Abstract
Objective(s):
Particulate matter (PM) exposure can promote cardiac ischemia and myocardial damage. The effects of PM10 on hemodynamic parameters, lipid peroxidation, and infarct size induced by ischemia-reperfusion injury and the protective effects of vanillic acid (VA) in isolated rat heart were investigated.
Materials and Methods:
Eighty male Wistar rats (250–300 g) were divided into 8 groups (n=10): Control, Sham, VAc, VA, PMa (0.5 mg/kg PM, intratracheal instillation), PMb (2.5 mg/kg PM, intratracheal instillation), PMc (5 mg/kg PM, intratracheal instillation), and PMc + VA (5 mg/kg PM, intratracheal instillation; and 10 mg/kg vanillic acid, gavage for 10 days). PM10 was instilled into the trachea in two stages, within 48 hr. After isolating the hearts and transfer to a Langendorff apparatus, hearts were subjected to 30 min ischemia and 60 min reperfusion. Hemodynamic parameters (±dp/dt, LVSP, LVDP, and RPP), production of lipid peroxidation (MDA), and infarct size were assessed.
Results:
A significant decrease in ±dp/dt, LVSP, LVDP and RPP occurred in PM groups. A significant increase in MDA and myocardial infarct size occurred in PM groups. A significant increase in LVDP, LVSP, ±dp/dt, RPP and decrease in infarct size, MDA, and myocardial dysfunction was observed in groups that received vanillic acid after ischemia–reperfusion.
Conclusion:
It was demonstrated that PM10 increases MDA, as well as the percentage of cardiac infarct size, and has negative effects on hemodynamic parameters. This study suggests that vanillic acid may serve as an adjunctive treatment in delaying the progression of ischemic heart disease.
Keywords: Hemodynamic parameters, Infarct size, Ischemia–reperfusion, Malondialdehyde Particulate matter, Vanillic acid
Introduction
Myocardial damage, a primary pathological change of coronary artery disease due to ischemia-reperfusion (I/R), is a major problem that needs attention (1). Although reperfusion after coronary occlusion improves heart functions and reduces infarct size, reperfusion after a certain time period of ischemia may aggravate cardiac contractile dysfunction and could change myocardial metabolism. The development of new therapeutic methods is important for I/R injury (2).
Studies show isolated hearts subjected to I/R have been associated with some changes in hemodynamic parameters such as decreased rate of pressure development during myocardial contraction (+dP/dt), left ventricular developed pressure (LVDP), rate of pressure decline during myocardial relaxation (-dP/dt), and increased left ventricular end diastolic pressure (LVEDP) (3).
There is some evidence indicating that particulate matter exposure can promote cardiac ischemia in animal (4, 5) and susceptible individuals (6). Acute increases in ischemic heart disease events have been observed consistently, 1 to 2 hr after exposure to elevated particulate matter (PM). Traffic-related PM is related to the incidence of ST-segment depression and the risk for ischemia increases within the first month after a cardiac event in diabetics (7).
Among the cohort studies that provided relevant results, a relationship was found between long-term exposure to high PM2.5 levels and increased risk for ischemic heart disease (8).
Heavy metals which are transported by particles (such as chromium, lead, arsenic, and cadmium) anions and cations are able to cause significant cardiovascular effects. Heavy metals associated with PM10 play a significant role in air pollution. Natural (minerals, volcanic dust, and so forth) and anthropogenic (dyeing industries, batteries, and metal plating) sources release various chemical forms of heavy metals into the environment through different routes (9).
Ahvaz is located in the southwest of Iran with about 250 mm average yearly rainfall (10). This city is located in an arid area neighboring Iraq, Kuwait, and Saudi Arabia, which are the major sources of dust storms in the Middle East (11).
Ischemia-reperfusion is the outcome of imbalance between the formation of ischemic factors and the accessibility of defensive factors such as endogenous antioxidants in the heart (12).
Vanillin, a compound used in drugs, cosmetics, and foods, has multiple functions as anti-metastatic (13), anti-melanogenesis (14) and anti-angiogenetic (15). Vanillic acid (VA, C8H8O4) is an oxidized form of vanillin and a phenolic acid. Vanillic acid was recognized in many plants such as Juglans regia L, C. murale, orchard grass, and Melilotus messanensis. Vanallic acid may be used as a natural antioxidant instead of synthetic ones which have a toxic potential (16). Researchers demonstrated the effectiveness of VA on lipid peroxidation, indicated by malondialdehyde (MDA) reduction, and endogenous antioxidant enzymes improvement, indicated by increased superoxide dismutase (SOD), catalase (CAT) glutathione peroxidase (GPx), and total antioxidant capacity (TAC) in the isolated rat hearts exposed to I/R (17). The aim of this study was to evaluate the effects of PM on hemodynamic parameters, MDA, and infarct size induced by global I/R injury in isolated rat heart and also to examine the cardioprotective effects of VA.
Materials and Methods
Materials
VA and heparin sodium were purchased from Sigma-Aldrich Co. (USA) and ketamine HCl (10%) and xylazine (2%) from Alfasan Co. (Netherlands). Krebs salts were obtained from Merck Co. (Germany). MDA kits were purchased from Zell Bio GmbH Co. (Germany), triphenyltetrazolium chloride (TTC) from Sigma-Aldrich Co. (USA), and PM10 (particles with aerodynamic diameter<10 μm) were collected from Ahvaz (18).
Animals and treatments
Eighty adult male Wistar rats (body weight, 250–300 g) were randomly divided into 8 experimental groups (n=10) as follows: Control group (1 ml normal saline, gavage, 10 days), VAc group (10 mg/kg of VA, gavage, 10 days) (14), sham group (0.1 ml normal saline, intratracheal instillation), VA group (10 mg/kg vanillic acid, gavage for 10 days; and 0.1 ml intratracheal instillation of saline), PMa group (0.5 mg/kg PM, intratracheal instillation), PMb group (2.5 mg/kg PM, intratracheal instillation), PMc group (5 mg/kg PM, intratracheal instillation) (15), PMc + VA group (5 mg/kg PM, intratracheal instillation; and 10 mg/kg vanillic acid, gavage for 10 days).
The groups were maintained under similar conditions (12 hr dark-light cycle at 22 ± 2 °C) and provided food and water ad libitum. VA was separately suspended in normal saline and was given to the rats by a gavage needle for ten days. The control group received normal saline for the same duration by gavage. Animals were maintained in the animal house of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. They were treated in accordance with the guidelines of Animal Care and Use of the Ethical Committee of Laboratory Animals of Ahvaz Jundishapur University of Medical Sciences (no. ajumsAPRC-9316).
Area of study
Ahvaz, well-known for PM equal or less than 10 micrometers (19), is located in the southwest of Iran. It is the capital of Khuzestan province, which is very close to Iraq, Kuwait, and Saudi Arabia. It is located at 31° 20 N, 48 ° 40 E geographically and has 18 meters elevation above sea level. Industrial activities due to Ahvaz Steel Company, carbon black, National Drilling Company, gas, oil, and petroleum refineries are gathered together in this city so the ambient air is polluted. Ahvaz has suffered from dust storms during the last decade (20).
Sampling procedure
One sample was taken by using a high volume PM10 sampler (Tisch Environmental, INC.145 South miamai AVE) on July 13, 2014, a dust storm day. Dust storm days were defined based on speed, visibility, wind, and PM10 concentration that were reported by Hoffmann et al (21). This type of dust storm is categorized as DS2 according to Hoffmann classification.
The high volume PM10 sampler was equipped with a quartz filter. The sampling device filled with the filter was placed on the rooftop of the Health Faculty of Ahvaz Jundishapur University of Medical Sciences at an approximate height of 10 m above the ground in order to assuage any barrier to the air flow.
It was calibrated by an orifice manometer and changeable framework. The device was operated with a flow rate of 1.2-1.8 m3/min for 16 hr. Filter preparation was conducted based on the procedure presented by researchers (22, 23).
Sample preparation for elemental analysis
After sampling and incision of exposed fiberglass filter it was placed in a Teflon container, then a mixture of hydrofluoric acid, HCL, and nitric acid was added. Afterward, the filter was digested in a hot oven at 170 °C for 4 hr. Then, the head piece of the Teflon container, places on a heater at 95-100 °C, was opened to evaporate all the remaining acids inside it. After cooling, distilled water and concentrated nitric acid (ratio 9:1 V %) were added and shaken for 15 min. The solution was filtered through a Whatman-42 filter paper. By distilled water, dilatation of the resultant solution was performed to 25 ml and stored in a sterile, clean plastic bottle at 4 °C until use (24). The digested specimen were analyzed for purpose of heavy metal detection by inductively coupled plasma atomic emission spectroscopy (ICP-AES; model: ARCOUS, Germany) (20).
PM intratracheal instillation
At first, the PM was recovered from the filter using a surgical blade (25). Each particle sample was suspended in saline at the necessary concentration and mixed continuously for 20 min before use (26). Animals were anesthetized with intraperitoneal (IP) injection of ketamine-xylazine, and then were intubated, and ventilated. 0.1 ml of saline and PM, was instilled into the trachea through the intubation tube. The rats were conjunct to the ventilator for 5 min to ensure the efficiency of the intratracheal instillation. 48 hr later, animals were anesthetized with IP injection of 50 mg/kg ketamine and 5 mg/kg xylazine. Heparin sodium, 1000 units, was injected IP into the rats to avoid blood coagulation and 0.1 ml of saline and PM, was instilled into the trachea through a fine intubation tube. The rats were conjunct to the ventilator for 5 min to make confident the efficiency of the intratracheal instillation. Thirty minutes later, the hearts were isolated.
Preparation of isolated hearts
After cannulation of the trachea, the rats were ventilated with room air using a rodent ventilator (UGO BASILE, model: 7025). The thoracic cage was opened and a steel cannula was inserted into the aorta and tightened with a suture. The hearts were quickly excised and transmitted to a Langendorff perfusion apparatus. The isolated hearts were perfused at 37±0.1°C and a constant 10 ml/min flow. The perfusion Krebs Henseleit buffer consisted of KCl (4.75 mM mM/l), NaCl (118 mM/l), CaCl2 (1.75 mM/l), MgSO4 (1.2 mM/l), KH2PO4 (1.18 mM/l), Glucose (11.1 mM/l), and NaHCO3 (25 mM/l) in distilled water at pH of 7.4, equilibrated by 95% O2 and 5% CO2. For each experiment, new perfusion buffer was filtered via 1.2-µm microfiber filter (GF/C glass filters; Whatman). Hearts were perfused for thirty min before the induction of ischemia to allow stabilization of coronary perfusion pressure and then subjected to 30 min of no-flow ischemia followed by 60 min of reperfusion (27). By a latex balloon inflated into the left ventricle, LVP was measured at the diastolic pressure of 5–10 mmHg, connected to a transducer. Hemodynamic parameters, such as LVDP (LVSP-LVEDP), ±dp/dt, left ventricular systolic pressure (LVSP), and rate pressure product [LVDP * heart rate) =RPP], were monitored continuously by a Power Lab system (AD-Instruments) (28).
Measurement of infarct size
At the end of each experiment, the hearts were extracted and frozen at -20 °C for 2 hr and cut into 2 mm-thick slices and incubated with 1% TTC (Sigma-Aldrich Co.) for 15 min at 37 °C. Then, 2 mm of the slices were incubated in formalin (10%) for 60 min. By using image J software, the infarct size was expressed as the ratio (%) of the infarct zone to the risk zone (29).
Lipid peroxidation
The hearts were removed and frozen in liquid nitrogen and stored at -80 °C for measuring MDA. Frozen tissue samples were quickly homogenized in 50 Mm ice cold 10% phosphate -buffers saline. Then they were centrifuged at 14000×g for 15 min at 4°C. The supernatants were separated and used for enzyme activities assays by kit MDA Zell Bio. Absorbance was read at 532 nm.
Statistical analysis
Results were analyzed using Graph Pad Prism6 and shown as mean±SEM. Between groups, One-way ANOVA or repeated measurement ANOVA followed by LSD were performed for multiple comparison tests. P<0.05 was considered statistically significant.
Results
PM10 level and its heavy metal contents
Heavy metal content of PM10 and its concentration that was used in this study are presented in Table 1. Dust storm days were defined based on visibility, wind speed, and concentration of PM10 which were reported by Hoffmann et al. (21) and categorized as DS2. In the present study, Cadmium had the lowest and aluminum showed the highest concentration during this dust storm (Table 1). Although the concentrations of cobalt and chromium are negligible and near to cadmium, the concentrations of zinc and lead are notable. The HYSPLIT model indicated that the dust had originated in Iraq (31).
Table 1.
Concentration and heavy metal composition of PM10 during a dust storm day in Ahvaz (July 13, 2014)
| Pollutant | (µg/m3) |
|---|---|
| PM10 | 575 |
| Cd | 0.7 |
| Co | 1.37 |
| Cr | 1.7 |
| Ni | 2.07 |
| Pb | 4.75 |
| Zn | 6.2 |
| Al | 27.9 |
The hemodynamic parameters
Eighty hearts with 1.06±0.07 g weight average were used in this experiment. To analyze whether vanillic acid was capable of preserving cardiac performance, hemodynamic parameters such as LVDP, ±dp/dt, LVSP, and RPP were measured.
There were significant differences in LVSP before and after ischemia between the sham and receiving PM groups and between VA and PM groups. The PM caused a significant decrease in LVSP before and after I/R, and this decline was more severe after ischemia. A significant increase in LVSP before and after ischemia was shown by using vanillic acid (Figure 1).
Figure 1.
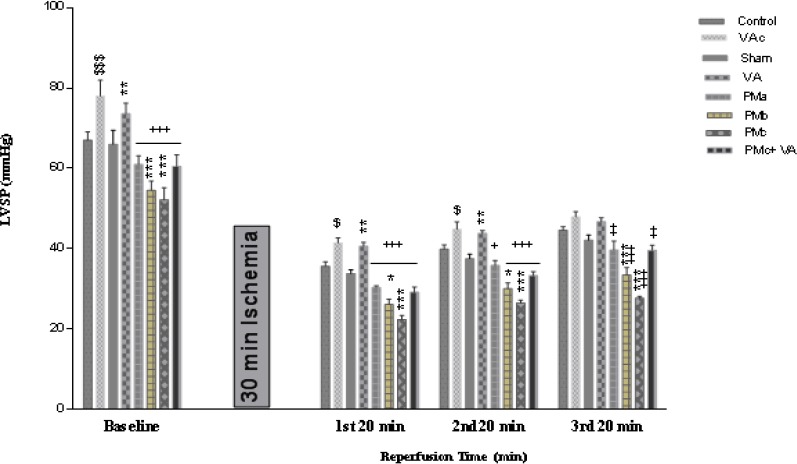
Effect of PM and vanillic acid on LVSP. Results are expressed as mean±SEM of 10 hearts per group. Control (1 ml normal saline, gavage, 10 days), VAc (10 mg/kg of VA, gavage, 10 days), Sham (0.1 ml normal saline, intratracheal instillation), VA (10 mg/kg VA, gavage, 10 days +0.1 ml normal saline, intratracheal instillation), PMa (0.5 mg/kg PM), PMb (2.5 mg/kg PM), PMc group (5 mg/kg PM), PMc + VA group (5 mg/kg PM + 10 mg/kg VA, gavage, 10 days). Repeated measurement ANOVA was used followed by the LSD test. $ P<0.05, $$$ P<0.001 vs. Control group. *P<0.05, **P<0.01, ***P<0.001 vs. Sham group. +P<0.05, ++P<0.01, +++P<0.001 vs. VA group
A significant reduction in LVDP before and after ischemia in PM groups was observed. After ischemia, this reduction effect was more noticeable than before ischemia. This decline was more severe in the PMc (5 mg/kg PM) group. The VA group in comparison with the rest of the groups showed a significant increase in LVDP before and after I/R (Figure 2).
Figure 2.
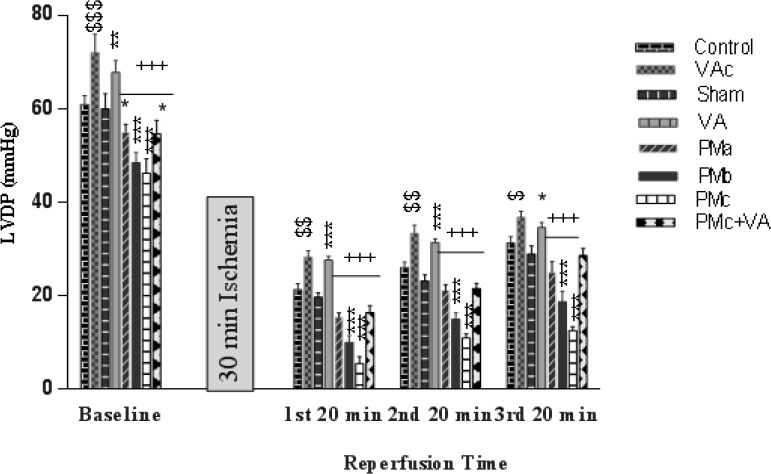
Effect of PM and vanillic acid on LVDP. Results are expressed as mean ± SEM of 10 hearts per group. Control (1 ml normal saline, gavage, 10 days), VAc (10 mg/kg of VA, gavage, 10 days), Sham (0.1 ml normal saline, intratracheal instillation), VA (10 mg/kg VA, gavage, 10 days +0.1 ml normal saline, intratracheal instillation), PMa (0.5 mg/kg PM), PMb (2.5 mg/kg PM), PMc group (5 mg/kg PM), PMc + VA group (5 mg/kg PM + 10 mg/kg VA, gavage, 10 days). Repeated measurement ANOVA was used followed by the LSD test. $ P<0.05, $$ P<0.01, $$$ P<0.001 vs. Control group. *P<0.05, **P<0.01, ***P<0.001 vs. Sham group. + P<0.05, ++ P<0.01, +++ P<0.001 vs. VA group
Myocardial contractility (±dp/dt) almost decreased in groups receiving PM before and after I/R, this reduction effect was more noticeable after ischemia than before it. Although this decline was more severe in the PMc (5 mg/kg PM) group before Ischemia, after ischemia all doses of PM similarly reduced it in comparison with the sham group. Vanillic acid increased the myocardial contractility significantly (Figures 3, 4).
Figure 3.
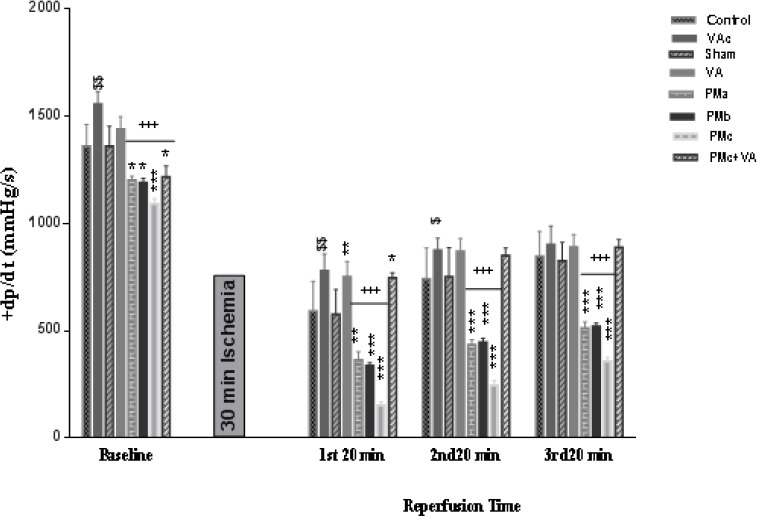
Effect of PM and vanillic acid on +dp/dt. Results are expressed as mean±SEM of 10 hearts per group. Control (1 ml normal saline, gavage, 10 days), VAc (10 mg/kg of VA, gavage, 10 days), Sham (0.1 ml normal saline, intratracheal instillation), VA (10 mg/kg VA, gavage, 10 days +0.1 ml normal saline, intratracheal instillation), PMa (0.5 mg/kg PM), PMb (2.5 mg/kg PM), PMc group (5 mg/kg PM), PMc + VA group (5 mg/kg PM + 10 mg/kg VA, gavage, 10 days). Repeated measurement ANOVA was used followed by the LSD test. $ P<0.05, $$ P<0.01 vs. Control group. *P<0.05, **P<0.01, ***P<0.001 vs. Sham group. +P<0.05, ++P<0.01, +++P<0.001 vs. VA group
Figure 4.
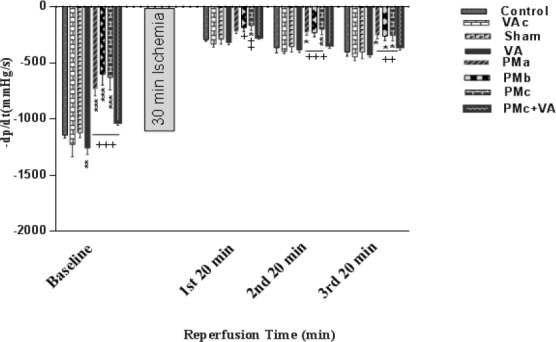
Effect of PM and vanillic acid on (-dp/dt). Results are expressed as mean ± SEM of 10 hearts per group. Control (1 ml normal saline, gavage, 10 days), VAc (10 mg/kg of VA, gavage, 10 days), Sham (0.1 ml normal saline, intratracheal instillation), VA (10 mg/kg VA, gavage, 10 days +0.1 ml normal saline, intratracheal instillation), PMa (0.5 mg/kg PM), PMb (2.5 mg/kg PM), PMc group (5 mg/kg PM), PMc + VA group (5 mg/kg PM + 10 mg/kg VA, gavage, 10 days). Repeated measurement ANOVA was used followed by the LSD test. *P<0.05, **P<0.01, ***P<0.001 vs. Sham group. + P<0.05, ++ P<0.01, +++P<0.001 vs. VA group
RPP decreased in groups receiving PM after, but before ischemia, in comparison with the sham groups, no change was observed. Pretreatment with vanillic acid (10 mg/kg) increased RPP significantly before and after I/R (Figure 5).
Figure 5.
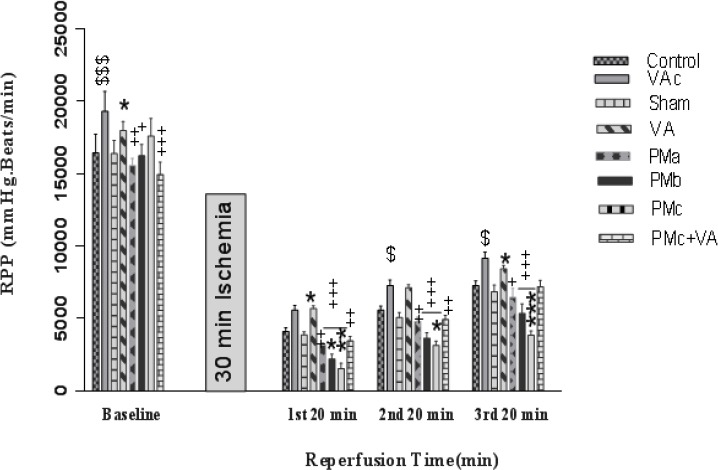
Effect of PM and vanillic acid on RPP. Results are expressed as mean±SEM of 10 hearts per group. Control (1 ml normal saline, gavage, 10 days), VAc (10 mg/kg of VA, gavage, 10 days), Sham (0.1 ml normal saline, intratracheal instillation), VA (10 mg/kg VA, gavage, 10 days +0.1 ml normal saline, intratracheal instillation), PMa (0.5 mg/kg PM), PMb (2.5 mg/kg PM), PMc group (5 mg/kg PM), PMc + VA group (5 mg/kg PM + 10 mg/kg VA, gavage, 10 days) Repeated measurement ANOVA was used followed by the LSD test. $ P<0.05, $$$ P<0.001 vs. Control group. *P<0.05, **P<0.01, ***P<0.001 vs. Sham group. +P<0.05, ++P<0.01, +++P<0.001 vs. VA group
In general, all groups receiving PM had destructive effects on mentioned hemodynamic parameters but was shown to have the greatest effect on the PMc group, and vanillic acid could improve them.
Infarct size
Infarct size is expressed as the percentage ratio of the infarct area to the total area. The infarct size, in the sham group, was 24±0.18%. A significant increase was observed in the infarct size for those groups that received PM. The administration of vanillic acid reduced the area of the infarct size to 19.56 ± 0.11 in the PMc+VA group (Figure 6).
Figure 6.
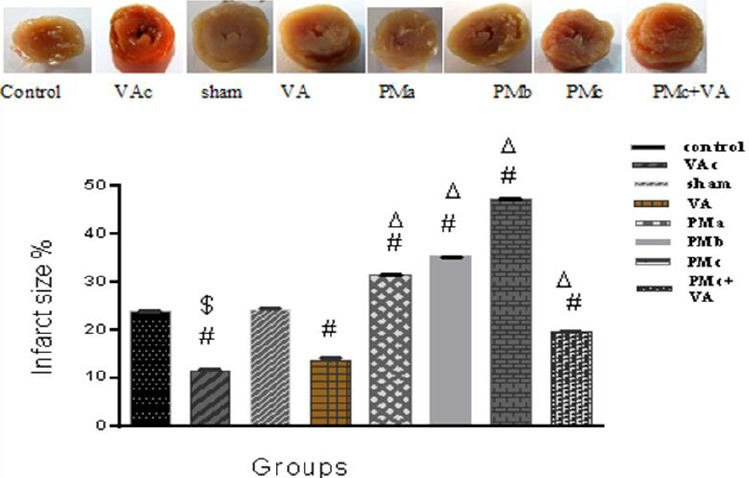
Effect of vanillic acid and PM on myocardial infarct size. Results are expressed as mean ± SEM of 10 hearts per group. Control (1 ml normal saline, gavage, 10 days), VAc (10 mg/kg of VA, gavage, 10 days), Sham (0.1 ml normal saline, intratracheal instillation), VA (10 mg/kg VA, gavage, 10 days +0.1 ml normal saline, intratracheal instillation), PMa (0.5 mg/kg PM), PMb (2.5 mg/kg PM), PMc group (5 mg/kg PM), PMc + VA group (5 mg/kg PM + 10 mg/kg VA, gavage, 10 days). One-way ANOVA was used followed by the LSD test. $ P<0.001 vs. Control group, # P<0.001 vs. Sham group, Δ P<0.001 vs. VA group
Level of MDA
The levels of MDA in the groups that were exposed to I/R are demonstrated in Figure 7. The obtained data in the group receiving PM showed that the amounts of MDA had a significant increase. This increase in the PM3 group was more than in other groups. The amounts of MDA obtained from all three groups which received VA showed a more significant decline in comparison with the other groups (Figure 7).
Figure 7.
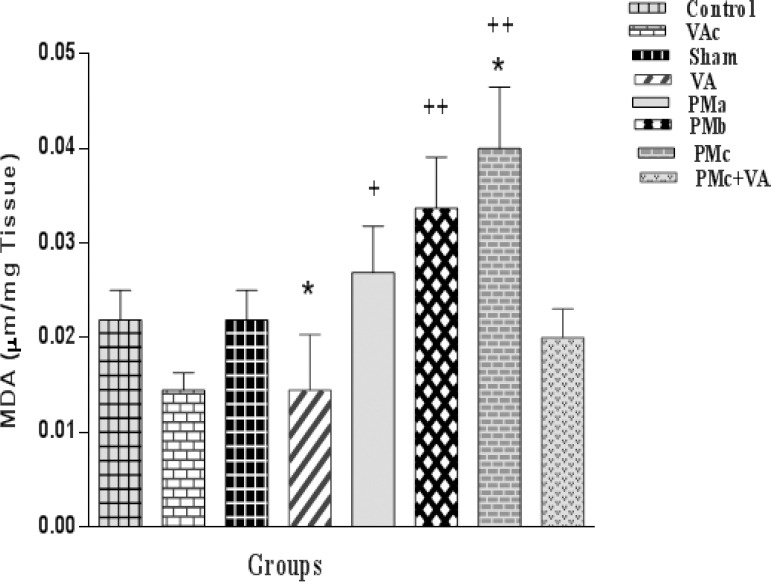
Effect of PM and vanillic acid on MDA. Results are expressed as mean±SEM of 10 hearts per group. Control (1 ml normal saline, gavage, 10 days), VAc (10 mg/kg of VA, gavage, 10 days), Sham (0.1 ml normal saline, intratracheal instillation), VA (10 mg/kg VA, gavage, 10 days +0.1 ml normal saline), PMa (0.5 mg/kg PM), PMb (2.5 mg/kg PM), PMc group (5 mg/kg PM), PMc + VA group (5 mg/kg PM + 10 mg/kg VA, gavage, 10 days). One way ANOVA was used followed by the LSD test. *P<0.05 vs. the Sham group. + P<0.05, + +P<0.001 vs. VA group
Discussion
Ischemia-reperfusion was related to the contractile function suppression as indicated by a decreased LVDP, impairment of the inotropic force of myocardial contraction and relaxation, and an increased LVEDP in isolated hearts (30).
In our study, PM10 was collected from Ahvaz, Iran. PM exposure had destructive effects on hemodynamic parameters such as significant reduction in LVSP, LVDP, and ±dp/dt, and a significant reduction in RPP after ischemia, and this decline was more severe after ischemia. The increase in production of lipid peroxidation and cardiac infarct size was shown in rats of PM groups.
The data suggest PM exposure may potentially increase the sensitivity of the myocardium to heart ischemia, possibly through impairing blood flow and perfusion (7).
In the Bagate et al. study in 2006 on I/R in rat hearts of spontaneously hypertensive rats that were exposed to PM, a significant reduction was shown in LVDP before and after at 4 hr post exposure. Bagate et al’s. study found evidence that exposure to PM affects the recovery of some cardiac parameters after ischemia. It may be caused by direct effect of soluble metals on the heart as a result of calcium homeostasis, or a significant role of pulmonary inflammation (31). In the study of the potential for ultrafine PM isolated from ambient air to exert an effect on the vascular reactivity, recovery of the myocardium, and oxidative state of the myocardium from Ischemia-reperfusion, it was shown that it has noteworthy factors in determining the outcome of an adverse cardiac event (32). Cardiovascular effects of PM may be linked to pulmonary, endothelial cell dysfunction, systemic inflammation, and oxidative stress accelerated atherosclerosis and changed cardiac autonomic function (33-34).
Although PM is controlled by mass concentration, the facet of PM most unsafe to cardiovascular health may not be known only by mass measurement. The properties of PM (e.g. oxidative stress potential, surface area, solubility, particle, charge, lung deposition, stability within the biological tissues, and atmosphere) are related to chemical composition and size/morphology (35,7). PM is composed of many compounds including elemental or black carbon, organic carbon species, and trace metals (e.g. arsenic and lead) (7).
Heavy metals can lead to diseases in human. The amounts in dust storm may arise from different sources such as soil, industries, and transportation systems. In Ahvaz, predominant emitters such as the steel factory, private administrative sectors, and transportation activities were seized during dust storm days. As a result, the heavy metals originated from erosion of crustal sources of ground nearby Ahvaz and countries adjoining Iran (36).
n the current study, as it is mentioned in Table 1, cadmium had the lowest and aluminum had the maximum concentration during this dust storm. Although the concentrations of zinc and lead were notable, the concentrations of cobalt and chromium were negligible and near to that of cadmium. This category of dust storm is determined as DS2, according to Hoffmann’s classification (21). Origination of these heavy metals was erosion of crustal sources of grounds nearby Ahvaz and countries neighboring Iran. The HYSPLIT model indicated the dust storm had originated in Iraq (22).
Phenolic acids such as vanillic acid, as natural antioxidant compounds, have been identified in vegetables, fruits and some other plants (34). The high capability of phenolic compounds to neutralize the active oxygen species is related to the number of hydroxyl groups in the aromatic ring and conjugated double bonds, mostly attributed to flavonoids and cinnamic acid derivatives. In one study vanillic acid pretreatment showed significant protective effects on the antioxidant system, cardiac troponins, electrocardiogram, lipid peroxidation, and expressions of interleukin-1β, interleukin-6, and tumor necrosis factor-α gene in the heart of isoproterenol-induced cardiotoxic rats. (38-40).
In our study, vanillic acid significantly preserved the hemodynamics and maintained ventricular functions. This was indicated by the amelioration in −dP/dt and +dP/dt, which indirectly suggests an increased ±dp/dt and ventricular filling resulting in increased cardiac output. PM10 had a significant reduction in LVDP, LVSP, ±dp/dt, and RPP after ischemia, but in groups that received PM and vanillic acid together, the above parameters were partly improved, which indicates the cardioprotective effect of vanillic acid on hemodynamic parameters.
Lipid peroxidation refers to the oxidative reduction of lipids when the free radicals steal electrons from the lipids in the cell membrane following cell damage (13). SOD, CAT, and GPx are the main endogenous antioxidants that have a vital effect in the cellular defense against oxidative stress (17). Our previous study demonstrated that VA decreases MDA and modifies antioxidant enzymes such as increased SOD, GPx, CAT, and TAC in the hearts exposed to I/R (17).
In this study, a significant decline in the size of the infarcted area and MDA level in three groups that received vanillic acid was observed. The cardioprotective effect of vanillic acid is probably due to its antioxidant properties against harmful effects of PM.
Further research needs to be done to find other possible mechanisms and pathways that might be directly or indirectly connected with the cardioprotec-tive role of vanillic acid.
Conclusion
In this study, it was shown that PM10 has damaging effects on hemodynamic parameters, and increased percentage of cardiac infarct size was seen. A significant increase was shown in the lipid peroxidation. This indicates that the mechanism of PM action is possibly related to free radicals Production. The present outcomes suggest that vanillic acid may help as an adjunctive therapy in delaying the progression of ischemic heart disease.
Acknowledgment
The source of data used in this paper was the PhD thesis of Mrs Esmat Radmanesh, a student of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Authors gratefully acknowledge the help and financial support of Physiology Research Center of Ahvaz Jundishapur University of Medical Sciences (no. ajums APRC-9316).
References
- 1.Canbaz S, Duran E, Ege T, Sunar H, Cikirikcioglu M, Acipayam M. The effects of intracoronary administration of vitamin E on myocardial ischemia-reperfusion injury during coronary artery surgery. Thorac Cardiovasc Surg. 2003;51:57–61. doi: 10.1055/s-2003-38983. [DOI] [PubMed] [Google Scholar]
- 2.Kinugasa Y, Ogino K, Furuse Y, Shiomi T, Tsutsui H, Yamamoto T, et al. Allopurinol improves cardiac dysfunction after ischemia-reperfusion via reduction of oxidative stress in isolated perused rat hearts. Circ J. 2003;67:781–82. doi: 10.1253/circj.67.781. [DOI] [PubMed] [Google Scholar]
- 3.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000;47:446–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 4.Holland NA, Fraiser CR, Sloan RC, 3rd, Devlin RB, Brown DA, Wingard CJ. Ultrafine particulate matter increases cardiac ischemia/reperfusion injury via mitochondrial permeability transition pore. Cardiovasc Toxicol. 2017 doi: 10.1007/s12012-017-9402-6. doi:10.1007/s12012-017-9402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cozzi E, Hazarika S, Stallings HW, 3rd, Cascio WE, Devlin RB, Lust RM, et al. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2006;291:H894–903. doi: 10.1152/ajpheart.01362.2005. [DOI] [PubMed] [Google Scholar]
- 6.Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis. 2016;8:E8e19. doi: 10.3978/j.issn.2072-1439.2015.11.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook RD, Rajagopalan S, Pope C A, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, et al. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 8.Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 9.Bajpai R, Mishra G K, Mohabe S, Upreti D K, Nayaka S. Determination of atmospheric heavy metals using two lichen species in Katni and Rewa cities, India. J Environ Biol. 2011;32:195–159. [PubMed] [Google Scholar]
- 10.Goudarzi GH, Zallaghi E, Neissi A, Ahmadi Ankali K, Sakid A, Babaeia A, et al. Cardiopulmonary mortalities and chronic obstructive pulmonary disease attributed to ozone air pollution. Arch Hyg Sci. 2013;2:62–72. [Google Scholar]
- 11.Goudarzi GH, Shirmardi M, Khodarahmi F, Hashemi-Shahraki A, Alavi N, AhmadiAnkali K, et al. Particulate matter and bacteria characteristics of the Middle East Dust (MED) storms over Ahvaz, Iran. Aerobiologia. 2014;30:345–356. [Google Scholar]
- 12.Singh G, Rohilla A, Singh M, Balakumar P. Possible role of JAK-2 in attenuated cardioprotective effect of ischemic preconditioning in hyper homocysteinemic rat hearts. Yakugaku Zasshi. 2009;129:523–535. doi: 10.1248/yakushi.129.523. [DOI] [PubMed] [Google Scholar]
- 13.Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005;25:57–65. doi: 10.1016/j.ejps.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Chou TH, Ding HY, Hung WJ, Liang CH. Antioxidative characteristics and inhibition of α-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanumvulgare. Exp Dermatol. 2010;19:742–750. doi: 10.1111/j.1600-0625.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 15.Lirdprapamongkol K, Kramb JP, Suthiphongchai T, Surarit R, Srisomsap C, Dannhardt G, et al. Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. Agric Food Chem. 2009;57:3055–3063. doi: 10.1021/jf803366f. [DOI] [PubMed] [Google Scholar]
- 16.Hegab MM, Ghareib HR. Antioxidative effects of acetone fraction and vanillic acid from Chenopodium murale L on tomato plant. Weed Biol Manag. 2010;10:64–72. [Google Scholar]
- 17.Dianat M, Hamzavi GH, Badavi M, Samarbafzadeh A. Effects of Losartan and vanillic acid co-administration on ischemia-reperfusion-induced oxidative stress in isolated rat heart. Iran Red Crescent Med J. 2014;16e16664:1–7. doi: 10.5812/ircmj.16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golomb E, Matza D, Cummings CA, Schwalb H, Kodavanti UP, Schneider A, et al. Myocardial mitochondrial injury induced by pulmonary exposure to particulate matter in rats. Toxicol Pathol. 2012;40:779–788. doi: 10.1177/0192623312441409. [DOI] [PubMed] [Google Scholar]
- 19.Goudie AS. Desert dust and human health disorders. Environ Int. 2014;63:101–113. doi: 10.1016/j.envint.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Heidari-Farsani M, Shirmardi M, Goudarzi GH, Alavi-Bakhtiarivand N, Ahmadi-Ankali K, Zallaghi E. The evaluation of heavy metals concentration related to PM10 in ambient air of Ahvaz city, Iran. J Adv Environ Health Res. 2014;1:120–128. [Google Scholar]
- 21.Hoffmann C, Funk R, Wieland R, Li Y, Sommer M. Effects of grazing and topography on dust flux and deposition in the Xilingele grassland, Inner Mongolia. J Arid Environ. 2008;72:792–807. [Google Scholar]
- 22.Shahsavani A, Naddafi K, Jaafarzadeh HN, Mesdaghinia A, Yunesian M, Nabizadeh R, et al. Characterization of ionic composition of TSP and PM10 during the Middle Eastern Dust (MED) storms in Ahvaz, Iran. Environ Monit Assess. 2012;184:6683–6692. doi: 10.1007/s10661-011-2451-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Zhuang G, Guo J, Xu D, Wang W, Baumgardner D, et al. Sources of aerosol as determined from elemental composition and size distributions in Beijing. Atmos Res. 2010;95:197–209. [Google Scholar]
- 24.Mohd TN, Poh SC, Suratman S, Ariffin MM, Shazali NA, Yunus K. Determination of trace metals in air borne particulate matter of Kuala Terengganu, Malaysia. Bull Environ ContamToxicol. 2009;83:199–203. doi: 10.1007/s00128-009-9751-3. [DOI] [PubMed] [Google Scholar]
- 25.Manzano-Leon N, Quintana R, Sanchez B, Serrano J, Vega E, Vazquez-Lopez I, et al. Variation in the composition and in vitro pro inflammatory effect of urban particulate matter from different sites. J Biochem Mol Toxicol. 2013;27:87–97. doi: 10.1002/jbt.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kodavanti UP, Schladweiler MC, Gilmour PS, Wallenborn JG, Mandavilli BS, Ledbetter AD, et al. The role of particulate matter-associated zinc in cardiac injury in rats. Environ Health. 2008;116:13–20. doi: 10.1289/ehp.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dianat M, Esmaeilizadeh M, Badavi M, Samarbafzadeh A, Naghizadeh B. Protective effects of crocin on hemodynamic parameters and infarct size in comparison with vitamin E after ischemia reperfusion in isolated rat hearts. Planta Med. 2014a;80:393–398. doi: 10.1055/s-0033-1360383. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Villalon AL, Monge L, Fernandez N, Salcedo A. Coronary response to di adenosine penta phosphate after ischaemia–reperfusion in the isolated rat heart. Cardiovasc Res. 2009;81:336–343. doi: 10.1093/cvr/cvn321. [DOI] [PubMed] [Google Scholar]
- 29.Price AN, Cheung KK, Lim SY, Yellon DM, Hausenloy DJ, Lythgoe MF. Rapid assessment of myocardial infarct size in rodents using multi-slice inversion recovery late gadolinium enhancement CMR at 9.4T. J Cardiovasc Magn Reson. 2011;13:44. doi: 10.1186/1532-429X-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osada M, Netticadan T, Tamura K, Dhalla NS. Modification of ischemia reperfusion induced changes in cardiac sarcoplasmic reticulum by preconditioning. Am J Physiol. 1998;274:2025–2034. doi: 10.1152/ajpheart.1998.274.6.H2025. [DOI] [PubMed] [Google Scholar]
- 31.Bagate K, Meiring JJ, Gerlofs-Nijland ME, Cassee FR, Wiegand H, Osornio-Vargas A, Borm PJA. Ambient particulate matter affects cardiac recovery in a langendorff ischemia model. Inhal Toxicol. 2006;18:633–643. doi: 10.1080/08958370600742706. [DOI] [PubMed] [Google Scholar]
- 32.Cozzi E, Hazarika S, Stallings HW, 3rd, Cascio WE, Devlin RB, Lust RM, et al. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2006;291:H894–H903. doi: 10.1152/ajpheart.01362.2005. [DOI] [PubMed] [Google Scholar]
- 33.Pope CA, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 34.Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep. 2010;12:291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- 35.Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soleimani Z, Parhizgari N, Dehdari Rad H, Akhoond MR, Kermani M, Bagherian M, et al. Normal and dusty days comparison of culturable indoor airborne bacteria in Ahvaz, Iran. Aerobiologia. 2015;31:127–141. [Google Scholar]
- 37.Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–436. [Google Scholar]
- 38.Natella F, Nardini M, Di Felice M, Saccini C. Benzoic and cinnamic acid derivatives as antioxidants: structure–activity relation. J Agric Food Chem. 1999;47:1453–1459. doi: 10.1021/jf980737w. [DOI] [PubMed] [Google Scholar]
- 39.Silva FA, Borges F, Guimaraes C, Lima JL, Matos C, Reis S. Phenolic acids and derivatives: studies on the relationship among structure, radical scavenging activity and physicochemical parameters. J Agric Food Chem. 2000;48:2122–2126. doi: 10.1021/jf9913110. [DOI] [PubMed] [Google Scholar]
- 40.Stanely Mainzen Prince P, Rajakumar S, Dhanasekar K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur J Pharmacol. 2011;668:233–240. doi: 10.1016/j.ejphar.2011.06.053. [DOI] [PubMed] [Google Scholar]


