, number needed to treat [NNT] = 1) (level Ia evidence) (37). On the other hand, there is no evidence for the efficacy of other drugs, such as methylnaltrexone or erythromycin (35).
If pharmacotherapy brings no improvement in 2 to 3 days, endoscopic deflation and insertion of a decompressive tube in the right hemicolon is recommended (level IIa evidence) (38, 39).
Surgery—The indications for surgical treatment in Ogilvie syndrome should be viewed critically, as the perioperative mortality is 25–31%. Absolute indications for surgery are typically stated as imminent or existing bowel perforation, ischemia, or persistent dilatation of the colon with a diameter larger than 12 cm for several days. As long as there is no perforation or ischemia, a cecostomy should be performed; percutaneous cecostomy is an alternative for critically ill patients. In the presence of perforation or ischemia, a discontinuity resection is recommended (level IIa evidence) (39).
Peritoneal carcinosis: a special case
Ileus and peritoneal carcinosis.
Patients with a peritoneal tumor burden and the new onset of ileus should be evaluated at least with a CT of the abdomen to determine the cause of ileus (mechanical or functional) and assess the extent of neoplastic disease.
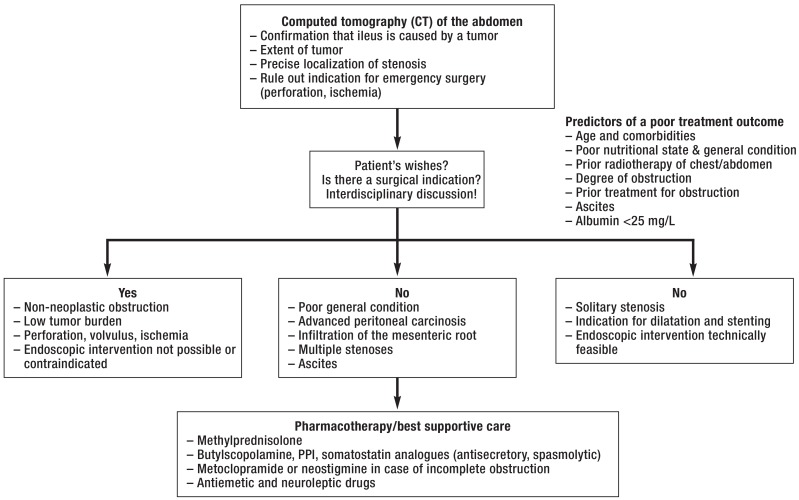
Cancer of the gastrointestinal tract or of the female reproductive tract often causes peritoneal carcinosis presenting with ileus. There are varying reasons for the lack of passage of bowel contents, and the determination of the pathophysiology in the individual case has major implications for treatment. Chronic use of opioid analgesics, micronodular peritoneal carcinosis of the bowel, and mesenteric infiltration by tumor masses generally cause functional ileus, while the infiltrative growth of a tumor into the lumen of the bowel or adhesions after prior abdominal surgery generally cause mechanical ileus. It is essential to determine whether the bowel is obstructed or only paralyzed, as surgery in the latter case is of little benefit and may well harm the patient. It is, therefore, recommended that patients with a peritoneal tumor burden and the new onset of ileus should be evaluated at least with a CT of the abdomen. This can help rule out absolute indications for surgery (ischemia, strangulation, perforation) while also enabling assessment of the extent of tumor, which is of prognostic significance. Depending on the radiologic findings and the patient’s age and general condition, various treatment options are available: pharmacotherapy (secretolytic, analgesic, and prokinetic drugs), interventional treatment (drainage PEG in case of proximal stenosis, dilatation + stent in case of distal stenosis), or surgery (resection if the tumor burden is manageable, colostomy) (Figure 4, level IV evidence) (40).
Figure 4.
The treatment of suspected ileus and known peritoneal carcinosis (after [40]); PPI, proton-pump inhibitors
Further Information On Cme.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 15 October 2017, and earlier CME units until the dates indicated:
-
“Latent Hypothyroidism in Adults” (Issue 25)
until 17 September 2017
-
“The Diagnosis and Treatment of Hemoptysis” (Issue 21/2017)
until 20 August 2017
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the approximate lifetime prevalence of mechanical ileus after colectomy?
6%
11%
16%
21%
26%
Question 2
What is the gold standard for the diagnosis of mechanical ileus?
Colonoscopy
Abdominal MRI
Abdominal CT
Plain abdominal x-ray
Endoscopic ultrasonography of the small bowel
Question 3
Which of the following can be of use in the decision whether to treat suspected mechanical ileus conservatively or by surgery?
The Thure-Brandt method
The Sachtleben method
The Dorn method
The Schwenter risk factor assessment
The Sanger score
Question 4
Which of the following has been shown by a meta-analysis to be helpful in the treatment of mechanical ileus, in that it makes the need for laparotomy with adhesiolysis less likely and shortens the average duration of hospital stay?
Oral contrast medium
Oral psyllium seed husks
Oral macrogol
Oral lactulose
Oral bisacodyl
Question 5
Which of the following, if found in an operation for mechanical ileus of the large bowel, implies that a two-staged surgical approach would be best?
A young, otherwise healthy patient
Pperitonitis
A stenosis in the ascending colon
An experienced surgeon
Absence of distention of the prestenotic segment
Question 6
What are the typical main manifestations of postoperative ileus?
Night sweats and agitation
Chest pain
Nausea and vomiting
Fever above 39°C and shaking chills
Dizziness and apnea
Question 7
Which of the following measures is a component of so-called fast-track surgery?
Nil per os for at least 24 hours before surgery
Preoperative bowel irrigation
Early mobilization
Opioid analgesia if possible
Routine postoperative placement of a nasogastric tube
Question 8
Which of the following measures shortens the duration of postoperative ileus?
Prophylactic antibiotic coverage
Postoperative sedation
Postoperative opioid analgesia
Cautious reintroduction of liquid and then solid nutrition after surgery
Minimally invasive surgery
Question 9
Which of the following drugs was found in a meta-analysis to be effective in the treatment of Ogilvie syndrome?
Neostigmine i.v.
Methylnaltrexone s.c.
Erythromycin i.v.
Azithromycin p.o.
Clarithromycin p.o.
Question 10
Which of the following are reasonable treatments for ileus due to peritoneal carcinosis?
Radical resection of all peritoneal cancerous lesions
If there are multiple stenoses in the mid-small bowel, endoscopic stenting
If the patient is vomiting and has a stenosis in the proximal portion of the small bowel, percutaneous endoscopic gastrostomy (PEG)
Administration of tincture of opium
Radiotherapy of the abdomen
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Confict of interest statement
The authors state that they have no conflict of interest.
References
- 1.Nieuwenhuijzen M, Reijnen MM, Kuijpers JH, van Goor H. Small bowel obstruction after total or subtotal colectomy: a 10-year retrospective review. Br J Surg. 1998;85:1242–1245. doi: 10.1046/j.1365-2168.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 2.Drozdz W, Budzynski P. Change in mechanical bowel obstruction demographic and etiological patterns during the past century: observations from one health care institution. Arch Surg. 2012;147:175–180. doi: 10.1001/archsurg.2011.970. [DOI] [PubMed] [Google Scholar]
- 3.Markogiannakis H, Messaris E, Dardamanis D, et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432–437. doi: 10.3748/wjg.v13.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarr MG, Bulkley GB, Zuidema GD. Preoperative recognition of intestinal strangulation obstruction. Prospective evaluation of diagnostic capability. Am J Surg. 1983;145:176–182. doi: 10.1016/0002-9610(83)90186-1. [DOI] [PubMed] [Google Scholar]
- 5.Leung AM, Vu H. Factors predicting need for and delay in surgery in small bowel obstruction. Am Surg. 2012;78:403–407. [PubMed] [Google Scholar]
- 6.Cosse C, Regimbeau JM, Fuks D, Mauvais F, Scotte M. Serum procalcitonin for predicting the failure of conservative management and the need for bowel resection in patients with small bowel obstruction. J Am Coll Surg. 2013;216:997–1004. doi: 10.1016/j.jamcollsurg.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 7.Suri S, Gupta S, Sudhakar PJ, Venkataramu NK, Sood B, Wig JD. Comparative evaluation of plain films, ultrasound and CT in the diagnosis of intestinal obstruction. Acta Radiol. 1999;40:422–428. doi: 10.3109/02841859909177758. [DOI] [PubMed] [Google Scholar]
- 8.Thompson WM, Kilani RK, Smith BB, et al. Accuracy of abdominal radiography in acute small-bowel obstruction: does reviewer experience matter? Am J Roentgenol. 2007;188:W233–W238. doi: 10.2214/AJR.06.0817. [DOI] [PubMed] [Google Scholar]
- 9.Branco BC, Barmparas G, Schnuriger B, Inaba K, Chan LS, Demetriades D. Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg. 2010;97:470–478. doi: 10.1002/bjs.7019. [DOI] [PubMed] [Google Scholar]
- 10.Mullan CP, Siewert B, Eisenberg RL. Small bowel obstruction. Am J Roentgenol. 2012;198:W105–W117. doi: 10.2214/AJR.10.4998. [DOI] [PubMed] [Google Scholar]
- 11.Oyasiji T, Angelo S, Kyriakides TC, Helton SW. Small bowel obstruction: outcome and cost implications of admitting service. Am Surg. 2010;76:687–691. [PubMed] [Google Scholar]
- 12.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aquina CT, Becerra AZ, Probst CP, et al. Patients with adhesive small bowel obstruction should be primarily managed by a surgical team. Ann Surg. 2016;264:437–447. doi: 10.1097/SLA.0000000000001861. [DOI] [PubMed] [Google Scholar]
- 14.Bilderback PA, Massman JD 3rd, Smith RK, La Selva D, Helton WS. Small bowel obstruction is a surgical disease: patients with adhesive small bowel obstruction requiring operation have more cost-effective care when admitted to a surgical service. J Am Coll Surg. 2015;221:7–13. doi: 10.1016/j.jamcollsurg.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Burge J, Abbas SM, Roadley G, et al. Randomized controlled trial of Gastrografin in adhesive small bowel obstruction. ANZ J Surg. 2005;75:672–674. doi: 10.1111/j.1445-2197.2005.03491.x. [DOI] [PubMed] [Google Scholar]
- 16.Kendrick ML. Partial small bowel obstruction: clinical issues and recent technical advances. Abdom Imaging. 2009;34:329–334. doi: 10.1007/s00261-008-9436-0. [DOI] [PubMed] [Google Scholar]
- 17.Schraufnagel D, Rajaee S, Milham FH. How many sunsets? Timing of surgery in adhesive small bowel obstruction: a study of the Nationwide Inpatient Sample. J Trauma Acute Care Surg. 2013;74:181–187. doi: 10.1097/TA.0b013e31827891a1. [DOI] [PubMed] [Google Scholar]
- 18.Keenan JE, Turley RS, McCoy CC, Migaly J, Shapiro ML, Scarborough JE. Trials of nonoperative management exceeding 3 days are associated with increased morbidity in patients undergoing surgery for uncomplicated adhesive small bowel obstruction. J Trauma Acute Care Surg. 2014;76:1367–1372. doi: 10.1097/TA.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 19.Schwenter F, Poletti PA, Platon A, Perneger T, Morel P, Gervaz P. Clinicoradiological score for predicting the risk of strangulated small bowel obstruction. Br J Surg. 2010;97:1119–1125. doi: 10.1002/bjs.7037. [DOI] [PubMed] [Google Scholar]
- 20.Breitenstein S, Rickenbacher A, Berdajs D, Puhan M, Clavien PA, Demartines N. Systematic evaluation of surgical strategies for acute malignant left-sided colonic obstruction. Br J Surg. 2007;94:1451–1460. doi: 10.1002/bjs.6007. [DOI] [PubMed] [Google Scholar]
- 21.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 22.Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm. 2009;15:485–494. doi: 10.18553/jmcp.2009.15.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg. 2013;17:962–972. doi: 10.1007/s11605-013-2148-y. [DOI] [PubMed] [Google Scholar]
- 24.Vilz TO, Wehner S, Pantelis D, Kalff JC. Immunomodulatory aspects in the development, prophylaxis and therapy for postoperative ileus. Zentralbl Chir. 2014;139:434–444. doi: 10.1055/s-0033-1350678. [DOI] [PubMed] [Google Scholar]
- 25.Vilz TO, Pantelis D, Kalff JC. Prophylaxis and therapy of postoperative ileus. Chirurgische Praxis. 2013;76:407–420. [Google Scholar]
- 26.Kehlet H. Fast-track surgery-an update on physiological care principles to enhance recovery. Langenbecks Arch Surg. 2011;396:585–590. doi: 10.1007/s00423-011-0790-y. [DOI] [PubMed] [Google Scholar]
- 27.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 28.Popping DM, Elia N, Van Aken HK, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259:1056–1067. doi: 10.1097/SLA.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 29.Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 30.Short V, Herbert G, Perry R, et al. Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD006506.pub3. CD006506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traut U, Brugger L, Kunz R, et al. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD004930.pub3. CD004930. [DOI] [PubMed] [Google Scholar]
- 32.Candrilli SD, Davis KL, Iyer S. Impact of constipation on opioid use patterns, health care resource utilization, and costs in cancer patients on opioid therapy. J Pain Palliat Care Pharmacother. 2009;23:231–241. doi: 10.1080/15360280903098440. [DOI] [PubMed] [Google Scholar]
- 33.AWMF. Stoffwechselkrankheiten S2k Leitlinie Chronische Obstipation AWMF Online 2013. www.awmf.org/uploads/tx_szleitlinien/021-019l_S2k_Chronische_Obstipation_2013-06_01.pdf (last accessed on 20 June 2017) [Google Scholar]
- 34.Ogilvie WH. William Heneage Ogilvie 1887-1971 Large-intestine colic due to sympathetic deprivation. A new clinical syndrome. Dis Colon Rectum. 1987;30:984–987. doi: 10.1007/BF02554291. [DOI] [PubMed] [Google Scholar]
- 35.Pereira P, Djeudji F, Leduc P, Fanget F, Barth X. Ogilvie’s syndrome-acute colonic pseudo-obstruction. J Visc Surg. 2015;152:99–105. doi: 10.1016/j.jviscsurg.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie’s syndrome) An analysis of 400 cases. Dis Colon Rectum. 1986;29:203–210. doi: 10.1007/BF02555027. [DOI] [PubMed] [Google Scholar]
- 37.Valle RG, Godoy FL. Neostigmine for acute colonic pseudo-obstruction: A meta-analysis. Ann Med Surg (Lond) 2014;3:60–64. doi: 10.1016/j.amsu.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geller A, Petersen BT, Gostout CJ. Endoscopic decompression for acute colonic pseudo-obstruction. Gastrointest Endosc. 1996;44:144–150. doi: 10.1016/s0016-5107(96)70131-1. [DOI] [PubMed] [Google Scholar]
- 39.De Giorgio R, Knowles CH. Acute colonic pseudo-obstruction. Br J Surg. 2009;96:229–239. doi: 10.1002/bjs.6480. [DOI] [PubMed] [Google Scholar]
- 40.Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48:75–91. doi: 10.1016/j.jpainsymman.2013.08.022. [DOI] [PubMed] [Google Scholar]