Abstract
Objective(s):
Epilepsy establishment gives rise to biochemical and morphological changes in the hippocampus. Oxidative stress, morphological changes, and mossy fiber sprouting (MFS) in the hippocampus underpin the epilepsy establishment. Eugenol is the main component of the essential oil extracted from cloves with the potential to modulate neuronal excitability. Therefore, we investigated the effect of eugenol on convulsive behavior, oxidative stress, and histological changes of the hippocampus in lithium-pilocarpine model of epilepsy.
Materials and Methods:
Male Wistar rats weighing 220–250 g were divided into 4 groups; Control, Pilocarpine, Eugenol-Pilocarpine, and Eugenol. Oxidative stress markers were assayed by a biochemical method. Nissl and Timm staining were used to show neuronal survival and MFS, respectively. Behavioral convulsions were evaluated using the modified Racine scale.
Results:
Eugenol decreased seizure stage and duration as well as mortality. Neuronal numbers were preserved by eugenol treatment in epileptic animals, while eugenol alone reduced the number by itself in all hippocampal sub-regions including DG, CA3, and CA1. Furthermore, eugenol alone increased MDA, GPx and SOD markers, while it increased MDA not only in combined treatment with pilocarpine but also in pilocarpine-treated animals. In contrast to MFS enhancement in naïve animals, eugenol partially reversed the MFS enhancement induced by pilocarpine.
Conclusion:
Eugenol could prevent behavioral convulsions and show neuroprotective effects through increasing neuronal survival probably by decreasing MFS and increasing the GPx antioxidant marker.
Keywords: Epilepsy, Eugenolm Hippocampus, Mossy fiber sprouting, Oxidative stress
Introduction
Epilepsy and epileptic disorders are distinguished by recurrent and unpredictable seizures (1) affecting one percent of people worldwide (2). Almost 30–40% of cases seem to be intractable despite various treatments (3). Mesial temporal lobe epilepsy (mTLE) is the most common type of epilepsy in the population (4) with high economical consequences every year (5). Various cellular abnormalities such as loss of pyramidal neurons of CA1 and CA3 regions of the hippocampus, mossy fiber sprouting (MFS), and granule cell layer dispersion (5) may be responsible for the pathology. Among these, MFS is the best-known form of axonal plasticity in epilepsy (6, 7), which leads to hyperexcitability and increases seizure intensity (5). On the other hand, oxidative stress, which results from an imbalance of oxidant and antioxidant factors (8, 9), is another phenomenon involved in epileptogenesis (10-13). A study showed that ROS (Reactive Oxygen Species) plays a role in neuronal toxicity-induced apoptosis and increased seizures (14).
An increasing interest is devoted to herbal remedies as a treatment for epilepsy disorders (15). Eugenol (4-allyl-2-methoxyphenol), is an aromatic compound found in the essential oils of several plants such as Rosa spp, Eugenia caryophyllata, Cinnamomum verum, Valeriana officinalis (16, 17), allspice, nutmeg, basil, and bay leaf (18, 19). It is increasingly used in medicine (20, 21), and because of its ability in modulating neuronal excitability (22) may help to cure some diseases. Gorji and Khaleghi Ghadiri in 2001 and 2002 reported that plants containing eugenol can alleviate the symptoms of some neurological disorders like recurrent unilateral and bilateral headaches and epilepsy (23, 24). Furthermore, Laurus nobilis essential oil containing eugenol has a usage in traditional medicine of Iran as an anticonvulsant agent (25, 26). Eugenol has shown an anticonvulsant effect against seizures induced by maximal electroshock and PTZ (27), which are both acute in nature without chronic precipitant consequences. Therefore, in this study, the effect of eugenol on acute convulsive behavior as well as consequent oxidative stress and histological changes in the hippocampus of the lithium-pilocarpine rat model of epilepsy were explored.
Materials and Methods
Experimental animals
Male 2.5 month old Wistar rats weighing 220–250 g were purchased (Razi Institute, Karaj, Iran) and used in this study. All experiments were done in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 23-80, revised 1996) and were approved by the research ethical standards for the care and use of animals in Damghan University. Rats were kept 5 per cage, under normal 12- 12 hr light- dark cycle, with lights on at 07:00 am, and food and water ad libitum.
Animal groups
Male rats were divided into 4 groups. 1. The Control group (Ctrl; n=12) received lithium chloride (3 meq/kg, intraperitonealy (IP)) to become comparable with the pilocarpine group. 2. Pilocarpine group (PL; n=14) received pilocarpine (30 mg/kg, IP (28)). 3. Eugenol-Pilocarpine group (PL-EUG; n=17) received eugenol (100 mg/kg, IP (22)) for 7 days (29) followed by lithium-pilocarpine on the 3rd day. 4. Eugenol group (EUG; n=16) received eugenol (100 mg/kg, IP) for 7 days. All groups received lithium and atropine at the time mentioned in the next section for the pilocarpine group.
The lithium-pilocarpine model of epilepsy
Lithium chloride (3 meq/kg, IP) was injected 20 hr before pilocarpine injection (30 mg/kg, IP), which was given repeatedly (limited to three injections per rat) every 30 min until the rats developed seizures (30). Atropine sulfate was (1 mg/kg, IP) injected 5 min prior to pilocarpine to reduce peripheral cholinergic effects (31). Then, diazepam was administered around 60 min after the onset of the status epilepticus to improve survival after the convulsion in PL and PL-EUG groups and in control and EUG groups for comparison, all in doses of 10, followed by 2.5 mg/kg, IP half an hour later. Seizures were scored in each rat upon modified Racine’s scale based on behavioral assessment of only convulsive (motor) seizures: stage 1, facial automatisms; stage 2, facial and head clonus; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing plus loss of balance and falling that was accompanied by generalized clonic (GC) convulsions (30). During one hour of behavioral observation, seizure stages (modified from Racine scales (32)), seizure latency (the time to GC convulsions; GC Latency), and GC duration were evaluated. Four weeks later, following the latent period, all hippocampal tissues were collected for biochemical and fixed using paraformaldehyde for histological evaluations.
Assessment of oxidative stress markers
After sacrificing animals and extraction of the hippocampus on day 28, oxidative markers were measured using a biochemical method. The concentration of malondialdehyde (MDA), as a marker of lipid peroxidation, was calculated by measuring thiobarbituric acid reactive substances (TBARS). The contents of superoxide dismutase (SOD) and glutathione peroxidase (GPx), as antioxidant enzymes, were measured to determine oxidative stress levels. The concentrations of MDA, SOD, and GPx were expressed as nmol/mg protein, U/mg protein, and ΔA/min/mg protein, respectively.
The protein content was measured by the Lowry method, using bovine serum albumin as the standard.
Histological studies
For Nissl staining, animals (n=5 for each group) were deeply anesthetized, on day 28, with ketamine (90%) and xylazine (10%) and perfused through the ascending aorta with 50 ml of normal saline followed by formalin (10%). Following perfusion, the brains were removed from the skulls and immersed in formalin for 24–48 hr. Then, sections were cut with a microtome with a thickness of 10 μm in a range between −3.72 and −4.44 mm from the bregma. The sections were Nisslstained with cresyl violet (Sigma). The cells of CA1, CA3, and dentate gyrus of the hippocampus were counted in the six consecutive sections using a grid (250 µm length) - installed light microscopy at a 40x magnification. The average of six sections for every region was taken as the final value for that region.
Timm stainingroup were deeply anesthetized with ketamine (90%) and xylazine (10%) and perfused through the ascending aorta with 50 ml of normal saline followed by 100 ml of sulfide solution (1.2% Na2S and 1.0% NaH2PO4) until the extremities turned blue/gray and by 100 ml of 4% paraformal-dehyde in 0.1 M phosphate buffer (pH 7.4). Following perfusion, the hippocampi were removed from the skull and were immersed in formalin for 24–48 hr. Then, the sections were cut with a microtome at a thickness of 10 μm. The slices were washed in 100%, followed by 95% alcohol and deionized water. For labeling the zinc-containing axons of the granule cells, the sections were stained with Timm stain solution under continuous agitation in the dark at room temperature for 45 min, and then at 60 °C for 20 min. Slides were washed with deionized water and sealed with a coverslip.
The Timm solution contains 120 ml of 50% gum Arabic, 20 ml of 2 M sodium citrate buffer (pH 3.7), 60 ml of 5.6% hydroquinone, and one ml of 17% silver nitrate. Timm-stained slices were scored according to 0–3 rating scale of Tauck & Nadler (33), with a score of zero indicating little or no Timm staining in the granule cell layer; 1 indicated mild, patchy staining in the granule cell layer; 2 indicated moderate continuous staining through the granule cell layer with discontinuous, punctate staining in the inner molecular layer; and 3 indicated a continuous band of intense staining in the inner molecular layer. Finally, the Timm index was calculated as average of four sections per group. In addition to Timm index, we employed the mean coloring duration of hippocampal tissues as a new variable to show the extent of MFS.
Drugs
Pilocarpine hydrochloride (cat, P6503; Cholino-mimetic) and lithium chloride (cat, 203637) from Sigma-Aldrich and diazepam and atropine sulfate (anti-cholinergic), from a local market in IRAN, were used in this research.
Statistical analysis
For statistical comparisons between groups, significance was determined using one-way ANOVA followed by Tukey’s post hoc test and Kruskal-Wallis test for non-parametric data. For statistical comparison between two independent groups, significance was evaluated by unpaired two-tailed Student’s t-test. All statistical analyses were performed using SPSS software v.16. The level of significance was set at P<0.05. The data were expressed as means ± SEM.
Results
Eugenol reduced pilocarpine-induced behavioral seizures
Eugenol increased GC latency in PL-EUG group (unpaired two-tailed Student’s t-test, P<0.05, n=7) compared to the PL group and also decreased seizure stages in the PL-EUG group compared to the PL group. The data demonstrated a reduction in GC duration of the PL-EUG group (unpaired two-tailed Student’s t-test, P<0.05, n=7) compared to the PL group. Seizures caused 61.11% mortality in the PL group, while eugenol decreased mortality in PL-EUG by 21.83% (39.28% in this group). There was only 5.88% mortality in the EUG group (Figure 1).
Figure 1.
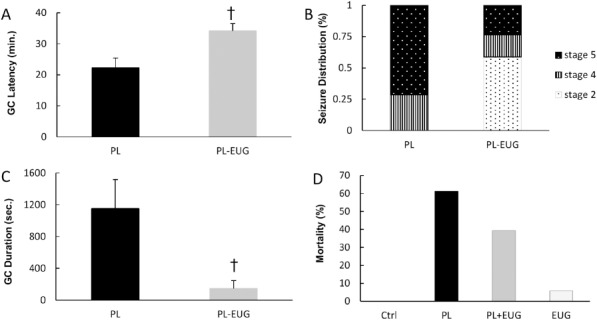
Effect of eugenol on behavioral convulsions. Eugenol increased latency to the clonic convulsions compared to pilocarpine (A). Seizure stages (B) and GC duration (C) decreased due to eugenol. Pilocarpine increased mortality, and eugenol partially reversed the effect (D). † P<0.05, PL; Pilocarpine, EUG; Eugenol, GC; Generalized Clonic Convulsions
Eugenol could reverse epilepsy-induced oxidative stress
Previous studies implied the antioxidant effect of eugenol (34, 35). To evaluate the effect of pilocarpine on oxidative stress and investigate therapeutic effects of eugenol, we assessed oxidative stress markers.
One way ANOVA showed a different MDA level between groups [F (3, 18) = 41. 799, P= 0. 0005]. There was an increase in MDA levels of PL (Tukey, P<0.05, n=6), PL-EUG (Tukey, P<0.01, n=6) and EUG (Tukey, P<0.001, n=6) groups compared to control. There were no differences in MDA levels between PL and PL-EUG groups (Figure 2A).
Figure 2.
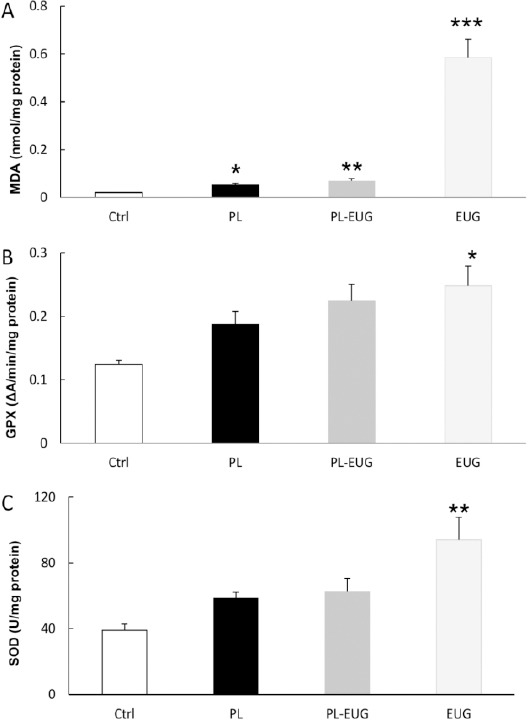
Eugenol activated the oxidative processes in the hippocampus. MDA increase was due to pilocarpine epilepsy and/or eugenol (A), while GPx increase was seen only in eugenol treated animals (B). SOD increase was shown due to eugenol in naïve animals (C).
* P<0.05, ** P<0.01, *** P<0.001, * compared to Ctrl, Ctrl; Control, PL; Pilocarpine, EUG; Eugenol
Comparing different groups using one-way analysis of variance showed a significant difference in the GPx level [F (3, 18) = 3.958, P= 0. 02]. GPx level increased in the EUG group (Tukey, P<0.05, n=6) compared to the control group. There was no difference in GPx level between PL and PL-EUG with the control group (Figure 2B).
One-way analysis of variance statistical comparison showed a different SOD content between groups [F (3, 18) = 5.865, P=0.006]. SOD content increased in the EUG group (Tukey, P<0.01, n=6) compared to controls. However, there was no difference in SOD levels between PL and PL-EUG group (Figure 2C).
Eugenol preserved neuronal population and prevented axonal sprouting
To evaluate surviving cell numbers after pilocarpine -induced seizures and the effect of eugenol, hippo-campal sections were Nissl stained after 28 days and neurons of CA1, CA3, and DG regions of the hippocampus were counted. One-way ANOVA comparison revealed a significant difference between different groups [F (3, 16)=15.703, P=0.0005]. Nissl staining showed that there was a reduction in total cell count in PL (Tukey, P<0.001, n=5) and EUG groups (Tukey, P<0.001, n=5) compared to the control group, while the neuronal count showed a reverse trend in the PL-EUG group (Tukey, P<0.01, n=5) compared to the PL group (Figure 3A).
Figure 3.
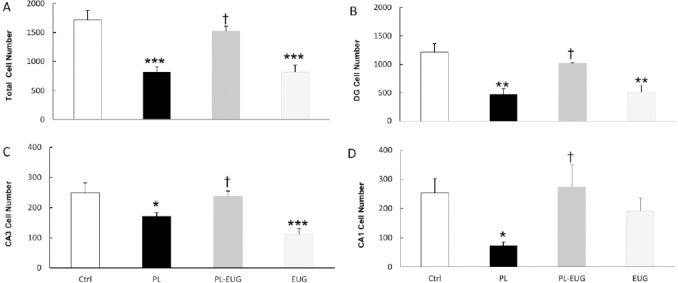
Eugenol prevented the hippocampal neuronal loss. While pilocarpine-induced neuronal loss in the total hippocampus, eugenol prevented that effect (A). Pilocarpine and eugenol increased and prevented, respectively, neuronal loss in DG (B), CA3 (C) and CA1 (D) Eugenol alone decreased neuronal survival in the total hippocampus, DG, and CA3
* P<0.05, ** P<0.01, *** P<0.001, †P<0.05, * compared to Ctrl and † compared to PL, Ctrl; Control, PL; Pilocarpine, EUG; Eugenol
Surviving neurons in DG region decreased due to pilocarpine (Tukey, P<0.01, n=5) and were compensated in the PL-EUG group (Tukey, P<0.05, n=5), while decreasing (Tukey, P<0.01, n=5) in the EUG only group (Figure 3B). Similarly, CA3 and CA1 (Tukey, P<0.05, n=5) cell count decreased in the PL group compared to control (Figures 3C and 3D) and were compensated due to EUG treatment. Moreover, Nissl staining represented cell dispersion in CA1 and CA3 regions of the hippocampus in the the PL group (Figure 4).
Figure 4.
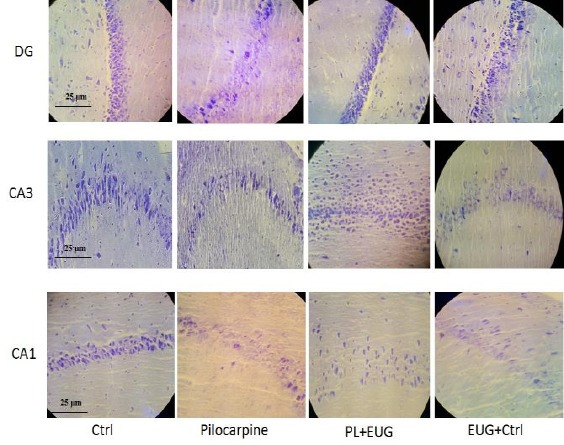
Photomicrographs showing Nissl staining of the hippocampal dentate and CA regions of epileptic animals. Control sections showed normal stained DG, CA3, and CA1 neurons (well-arranged and distributed neurons). PL sections reveal reduced and dispersed neuronal numbers, while eugenol restored the number, but not the dispersion except in DG. eugenol itself decreased neuronal survival and increased dispersion in DG, CA3, and CA1. Ctrl; Control, PL; Pilocarpine, EUG; Eugenol
To represent axonal sprouting, hippocampal sec-tions were Timm-stained and mossy fiber’s staining was scored according to Tauck & Nadler scale (33). Kruskal-Wallis comparison revealed a significant difference of the Timm index between different groups (P=0.006). Pairwise multiple comparisons of the groups showed a remarkable MSF in the PL group (P<0.001, n=4; Figure 5). Although MFS was also demonstrated in PL-EUG (P<0.01, n=4) and EUG groups (P<0.05, n=4) compared to control, there was a reduction in PL-EUG (P<0.01, n=4) compared to the PL group (Figure 5A).
Figure 5.
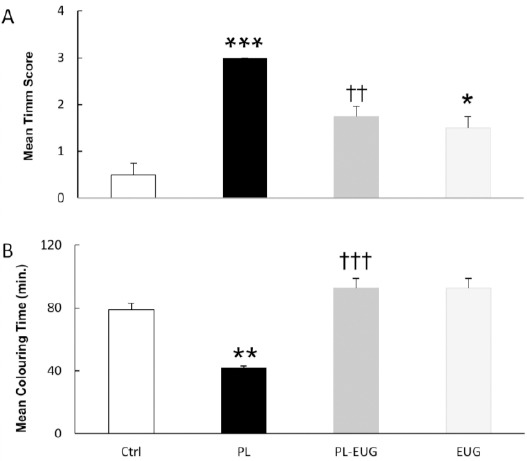
Eugenol prevented hippocampal mossy fiber sprouting in the dentate gyrus. The mean Timm index was increased due to epilepsy (PL) and was prevented after eugenol administration, while eugenol alone partially increased the index (A). Coloring time was reduced in epileptic animals while it took longer due to eugenol IP injections (B)
** P<0.01, *** P<0.001, †† P<0.01, ††† P<0.001, * compared to Ctrl and † compared to PL, Ctrl; Control, PL; Pilocarpine, EUG; Eugenol
As Timm index showed a higher grade due to epilepsy, we demonstrated a different capacity of coloring time between groups, therefore coloring time as a new variable for MFS intensity was employed. Mean coloring time of hippocampal sections using one-way ANOVA revealed different durations [F (3, 12) = 23.018, P=0.0005], with the PL group having the lowest time (colored faster) (Tukey, P<0.01, n=4) while the eugenol increased the mean coloring time in PL-EUG (Tukey, P<0.001, n=4) and EUG (Tukey, P<0.001, n=4) groups compared to the PL group (colored slowly) (Figure 5B).
Discussion
In this study, the effect of eugenol on convulsive behavior, oxidative stress, and consequent histological changes in a lithium-pilocarpine model of epilepsy were explored. We demonstrated that eugenol reduced seizure severity and animal mortality. In addition, eugenol treatment prevented the epilepsy induced reduction of neuronal numbers and MFS in all hippocampal sub-regions: DG and CAs, however, eugenol alone decreased neuronal survival. Furthermore, pilocarpine increased the MDA content, while eugenol increased not only MDA but also other markers in the naïve animals.
Eugenol revealed an increase of GC latency (the time from injection to the onset of tonic-clonic convulsions) and a decrease in GC duration. Moreover, animals developed lower seizure stages due to eugenol. These results are in agreement with previously published studies in the acute preparations (22, 36, 37). In accordance, previous studies suggested that eugenol can modulate neuronal excitability and can affect the firing of action potentials (22) through modulation of ionic channels involved in the seizure activity of pilocarpine-induced epilepsy; such as voltage gated sodium (38) and calcium channels (39-42). Therefore, it is assumed that eugenol has probably modulated these channels (22, 43) and hence decreased neuronal excitability. On the other hand, a study showed that eugenol potentiates the response of GABA in low concentrations (probably by increasing the affinity of GABA for the receptors) (44). Thus, anticonvulsant effects of eugenol may be due to its ability to decrease neuronal excitability and potentiating the inhibitory responses. This eugenol effect in modulating seizure intensity may be responsible for the decreased mortality in eugenol treated animals.
Some studies mentioned antioxidant effects of eugenol (20, 21) which was confirmed in our study with the increase of GPx and SOD antioxidant markers. In contrast, there was an elevation of the MDA lipid peroxidation index level due to eugenol in healthy and epileptic brains. Although the MDA increase in epileptic animals may indicate the behavioral and structural consequences of convulsions, its increase following eugenol treatment was unpredictable. There are reports concerning dose-related effects of eugenol from neuroprotection to neurodevastating, with low to high doses, respectively (45, 46), and the dose used in this study was assigned as high in some studies (45). It appears that in such a dose, eugenol facilitates the conversion of glucose to glycogen and hence the depletion of NADPH as the precursor of glutathione (47) which may indicate the increase of oxidative stress. On the other hand, eugenol increased SOD and GPx activity, but due to the depletion, they may not be able to convert the oxidative molecules to water. In contrast, combined pilocarpine and eugenol treatment increased GPx, which, along with the lower MDA levels demonstrated, may indicate the pilocarpine enhancing effect of the glucose production and hence conversion to NADPH shown in a study on status epilepticus induced long-term inhibition of glycogen phosphorylase (48). Therefore, we assumed that combined treatment may prevent the eugenol induced MDA buildup and the consequent decrease in oxidative outcomes.
Neuronal survival was demonstrated to be decreased after pilocarpine-induced epilepsy while treating with eugenol reversed the detrimental effect and increased the survival which indicates the neuroprotective effect of eugenol. In addition to its effects on GPx levels shown in this study and the consequent prevention of MDA buildup, eugenol may inhibit the intracellular calcium increase, cytochrome-c release, and caspase3 activation and also decrease caspase3 protein expression (34). Conversely, eugenol alone decreased neuronal population as strong as pilocarpine-induced epilepsy which may be attributable to MDA buildup and the resulting lipid peroxidation induced oxidative stress. Studies suggest that the eugenol induced ROS increase may lead to apoptosis, especially in high doses (49) and this cytotoxic effect is due to Quinone Methide production (50).
Moreover, Nissl staining revealed neuronal dispersion in CA1, CA3, and DG of the hippocampus due to pilocarpine and/or eugenol. This is previously explained by Reelin protein loss that is necessary for neural migration and lamination (51-54). The pilocarpine-induced glutamate release and prolonged BDNF gene expression promotion lead to Reelin loss and subsequent abnormal migration of hippocampal neurons (55, 56). Eugenol is also shown to increase BDNF gene expression (57), similar to seizure-induced BDNF increase (58), and therefore may lead to Reelin loss and neuronal dispersion in eugenol treated animals, however, it has shown an ameliorating effect on neuronal dispersion in lower doses through inhibition of mTORC1 expression (37).
While epilepsy results in various morphological changes in hippocampus such as MSF (7) that leads to increased tissue excitability (5), some studies suggest that inhibition of axonal sprouting decreases the excitability (5). Timm staining, a method for representing MFS, indicated seizure-induced MFS after pilocarpine treatment. Although MFS was obvious due to pilocarpine and/or eugenol, it was reduced after eugenol application, probably through increasing BDNF expression (57) and inhibiting voltage-gated calcium channels (43). The assumption of eugenol induced BDNF gene expression may explain the demonstrated MFS in control animals treated with eugenol (57).
Conclusion
Eugenol decreased pilocarpine-induced seizure intensity as well as mortality. It also increased cell survival in hippocampal sub-regions and prevented MSF in the molecular layer of the dentate gyrus in the epileptic animals. Conversely, eugenol, probably in the administered dose, could not reduce lipid peroxidation marker, MDA, and increased the neuronal loss in the hippocampus. Although eugenol exhibited anticonvulsive and neuroprotective effects at this dose in the combined treatment, it is needed to set a dose response study in future experiments, as it has shown a U-shaped curve in inhibiting stress effects, to clarify the responsible mechanisms. Moreover, measuring glutathione content in such a study will help to test its absence in eugenol neurodegeneration and its probable role in the establishment of new therapies for epilepsy induced structural damage.
Acknowledgment
This study was supported by Damghan University, Damghan, IRAN. The authors declare no conflicts of interest. The authors would like to thank Dr. Hossein Davari (Damghan University) for English editing of the manuscript.
References
- 1.Pitkanen A. Therapeutic approaches to epileptogenesis--hope on the horizon. Epilepsia. 2010;3:2–17. doi: 10.1111/j.1528-1167.2010.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodie MJ, Shorvon SD, Canger R, Halasz P, Johannessen S, Thompson P, et al. Commission on european affairs: appropriate standards of epilepsy care across Europe. ILEA. Epilepsia. 1997;38:1245–1250. doi: 10.1111/j.1528-1157.1997.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 4.Engel J., Jr Etiology as a risk factor for medically refractory epilepsy: a case for early surgical intervention. Neurology. 1998;51:1243–1244. doi: 10.1212/wnl.51.5.1243. [DOI] [PubMed] [Google Scholar]
- 5.Parent JM, Kron MM. Neurogenesis and Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 6.Paradiso B, Zucchini S, Su T, Bovolenta R, Berto E, Marconi P, et al. Localized overexpression of FGF-2 and BDNF in hippocampus reduces mossy fiber sprouting and spontaneous seizures up to 4 weeks after pilocarpine-induced status epilepticus. Epilepsia. 2011;52:572–578. doi: 10.1111/j.1528-1167.2010.02930.x. [DOI] [PubMed] [Google Scholar]
- 7.Buckmaster PS. Mossy Fiber Sprouting in the Dentate Gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 8.Castagne V, Gautschi M, Lefevre K, Posada A, Clarke PG. Relationships between neuronal death and the cellular redox status. Focus on the developing nervous system. Prog Neurobiol. 1999;59:397–423. doi: 10.1016/s0301-0082(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell BGJ. Free radicals in biology and medicine. 4th ed. New York: Oxford University Press; 2007. [Google Scholar]
- 10.Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2010;88:23–45. doi: 10.1016/j.eplepsyres.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry G, Nunomura A, Hirai K, Zhu X, Perez M, Avila J, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 12.Ashrafi MR, Shams S, Nouri M, Mohseni M, Shabanian R, Yekaninejad MS, et al. A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia. 2007;48:1750–1755. doi: 10.1111/j.1528-1167.2007.01143.x. [DOI] [PubMed] [Google Scholar]
- 13.Migliore L, Fontana I, Colognato R, Coppede F, Siciliano G, Murri L. Searching for the role and the most suitable biomarkers of oxidative stress in Alzheimer’s disease and in other neurodegenerative diseases. Neurobiol Aging. 2005;26:587–595. doi: 10.1016/j.neurobiolaging.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Radic Biol Med. 1995;18:993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- 15.Samuels N, Finkelstein Y, Singer SR, Oberbaum M. Herbal medicine and epilepsy: proconvulsive effects and interactions with antiepileptic drugs. Epilepsia. 2008;49:373–380. doi: 10.1111/j.1528-1167.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 16.Umezu T, Ito H, Nagano K, Yamakoshi M, Oouchi H, Sakaniwa M, et al. Anticonflict effects of rose oil and identification of its active constituents. Life Sci. 2002;72:91–102. doi: 10.1016/s0024-3205(02)02197-5. [DOI] [PubMed] [Google Scholar]
- 17.Cheng SS, Liu JY, Tsai KH, Chen WJ, Chang ST. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J Agric Food Chem. 2004;52:4395–4400. doi: 10.1021/jf0497152. [DOI] [PubMed] [Google Scholar]
- 18.Bender IB. Pulpal pain diagnosis--a review. J Endod. 2000;26:175–179. doi: 10.1097/00004770-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Koseoglu BG, Tanrikulu S, Subay RK, Sencer S. Anesthesia following overfilling of a root canal sealer into the mandibular canal: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:803–806. doi: 10.1016/j.tripleo.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Guenette SA, Beaudry F, Marier JF, Vachon P. Pharmacokinetics and anesthetic activity of eugenol in male Sprague-Dawley rats. J Vet Pharmacol Ther. 2006;29:265–270. doi: 10.1111/j.1365-2885.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 21.He M, Du M, Fan M, Bian Z. In vitro activity of eugenol against Candida albicans biofilms. Mycopathologia. 2007;163:137–143. doi: 10.1007/s11046-007-0097-2. [DOI] [PubMed] [Google Scholar]
- 22.Huang CW, Chow JC, Tsai JJ, Wu SN. Characterizing the effects of Eugenol on neuronal ionic currents and hyperexcitability. Psychopharmacology (Berl) 2012;221:575–587. doi: 10.1007/s00213-011-2603-y. [DOI] [PubMed] [Google Scholar]
- 23.Gorji A, Khaleghi Ghadiri M. History of epilepsy in Medieval Iranian medicine. Neurosci Biobehav Rev. 2001;25:455–461. doi: 10.1016/s0149-7634(01)00025-2. [DOI] [PubMed] [Google Scholar]
- 24.Gorji A, Khaleghi Ghadiri M. History of headache in medieval Persian medicine. Lancet Neurol. 2002;1:510–515. doi: 10.1016/s1474-4422(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 25.Aqili khorasani MS. Collection of drugs (Materia media) Tehran: Enqelab-e-Eslami Publishing and Educational Organization; 1992. [Google Scholar]
- 26.Zargari A. Medicinal Plants. Tehran: Tehran University press; 1990. [Google Scholar]
- 27.Sayyah M, Valizadeh J, Kamalinejad M. Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole- and maximal electroshock-induced seizures. Phytomedicine. 2002;9:212–216. doi: 10.1078/0944-7113-00113. [DOI] [PubMed] [Google Scholar]
- 28.Andre V, Dube C, Francois J, Leroy C, Rigoulot MA, Roch C, et al. Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia. 2007;48:41–47. doi: 10.1111/j.1528-1167.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 29.Garabadu D, Shah A, Ahmad A, Joshi VB, Saxena B, Palit G, et al. Eugenol as an anti-stress agent: modulation of hypothalamic-pituitary-adrenal axis and brain monoaminergic systems in a rat model of stress. Stress. 2011;14:145–155. doi: 10.3109/10253890.2010.521602. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Ren M, Guo J, Yang G, Long X, Hu R, et al. The inhibitory effects of Npas4 on seizures in pilocarpine-induced epileptic rats. PLoS One. 2014;9:e115801. doi: 10.1371/journal.pone.0115801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li YH, Li JJ, Lu QC, Gong HQ, Liang PJ, Zhang PM. Involvement of thalamus in initiation of epileptic seizures induced by pilocarpine in mice. Neural Plasticity. 2014;2014:1–15. doi: 10.1155/2014/675128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racine RJ. Modification of seizure activity by electrical stimulation II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 33.Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou HC, Chou FP, Lin TM, Yang CH, Sheu WH. Protective effects of eugenol against oxidized LDL-induced cytotoxicity and adhesion molecule expression in endothelial cells. Food Chem Toxicol. 2006;44:1485–1495. doi: 10.1016/j.fct.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Wie MB, Won MH, Lee KH, Shin JH, Lee JC, Suh HW, et al. Eugenol protects neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci Lett. 1997;225:93–96. doi: 10.1016/s0304-3940(97)00195-x. [DOI] [PubMed] [Google Scholar]
- 36.Muller M, Pape HC, Speckmann EJ, Gorji A. Effect of eugenol on spreading depression and epileptiform discharges in rat neocortical and hippocampal tissues. Neuroscience. 2006;140:743–751. doi: 10.1016/j.neuroscience.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 37.Jeong KH, Lee DS, Kim SR. Effects of eugenol on granule cell dispersion in a mouse model of temporal lobe epilepsy. Epilepsy Res. 2015;115:73–76. doi: 10.1016/j.eplepsyres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Su H, Yue C, Remy S, Royeck M, Sochivko D, et al. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. J Neurophysiol. 2011;105:117–129. doi: 10.1152/jn.00184.2010. [DOI] [PubMed] [Google Scholar]
- 39.Su H, Sochivko D, Becker A, Chen J, Jiang Y, Yaari Y, et al. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22:3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaari Y, Yue C, Su H. Recruitment of apical dendritic T-type Ca2+ channels by backpropagating spikes underlies de novo intrinsic bursting in hippocampal epileptogenesis. J Physiol. 2007;580:435–450. doi: 10.1113/jphysiol.2007.127670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, et al. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28:13341–13353. doi: 10.1523/JNEUROSCI.1421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graef JD, Nordskog BK, Wiggins WF, Godwin DW. An acquired channelopathy involving thalamic T-type Ca2+ channels after status epilepticus. J Neurosci. 2009;29:4430–4441. doi: 10.1523/JNEUROSCI.0198-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung ZH, Chin WH, Chen Y, Ng YP, Ip NY. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007;5:e63. doi: 10.1371/journal.pbio.0050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoshima H, Hamamoto K. Potentiation of GABAA receptors expressed in Xenopus oocytes by perfume and phytoncid. Biosci Biotechnol Biochem. 1999;63:743–748. doi: 10.1271/bbb.63.743. [DOI] [PubMed] [Google Scholar]
- 45.Won MH, Lee JC, Kim YH, Song DK, Suh HW, Oh YS, et al. Postischemic hypothermia induced by eugenol protects hippocampal neurons from global ischemia in gerbils. Neurosci Lett. 1998;254:101–104. doi: 10.1016/s0304-3940(98)00664-8. [DOI] [PubMed] [Google Scholar]
- 46.Liu Z, Niu W, Yang X, Wang Y. Effects of combined acupuncture and eugenol on learning-memory ability and antioxidation system of hippocampus in Alzheimer disease rats via olfactory system stimulation. J Tradit Chin Med. 2013;33:399–402. doi: 10.1016/s0254-6272(13)60186-7. [DOI] [PubMed] [Google Scholar]
- 47.Sartorius T, Peter A, Schulz N, Drescher A, Bergheim I, Machann J, et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS One. 2014;9:e92358. doi: 10.1371/journal.pone.0092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walling SG, Rigoulot MA, Scharfman HE. Acute and chronic changes in glycogen phosphorylase in hippo-campus and entorhinal cortex after status epilepticus in the adult male rat. Eur J Neurosci. 2007;26:178–189. doi: 10.1111/j.1460-9568.2007.05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidhya N, Devaraj SN. Induction of apoptosis by eugenol in human breast cancer cells. Indian J Exp Biol. 2011;49:871–878. [PubMed] [Google Scholar]
- 50.Pramod K, Ansari SH, Ali J. Eugenol: a natural compound with versatile pharmacological actions. Nat Prod Commun. 2010;5:1999–2006. [PubMed] [Google Scholar]
- 51.Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, et al. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- 53.Soriano E, Del Rio JA. The cells of cajal-retzius: still a mystery one century after. Neuron. 2005;46:389–394. doi: 10.1016/j.neuron.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 54.Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–119. doi: 10.1016/j.tins.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Guilhem D, Dreyfus PA, Makiura Y, Suzuki F, Onteniente B. Short increase of BDNF messenger RNA triggers kainic acid-induced neuronal hypertrophy in adult mice. Neuroscience. 1996;72:923–931. doi: 10.1016/0306-4522(96)00005-x. [DOI] [PubMed] [Google Scholar]
- 56.Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E, et al. BDNF regulates reelin expression and Cajal-Retzius cell development in the cerebral cortex. Neuron. 1998;21:305–315. doi: 10.1016/s0896-6273(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 57.Irie Y. Effects of eugenol on the central nervous system: its possible application to treatment of Alzheimer’s disease, depression, and Parkinson’s isease. Curr. Bioactive Compound; 2006;2:57–66. [Google Scholar]
- 58.Simo S, Pujadas L, Segura MF, La Torre A, Del Rio JA, Urena JM, et al. Reelin induces the detachment of postnatal subventricular zone cells and the expression of the Egr-1 through Erk1/2 activation. Cereb Cortex. 2007;17:294–303. doi: 10.1093/cercor/bhj147. [DOI] [PubMed] [Google Scholar]


