Abstract
Introduction: Cannabidiol (CBD) is a nonpsychoactive constituent of whole plant cannabis that has been reported to reduce anxiety-like behaviors in both pre-clinical and human laboratory studies. Yet, no controlled clinical studies have demonstrated its ability to reduce negative mood or dampen responses to negative emotional stimuli in humans. The objective of this study was to investigate the effects of CBD on responses to negative emotional stimuli, as a model for its potential anxiety-reducing effects.
Materials and Methods: The study used a double-blind, placebo (PLB)-controlled, within-subjects design in which 38 healthy, drug-free participants consumed oral CBD (300, 600, and 900 mg) or PLB before completing several behavioral tasks selected to assess reactivity to negative stimuli. Dependent measures included emotional arousal to negative and positive visual stimuli, perceptual sensitivity to emotional facial expressions, attentional bias toward emotional facial expressions, and feelings of social rejection. In addition, subjective drug effects and physiological data were also gathered during each experimental session to assess drug effects.
Discussion: CBD did not dampen responses to negative emotional stimuli and did not affect feelings of social rejection. The high dose of CBD (900 mg) marginally reduced attentional bias toward happy and sad facial expressions, and produced a slight increase in late-session heart rate. CBD did not produce detectable subjective effects or alterations in mood or anxiety.
Conclusion: These findings indicate that CBD has minimal behavioral and subjective effects in healthy volunteers, even when they are presented with emotional stimuli. Further research into the behavioral and neural mechanisms of CBD and other phytocannabinoids is needed to ascertain the clinical function of this drug.
Keywords: : behavior, cannabidiol, emotional stimuli, psychopharmacology
Introduction
Cannabidiol (CBD), a constituent of cannabis, has received enormous public and scientific attention over the past decade. CBD citations in PubMed increased from 40 in 2000–2002 to 458 in 2014–2016. Although many of these refer to the potential of CBD to treat psychiatric or neurological disorders,1,2 there have been promising reports of its efficacy for treatment-resistant epilepsy and, combined with delta-9 tetrahydrocannabinol (THC; Sativex®), for multiple sclerosis. There is also evidence that single doses of CBD alter mood or behavior, either alone or in combination with other cannabinoids.
Several studies suggest that CBD has anxiolytic effects. Zuardi et al.3 reported that CBD (about 35 mg) reduced anxiety provoked by oral THC (about 70 mg) in normal volunteers. Later, Zuardi et al.4 reported that CBD (300 mg) reduced anxiety during a stressful public speaking task in healthy adults, to a similar extent as diazepam (10 mg) and ipsaperone (5 mg). In other studies, CBD reduced anxiety during public speaking in individuals with social anxiety disorder,5 reduced amygdala responses to fearful faces in healthy men,6,7 and reduced anxiety in response to a stressful imaging procedure in men.8 CBD also produces anxiolytic-like effects in animal models.9 Together, these findings suggest that CBD may possess anxiolytic properties similar to those of known anxiolytic drugs.
The neural mechanisms by which CBD acts in the brain are poorly understood. CBD has low binding affinity for either CB1 or CB2 receptors. However, some in vivo studies indicate that the behavioral effects of CBD may be elicited indirectly through these receptors.10,11 CB1 inverse agonists can block the behavioral effects of CBD in mouse models of fear conditioning, extinction, and marble burying behaviors.12,13 CBD administered directly into key brain regions reduces anxiety-like behavior in rodents.14–16 CBD may also reduce anxiety and alleviate other neurological disorders by enhancing anandamide through fatty acid amide hydrolase inhibition17,18 or by altering serotonergic (5-HT) neurotransmission, including actions as an indirect 5-HT1A agonist.1,18–21
In this study, we examined the effects of CBD on responses to negative emotional stimuli in healthy human volunteers.22 Single doses of anxiolytic and antidepressant drugs produce subtle changes in perception and responses to emotional stimuli in healthy individuals, effects that appear to predict their therapeutic efficacy.23–26 In this study, we used such measures to study the effects of CBD. We tested participants' responses to images or words with negative affective content, reactivity to threatening emotional faces, and sensitivity to social rejection after oral CBD (0, 300, 600, and 900 mg) in a double-blind design. We hypothesized that CBD would reduce reactivity to negative emotional stimuli.
Materials and Methods
Design
Healthy men and women aged 18–35 years participated in this four-session, within-subjects double-blind study. On each session, they received a single oral dose of CBD (300, 600, and 900 mg) or placebo (PLB) in randomized order. The study was approved by the University of Chicago Biological Science Division Institutional Review Board, and procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2008.
Drug
Oral CBD (300 mg/ml solution) or PLB vehicle was provided by Insys Therapeutics, Inc. (IND 125302). On each session, participants received a total of 4 ml of fluid that included 1 ml of sugar-free syrup to enhance palatability and to improve blinding (Ora-Sweet; Paddock Laboratories, Minneapolis, MN), plus the appropriate volume of active CBD (0, 1, 2 or 3 ml) and vehicle (3, 2, 1 or 0 ml). The solutions for each dose were matched on taste, physical characteristics, and volume. The drug was administered 2.5 h before subjects completed the behavioral tasks, based on evidence that CBD plasma levels are rising at this time.27,28
Subjects
Volunteers (19 men and 19 women) were recruited through posters, advertisements, and word-of-mouth referrals. Screening included a physical examination, an electrocardiogram, a psychiatric screening interview, and detailed drug use history. Individuals were excluded if they had cardiovascular problems, used prescription medications (except hormonal contraception in women), had a current DSM-V Axis I29 mood, anxiety, eating, or substance dependence disorder, or a lifetime history of a psychotic disorder. Women who were pregnant, nursing, or planning to become pregnant, anyone with less than a high-school education, not fluent in English, body mass index (BMI) less than 19 or more than 30, or who reported using cannabis >100 times in their lifetime were also excluded to minimize possible tolerance to CBD.
Study procedures
Orientation
Participants who met criteria attended an orientation session to explain the study, obtain consent, and practice the study tasks. They agreed to fast after 11:00 am on the day of the sessions and abstain from alcohol and other drug use for 24 h before sessions. To mitigate expectancy effects, participants were told that they might receive a PLB, a stimulant, a sedative, or a cannabis-like drug (e.g., THC or CBD).
Study sessions
Sessions were conducted in a comfortable laboratory room from 1:00 pm to 5:45 pm, separated by at least 1 week. Upon arrival, subjects provided urine and breath samples to test for recent alcohol and drug use, and a pregnancy test (for women). Subjects who tested positive were rescheduled or dropped from the study. Participants then consumed a standardized snack (granola bar) and completed baseline (Time Point 1) measures of subjective mood, drug effects, and cardiovascular variables. These measures were obtained at regular 30–60 min intervals during the 4-h session. At 1:30 pm, participants ingested capsules containing CBD (300, 600, and 900 mg) or PLB. For the next 2.5 h until the drug effect reached its expected peak,27,28 participants relaxed and watched a movie or read a book. From 4 pm to 5 pm, they completed the tasks described hereunder to assess their reactivity to emotional stimuli. The tasks were presented in a counterbalanced order. Participants were discharged at 5:45 pm.
Physiological measures
Cardiovascular measures
Heart rate and blood pressure (BP) were measured using portable monitors (Omron Automatic Blood Pressure Monitor, Model No. BP791IT; Omron Healthcare, Inc., Lake Forest, IL). Mean arterial pressure (MAP; [systolic BP +2×diastolic BP]/3) was calculated.
Subjective measures
Profile of Mood States
The Profile of Mood States (POMS) is a validated measure of mood states consisting of 72 adjectives commonly used to describe momentary moods.30 The POMS is sensitive to the effects of mood-altering drugs in healthy volunteers.31,32
Drug Effects Questionnaire
The Drug Effects Questionnaire is a validated measure of subjective drug effects.33,34 Participants indicate on a visual analog scale the extent they feel a drug effect, whether they like or dislike the drug effect, and whether given a choice would they want to take more of the drug.
Behavioral tasks
We based our predictions of CBD on previous studies investigating other pharmacological interventions in similar behavioral tasks. Specifically, drugs that are known to improve anxiety or depression (tryptophan supplementation, antidepressant medication, steroids, and benzodiazepines) tend to reduce or direct an individual's attention away from negative emotional stimuli.26 Thus, we predicted that CBD would result in similar behavioral outcomes.
Emotional Stroop
The Emotional Stroop is a measure of emotional arousal adapted from the original Stroop color task.26,35,36 It consists of words with specific emotional connotations displayed in four different colors. It includes positive and negative words with religious, social, and emotional content. For example, religious-positive words included “angel,” “paradise,” and “divine,” and social-negative words included “alone,” “unwanted,” and “disliked.” It also includes a “color control” in which names of colors were presented in different colored font.36 Participants indicate the color of each word's text by pressing a corresponding key on a keyboard. Longer reaction times are expected for mismatched color and text, and longer reaction times with emotional words are indicative of a greater emotional response (longer fixation) to emotional words.37,38 Previous studies have shown that changes in tryptophan depletion can increase interference of words with negative connotations.39–41 Interestingly, CBD has also been shown to suppress tryptophan depletion in ways that suggest a neural mechanism by which antidepressant or anxiolytic effects of cannabinoids might be linked to serotonergic neurotransmission.42 Thus, it was predicted that CBD would blunt the emotional response toward words with negative connotations, resulting in faster reaction times toward negative words.
International Affective Picture System
The International Affective Picture System (IAPS) was used as a measure of emotional arousal43 to visual stimuli with affective content. Participants viewed standardized positive, negative, and neutral pictures from the IAPS. The negative and positive images were matched on degree of valence and arousal. Positive, negative, and neutral images were also categorized as “social” or “nonsocial” by the experimenters. Subjects rated both the positivity and the negativity of each image. It was predicted that CBD would reduce negativity ratings to negative pictures.
Dynamic Emotion Identification Task
The Dynamic Emotion Identification Task (DEIT) is a measure of sensitivity to detecting facial expressions, created for use in our laboratory.44 Participants viewed dynamically developing facial expressions comprising 2% morphs from a neutral face to a 100% expression of an emotion, happy, sad, angry, and fearful. Participants responded as soon as they could correctly identify the emotion expressed. Emotion identification was quantified as the intensity (0–100%) of the face when the participant responded on trials when they correctly identified the emotion. Based on previous studies investigating pharmacologically induced reductions in reactivity to negative emotional stimli,25,26 we predicted that CBD would reduce sensitivity to negative facial expressions (i.e., a higher intensity of expression needed to identify sadness, anger, and fear).
Attentional Bias Task
The Attentional Bias Task (ABT) measures attention toward emotional stimuli (facial expressions) and was adapted from Garner et al.45 Participants viewed a neutral face and one with an emotional expression46 side-by-side. After 500 msec viewing, a white dot appeared in place of one of the faces and participants were instructed to indicate its location. Trials were separated by 750–1,250 msec. Attentional bias toward emotional facial expressions was indicated by a longer shorter reaction time for the emotional expression.47 Based on previous studies in nonclinical participants investigating the effects of serotonergic antidepressants and anxiolytics on emotional visual probe tasks,26 we predicted that CBD would reduce attentional bias (i.e., lengthen reaction times) to negative facial expressions (anger, sadness, and fear).
Cyberball
This is a measure of social acceptance and ostracism.48–50 Participants played two virtual games of toss with two other “players” who were represented as animated icons on the computer screen. Participants were told that they represented one of the three players. During the first game (acceptance), the ball was tossed equally among the three players. During the second game (rejection), the participant was excluded. After each game, participants rated their levels of mood and self-esteem. We have previously found that MDMA, a drug that has prosocial and mood-enhancing effects, can blunt the effects of simulated social rejection.51 It was predicted here that CBD would blunt the rejection-induced reduction in mood and self-esteem. Participants also estimated the percentage of throws they received during each game.
Data analyses
The physiological and subjective effects of CBD were analyzed by two-way (drug×time) repeated measures analysis of variance (ANOVA). Behavioral tasks (Emotional Stroop, IAPS, DEIT, ABT, and Cyberball) were analyzed through a series of two-way mixed factorial ANOVAs. Significant effects were followed by post hoc comparisons of the estimated marginal means. Bonferroni corrections for multiple comparisons within each dependent measure were utilized to maintain a family-wise error rate of 0.05.
Results
Participants
One person tested positive for cannabis and was dropped from the study. Table 1 illustrates that most of the remaining 38 subjects were in their early 20s and reported light-to-moderate recent use of alcohol, nicotine, caffeine, and cannabis.
Table 1.
Characteristics of the 38 Participants Who Completed the Cannabidiol Study
| Gender (M:F) | 19:19 |
| Age (years) | 23.6 (0.66) |
| Education (years) | 15.2 (0.27) |
| Race | |
| Caucasian | 22 |
| African American | 10 |
| Other | 6 |
| Recent (past month) substance use | |
| Alcohol (drinks/week) | 5.28 (0.64); n=35 |
| Cigarettes (cigs/week) | 8.58 (5.32); n=7 |
| Caffeine (cups/day) | 1.32 (0.23); n=33 |
| Cannabis (times/month) | 5.12 (1.02); n=14 |
| Lifetime substance use (% ever used) | |
| Cannabis | 94.7 |
| Hallucinogens | 23.7 |
| Stimulants | 15.8 |
| Opiates | 7.9 |
| MDMA | 18.4 |
| Sedatives | 5.3 |
Age, education, and past month recent substance use are listed as mean (SEM). For recent substance use, the mean and SEM were calculated using only subjects who reported any recent use of the drug (n for each drug type is shown). Remaining subjects reported no recent use of the drugs. Race is number of individuals who identify as such (“Other” is mainly Asian). Lifetime substance use refers to nonmedical use only.
SEM, standard error of the mean.
Physiological response
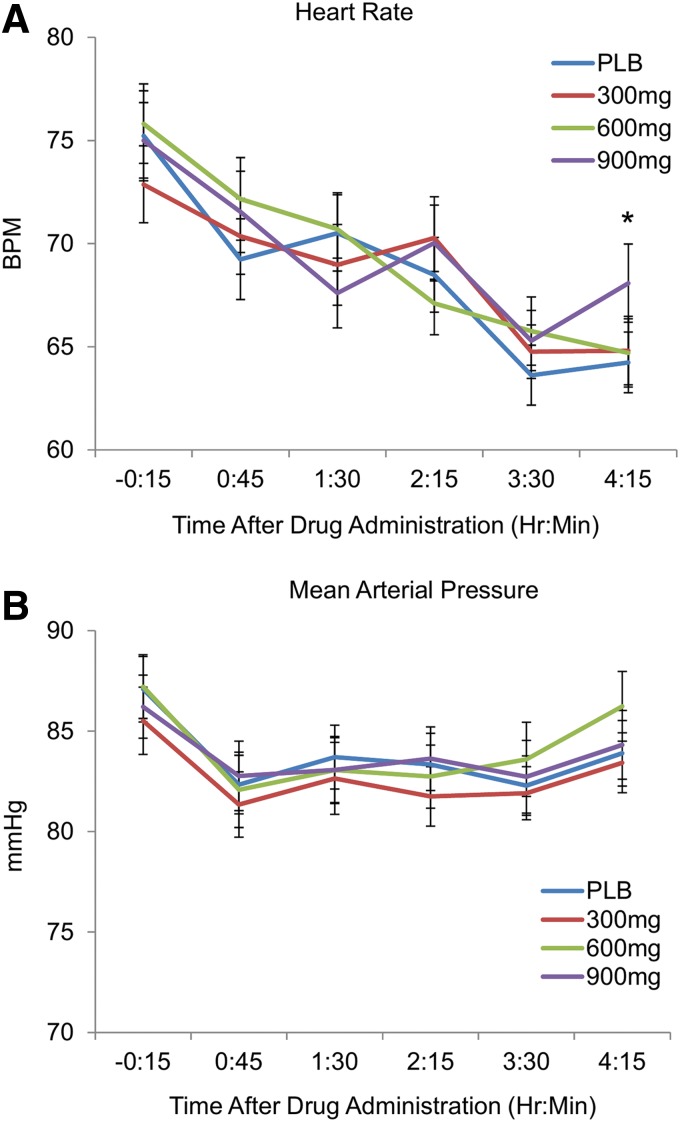
CBD had no effect on MAP (p>0.05). CBD (300 and 600 mg) did not affect heart rate, and only the highest dose of CBD (900 mg) resulted in a slight increase in heart rate at the last time point of the session, indicated by a significant drug×time interaction effect, F(15, 540)=1.77, p=0.036.
Subjective drug effects
CBD did not alter ratings of mood states (POMS) and subjects did not report feeling a drug effect, or liking or disliking it (all p's>0.05).
Emotional Stroop
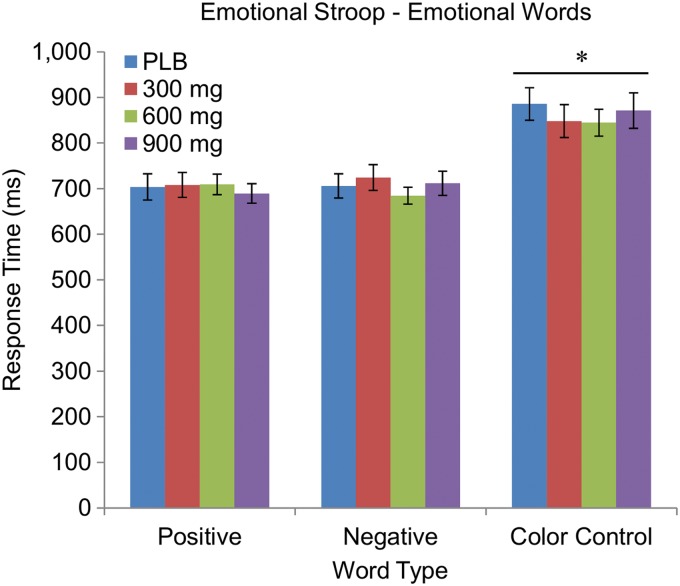
As expected, subjects took longer to identify the name of the colored words when the text of the word was in a different color, F(2, 70)=85.08, p<0.001. However, they did not exhibit longer reaction times with negative emotional words, and CBD had no effect on reaction times for any words (Fig. 1; all p's>0.05).
FIG. 1.
Mean±SEM reaction times for emotional words in the Emotional Stroop task. Asterisk (*) indicates that both the reaction times for the negative and positive words were significantly shorter than the reaction times for the color control words, but the reaction times for the negative and positive words did not significantly differ from one another. CBD had no effect on reaction times for any words. CBD, cannabidiol; SEM, standard error of the mean.
International Affective Picture System
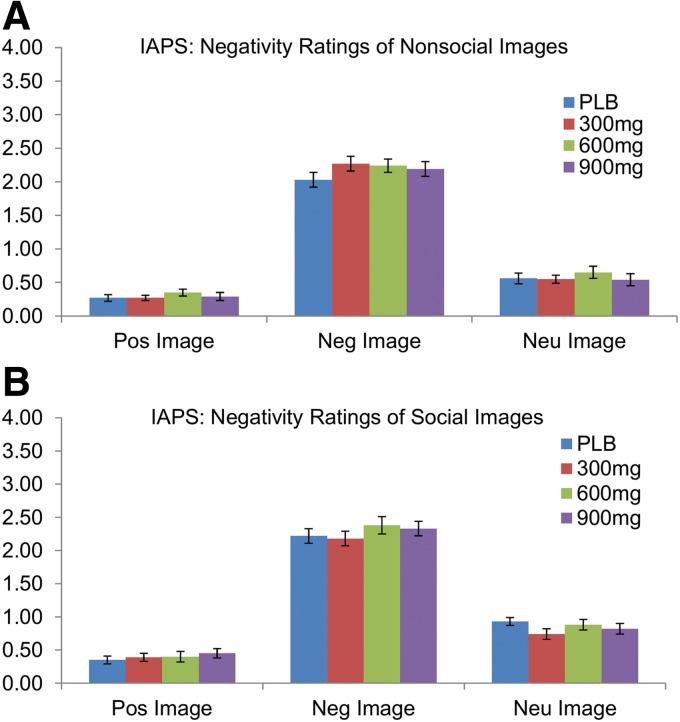
As expected, subjects rated positive images more positively (i.e., higher positivity ratings, F(2, 72)=182.89, p<0.001) and negative images more negatively [i.e., higher negativity ratings, F(2, 72)=393.24, p<0.001]. However, CBD did not alter ratings of either positive or negative stimuli (all p's>0.05; Fig. 2).
FIG. 2.
Mean±SEM negativity ratings for (A) nonsocial and (B) social images of positive, negative, or neutral valence in the IAPS picture rating task. All image valence groups significantly differed from all other image valence groups, but CBD did not alter ratings of either positive or negative stimuli. A similar trend in positivity ratings was observed for both nonsocial and social images. IAPS, International Affective Picture System.
Dynamic Emotion Identification Task
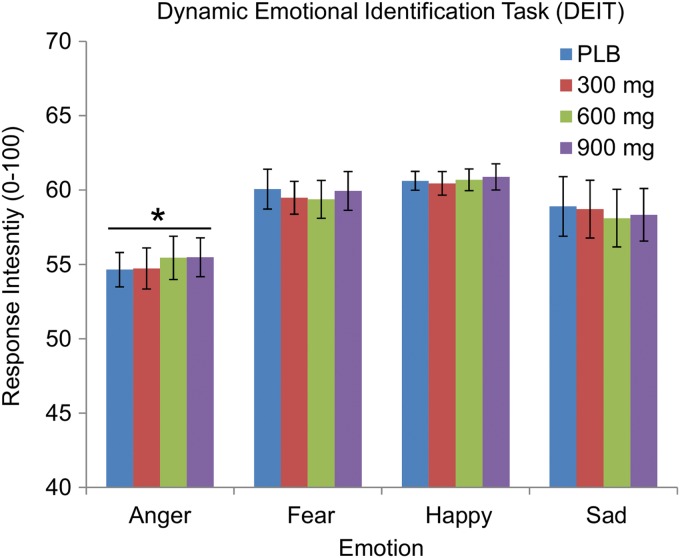
As expected, subjects identified angry faces significantly earlier than any of the other facial emotions, F(3, 108)=18.30, p<0.001. CBD did not affect the ability to identify any expressions, regardless of the emotion (Fig. 3; all p's>0.05).
FIG. 3.
Mean±SEM response intensity of facial expressions at time of identification in the DEIT. Asterisk (*) indicates that participants identified angry faces significantly faster than any of the other facial emotions. None of the other facial emotions differed from one another, and CBD did not affect the ability to identify facial expressions, regardless of emotion. DEIT, Dynamic Emotion Identification Task.
Attentional Bias Task
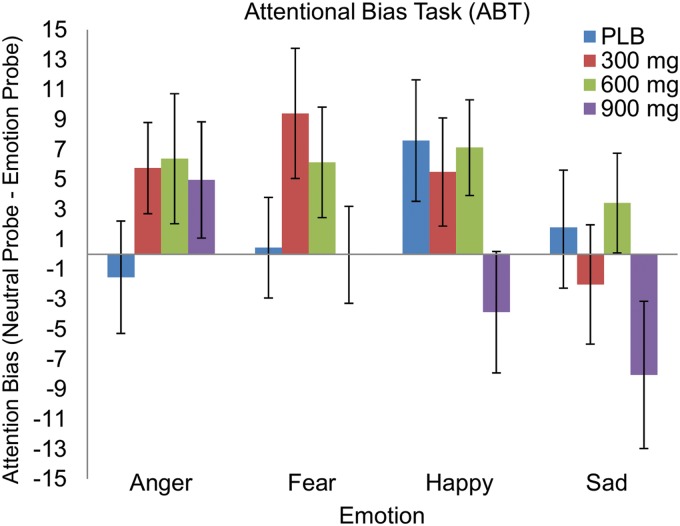
With all emotions taken together, CBD affected attentional bias [drug, F(3, 102)=3.07, p=0.031], but this effect was no longer significant after correcting for multiple comparisons (all p's>0.05). CBD did not affect attentional bias toward specific facial expressions, either negative (anger, sadness, and fear) or happy (Fig. 4). The absence of an interaction between drug and emotion (p>0.05) indicates that the effect of CBD on attentional bias was not significantly specific to certain emotions.
FIG. 4.
Mean±SEM attentional bias scores toward angry, fearful, happy, or sad facial expressions. With all emotions taken together, CBD affected attentional bias (p=0.031). This effect of CBD was not specific to certain emotions.
Cyberball
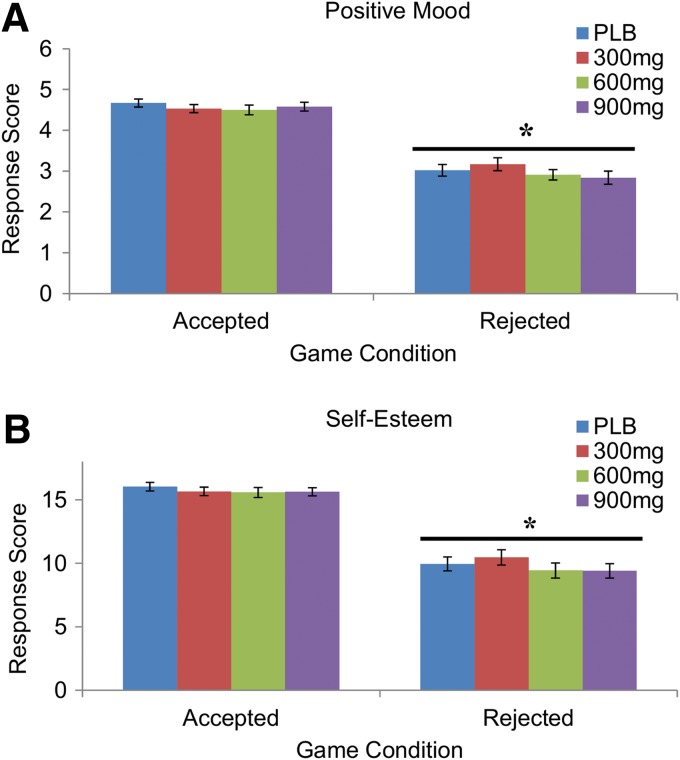
Subjects reported more negative mood, F(1, 34)=127.26, p<0.001 and lower self-esteem, F(1, 34)=112.54, p<0.001 during the “rejection” compared with the “acceptance” phase of the task, and correctly noted that they received fewer throws, F(1, 34)=440.26, p<0.001 (data not shown). However, CBD did not change their responses during either phase (Fig. 5; all p's>0.05).
FIG. 5.
Mean±SEM feelings of positive mood (A) and self-esteem (B) after the Cyberball games conditions of “accepted” and “rejected.” Asterisks (*) indicate that subjects reported reduced feelings of positive mood and self-esteem after the rejection game, but CBD did not change their responses.
Discussion
Single doses of CBD (300, 600, and 900 mg oral) did not dampen responses to negative emotional stimuli in healthy volunteers. The highest dose of CBD (900 mg) produced a slight, delayed increase in heart rate 240 min after administration (Fig. 6), and decreased attentional bias toward emotional facial expressions. But, CBD produced no detectable subjective effects (e.g., “feeling” a drug effect or ratings of momentary mood states) and had little effect on reactions to negative emotional stimuli on standardized tasks. The drug was well tolerated in these healthy young adults, and no participants experienced any adverse effects.
FIG. 6.
Mean±SEM (A) heart rate and (B) blood pressure (BP) throughout the experimental session before and after CBD (300, 600, and 900 mg) or placebo administration. Asterisk (*) indicates that the highest dose of CBD (900 mg) resulted in a slight increase in heart rate at the last time point of the session. CBD had no effect on BP (MAP, p>0.05). MAP, mean arterial pressure.
Previous findings on the effects of CBD on mood or subjective state have been mixed. Several previous studies have suggested that CBD has stress-dampening effects, either alone or when it is administered in combination with other constituents of the cannabis plant.38 Our finding that CBD had little or no effect on behavioral measures of negative emotionality is apparently inconsistent with the prior reports of anxiolytic effects. However, our findings are consistent with other recent reports that CBD has minimal psychological or behavioral effects. In particular, Babalonis et al.52 reported that oral CBD (200, 400, and 800 mg) produced no detectable subjective or behavioral effects in regular cannabis users on measures of psychomotor performance and selective attention. In a related report, Haney et al.53 reported that CBD also did not change responses to cannabis.
An important shortcoming of this study was the absence of pharmacokinetic data to confirm that the drug was absorbed and that the participants were exposed to the intended plasma concentrations of drug. Because the drug, unexpectedly, had little effect on any outcome measure, we cannot be assured that the appropriate dose was delivered. Furthermore, Haney et al.53 reported a great deal of inter-individual variability in the peak plasma levels of CBD: among the eight participants in their study who received 800 mg CBD, peak plasma levels ranged from 1.6 to 271.9 ng/ml for the 6-h session, and the time to peak ranged from 120 to 360 min. This level of variability makes it difficult to measure the effects of the drug, and, if present in our study, could have contributed to our negative findings. Unpublished data from the supplier of the CBD formulation used here indicated that peak plasma CBD concentrations after a single dose occurred 4.5 h after oral administration (standard deviation [SD] 1.62 in 20 healthy volunteers), suggesting that absorption here was adequate and plasma levels were rising when we implemented our behavioral tasks. On a separate issue, it also would be of interest to study the effects of chronic doses of CBD to determine whether steady-state concentrations of CBD produce detectable behavioral effects.
Little is known about effects of CBD on attentional bias. One study54 compared attentional bias to drug and food stimuli in individuals who reported smoking high CBD:THC strains compared with low CBD:THC ratios, and found that high CBD:THC smokers showed lower attentional bias to drug and food stimuli. However, it is difficult to relate those findings to the present results because of differences in drugs, participants, and study stimuli. It remains to be determined whether CBD truly alters attentional bias to emotional stimuli.
One question to be addressed in any study, especially one with “negative” findings, is whether the measures used were sensitive to the purported drug effect. In this case, we used four tasks assessing reactivity to emotional stimuli, and each task was previously shown to be sensitive to the effects of psychoactive drugs. Moreover, our tasks provided orderly and expected effects, independently of CBD administration. On the DEIT, subjects were quicker to identify angry facial expressions than other emotions, as previously reported,44 and other drugs are known to alter DEIT performance.44,55 Yet, we found no effect with CBD. On the IAPS task, participants rated positive pictures positively and negative pictures negatively, and previous studies have shown that stimulant drugs alter ratings of images.44,55 Again, CBD did not affect responses to images of any valence. In the Cyberball task, participants reported greater feelings of social rejection after the exclusion game in this study, and we have previously reported that certain psychoactive drugs reduce feelings of rejection.51 Yet, CBD did not blunt feelings of rejection in this study. Finally, on the ABT, the mean reaction times in this study were comparable to those in previous studies,47 and on the Stroop task, we detected the usual slowing on the color control condition, but CBD did not alter performance in either of these measures. Thus, CBD had little or no effect on tasks with known sensitivity.
This study had limitations. We assessed attentional bias using reaction time to detect a stimulus, whereas previous studies used eye gaze, measured with electrooculography44 (EOG). It is possible that the EOG measure provides a more sensitive index of attention. It is possible that the emotional stimuli used here were not salient enough to induce negative mood states or anxiety, or that the mood effects of CBD are only evident in individuals with high levels of anxiety, because of an underlying trait, or because of a contextual stressor (an anxiogenic drug or a stressful task4,5), as in prior reports of anxiolysis with CBD. Thus, it is possible that we did not detect a mood effect because participants were not experiencing anxiety at the time of CBD administration.
Conclusions
This study suggests that oral CBD does not alter responses to emotional stimuli, or produce anxiolytic-like effects in healthy human subjects. The absence of effect of CBD is consistent with another recent, carefully controlled laboratory study.52,53 Cannabinoids as a class of drugs are understudied, despite their widespread use for both recreational and therapeutic reasons. Yet, little is known about the active constituents and their effects on mood and behavior. Further research is needed to assess the therapeutic potential of cannabis constituents such as CBD. Studies of chronic, as well as acute administration of these drugs, as well as studies in at-risk individuals, are needed. Studies of the pharmacokinetic characteristics and the mechanisms of action are also essential. This research is critical to establish the brain actions, behavioral effects, and potential toxicity of this widely used class of drugs.
Abbreviations Used
- ABT
attentional bias task
- ANOVA
analysis of variance
- BP
blood pressure
- CBD
cannabidiol
- DEIT
Dynamic Emotion Identification Task
- EOG
electrooculography
- IAPS
International Affective Picture System
- MAP
mean arterial pressure
- POMS
Profile of Mood States
- THC
tetrahydrocannabinol
Acknowledgments
This study was supported by Insys Therapeutics, Inc., and by DA02812. Dr. Arndt was supported by T32GM007019. The authors thank Joel Cavallo for early contributions to this work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Campos AC, Fogaça MV, Sonego AB, et al. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–127 [DOI] [PubMed] [Google Scholar]
- 2.Zhornitsky S, Potvin S. Cannabidiol in humans—the quest for therapeutic targets. Pharmaceuticals. 2012;5:529–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuardi AW, Shirakawa I, Finkelfarb E, et al. Action of cannabidiol on the anxiety and other effects produced by Δ9-THC in normal subjects. Psychopharmacology (Berl). 1982;76:245–250 [DOI] [PubMed] [Google Scholar]
- 4.Zuardi AW, Cosme RA, Graeff FG, et al. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7:82–88 [DOI] [PubMed] [Google Scholar]
- 5.Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of [Delta]-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2009;35:764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fusar-Poli P, Crippa JA, Bhattacharyya S, et al. Distinct effects of delta 9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105 [DOI] [PubMed] [Google Scholar]
- 8.Crippa JAdS, Zuardi AW, Garrido GEJ, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2003;29:417–426 [DOI] [PubMed] [Google Scholar]
- 9.Schier ARM, Ribeiro NPO, Hallak JEC, et al. Cannabidiol, a Cannabis sativa constituent, as an anxiolytic drug. Rev Bras Psiquiatr. 2012;34:104–110 [DOI] [PubMed] [Google Scholar]
- 10.Thomas A, Ross RA, Saha B, et al. 6″-Azidohex-2″-yne-cannabidiol: a potential neutral, competitive cannabinoid CB 1 receptor antagonist. Eur J Pharmacol. 2004;487:213–221 [DOI] [PubMed] [Google Scholar]
- 11.Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casarotto PC, Gomes FV, Resstel LB, et al. Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors. Behav Pharmacol. 2010;21:353–358 [DOI] [PubMed] [Google Scholar]
- 13.Stern CA, Gazarini L, Takahashi RN, et al. On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology. 2012;37:2132–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes FV, Resstel LB, Guimarães FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl). 2011;213:465–473 [DOI] [PubMed] [Google Scholar]
- 15.Fogaca MV, Reis FMCV, Campos AC, et al. Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: involvement of 5HT 1A receptors and previous stressful experience. Eur Neuropsychopharmacol. 2014;24:410–419 [DOI] [PubMed] [Google Scholar]
- 16.Hsiao YT, Yi PL, Li CL, et al. Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elecated plus-maze in rats. Neuropharmacology. 2012;62:373–384 [DOI] [PubMed] [Google Scholar]
- 17.Deutsch DG. A personal retrospective: elevating anandamide (AEA) by targeting fatty acid amide hydrolase (FAAH) and the fatty acid binding proteins (FABPs). Front Pharmacol. 2016;7:37–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurd YL. Cannabidiol: swinging the marijuana pendulum from “weed” to medication to treat the opioid epidemic. Trends Neurosci. 2017;40:124–127 [DOI] [PubMed] [Google Scholar]
- 19.Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043 [DOI] [PubMed] [Google Scholar]
- 20.Scuderi C, Filippis DD, Iuvone T, et al. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res. 2009;23:597–602 [DOI] [PubMed] [Google Scholar]
- 21.Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl). 2008;199:22–3. [DOI] [PubMed] [Google Scholar]
- 22.Pringle A, Browning M, Cowen PJ, et al. A cognitive neuropsychological model of antidepressant drug action. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1586–1592 [DOI] [PubMed] [Google Scholar]
- 23.Harmer CJ, Heinzen J, O'Sullivan U, et al. Dissociable effects of acute antidepressant drug administration on subjective and emotional processing measures in healthy volunteers. Psychopharmacology (Berl). 2008;199:495–502 [DOI] [PubMed] [Google Scholar]
- 24.Harmer CJ, Bhagwagar Z, Perrett DI, et al. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28:148–152 [DOI] [PubMed] [Google Scholar]
- 25.Harmer CJ. Serotonin and emotional processing: does it help explain antidepressant drug action? Neuropharmacology. 2008;55:1023–1028 [DOI] [PubMed] [Google Scholar]
- 26.Browning M, Holmes EA, Harmer CJ. The modification of attentional bias to emotional information: a review of the techniques, mechanisms, and relevance to emotional disorders. Cogn Affect Behav Neurosci. 2010;10:8–20 [DOI] [PubMed] [Google Scholar]
- 27.Grotenhermen F. Cannabinoids for therapeutic use. Am J Drug Deliv. 2004;2:229–240 [Google Scholar]
- 28.Agurell S, Carlsson S, Lindgren JE, et al. Interactions of Δ11-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia. 1981;37:1090–1092 [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub.: Arlington, VA, 2013. [Google Scholar]
- 30.McNair D, Lorr M, Droppleman L. Profile of mood states. Educational and Industrial Testing Service: San Diego, CA, 1981, pp. 1–29 [Google Scholar]
- 31.de Wit H, Griffiths RR. Testing the abuse liability of anxiolytic and hypnotic drugs in humans. Drug Alcohol Depend. 1991;28:83–111 [DOI] [PubMed] [Google Scholar]
- 32.Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacology (Berl). 1980;71:269–273 [DOI] [PubMed] [Google Scholar]
- 33.Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570 [DOI] [PubMed] [Google Scholar]
- 34.Morean ME, de Wit H, King AC, et al. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology (Berl). 2013;227:177–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams JMG, Mathews A, MacLeod C. The Emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3. [DOI] [PubMed] [Google Scholar]
- 36.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:64–3. [Google Scholar]
- 37.Dresler T, Mériau K, Heekeren HR, et al. Emotional Stroop task: effect of word arousal and subject anxiety on emotional interference. Psychol Res. 2009;73:364–371 [DOI] [PubMed] [Google Scholar]
- 38.Gotlib IH, McCann CD. Construct accessibility and depression: an examination of cognitive and affective factors. J Pers Soc Psychol. 1984;47:42–7. [DOI] [PubMed] [Google Scholar]
- 39.Evers EAT, Van der Veen FM, Jolles J, et al. Acute tryptophan depletion improves performance and modulates the BOLD response during a Stroop task in healthy females. Neuroimage. 2006;32:248–255 [DOI] [PubMed] [Google Scholar]
- 40.Hayward G, Goodwin GM, Cowen PJ, et al. Low-dose tryptophan depletion in recovered depressed patients induces changes in cognitive processing without depressive symptoms. Biol Psychiatry. 2005;57:517–524 [DOI] [PubMed] [Google Scholar]
- 41.Munafò MR, Hayward G, Harmer C. Selective processing of social threat cues following acute tryptophan depletion. J Psychopharmacol. 2006;20:33–39 [DOI] [PubMed] [Google Scholar]
- 42.Jenny M, Schröcksnadel S, Überall F, et al. The potential role of cannabinoids in modulating serotonergic signaling by their influence on tryptophan metabolism. Pharmaceuticals. 2010;3:2647–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): technical manual and affective ratings. NIMH Center for the study of emotion and attention. University of Florida: Birmingham, AL, 1999 [Google Scholar]
- 44.Wardle M, Garner M, Munafò M, et al. Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacology (Berl). 2012;223:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. J Abnorm Psychol. 2006;115:760–770 [DOI] [PubMed] [Google Scholar]
- 46.Goeleven E, De Raedt R, Leyman L, et al. The Karolinska Directed Emotional Faces: a validation study. Cogn Emot. 2008;22:1094–1118 [Google Scholar]
- 47.Clark-Elford R, Nathan PJ, Auyeung B, et al. Effects of oxytocin on attention to emotional faces in healthy volunteers and highly socially anxious males. Int J Neuropsychopharmacol. 2015;18:pyu01–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. J Exp Soc Psychol. 2004;40:560–567 [Google Scholar]
- 49.Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. 2006;38:174–180 [DOI] [PubMed] [Google Scholar]
- 50.Liu JC, Mulick D, Chee MW. Odd one out: social ostracism affects self‐reported needs in both sleep-deprived and well-rested persons. J Sleep Res. 2014;23:448–457 [DOI] [PubMed] [Google Scholar]
- 51.Frye CG, Wardle MC, Norman GJ, et al. MDMA decreases the effects of simulated social rejection. Pharmacol Biochem Behav. 2014;117:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babalonis S, Haney M, Malcolm RJ, et al. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend. 2017;172:9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haney M, Malcolm RJ, Babalonis S, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41:1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan CJ, Freeman TP, Schafer GL et al. Cannabidiol attenuates the appetitive effects of Δ9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010;35:1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wardle M, de Wit H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology (Berl). 2012;220:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
Cite this article as: Arndt DL, de Wit H (2017) Cannabidiol does not dampen responses to emotional stimuli in healthy adults, Cannabis and Cannabinoid Research 2:1, 105-113, DOI: 10.1089/can.2017.0014.