Abstract
Objective(s):
Umbelliprenin is a prenyloxy-coumarin with pharmacologically polyvalent activity. Several studies have shown Several studies have been shown its anti-inflammatory, anti-tumor, antioxidant, and antigenotoxic activities. However, the exact mechanism of action of this compound on the immune response has not yet been shown. Here, we investigated umbelliprenin effects on the predominance of Th1 and Th2 responses in normal C57/BL6 mice.
Materials and Methods:
Umbelliprenin (2.5 mg/200 µl IP) were administered to six C57/BL6 mice every other day for 8 days. Paraffin and PBS-injected mice were enrolled as solvent and control groups, respectively (n=6 mice/group). IL-10, IFN-γ, and IL-4 levels were determined in sera and also in splenocytes culture supernatants in the presence of Con A (3 µg/ml) after 72 hr. H&E staining of paraffin embedded blocks was performed for lung and liver tissues of mice.
Results:
Umbelliprenin could significantly increase the secretion of IFN-γ and IL-4 in sera and IL-10 in splenocytes cultures. Comparison of IFN-γ/IL-4 in the sera and splenocytes culture supernatants showed lower ratios in umbelliprenin treated mice than in solvent and untreated groups.
Conclusion:
The in vivo study showed that umbelliprenin could induce anti-inflammatory responses via the predominance of Th2 cells and some regulatory responses in C57/BL6 mice.
Keywords: Anti-inflammatory, Concanavalin A (Con A), Interferon gamma (IFN-γ), Prenyloxy-coumarin, Umbelliprenin
Introduction
Umbelliprenin (C24H30O3, molecular weight: 366) is a naturally occurring compound related to the simple coumarins sub-type, which is synthesized by various Ferula species (1-3). It has also been found in higher plants such as Rutaceae and Umbelliferae (3). Umbelli-prenin shows various pharmacological functions such as in vitro (4-6) and in vivo (7, 8) cancer chemopreventive, antibacterial (1), antileishmanial (2), anti-inflammatory, and anti-oxidant activities (9). Umbelliprenin’s inhibitory effects have been shown on 5-lipoxygenase (9) and on matrix metalloproteinases (MMPs) activities (10). Also, its relaxant effect on smooth muscle has been revealed in the guinea-pig (11).
An in vivo study was published on immunological properties of a similar compound, auraptene, which has the nearest structure to umbelliprenin (containing a shorter 7-prenyloxy chain than umbelliprenin) (4). Oral administration of auraptene in normal BALB/c mice could increase the production of several cytokines such as TNF-α, IL-1, IFN-γ, and IL-2 (12). However, Niu X, et al. have shown auraptene inhibitory effects on mice T cell responses and proliferation (13). Although, these two compounds share some properties they have differences in their mechanisms (14). Previously, we demonstrated the anticancer activity of umbelliprenin in the mice model of lung cancer, in C57/BL6 mice (8). Although umbelliprenin has been studied in multiple in vivo and in vitro models previously, none of them mentioned the exact effects of this compound in normal in vivo situation. As we know the kinetics and distribution of in vivo administration of this compound have not been studied yet, especially in an extended reticuloendothelial system, such as the spleen. The effect of umbelliprenin administration on the other organs such as lungs and liver has not yet been determined. As new research is increasingly interested in this compound, here for the first time we focused on the immunological and also the histo-pathological aspects of umbelliprenin administration in C57/BL6 mice, in vivo.
To explore this, here we assessed the effect of umbelliprenin on the adaptive immune response in C57/BL6 mice. We have determined the cytotoxic and proliferative effect of umbelliprenin on mice splenocytes, previously (8). Here Th1/Th2 cells modulation were investigated as well as regulatory responses in C57/BL6 mice after the umbelliprenin treatment regimen. Also, the possible effects of liquid paraffin as an umbelliprenin solvent were examined. Th1/Th2 cytokines levels were evaluated in mice sera and also in the supernatants of splenocytes cultures after 72 hr. Moreover, H&E staining of paraffin-embedded blocks of lung and liver tissues in all groups were evaluated for the histopathological aspects of umbelliprenin administration.
Materials and Methods
Preparation of umbelliprenin
Umbelliprenin (C24H30O3, MW: 366) was synthesized as previously described (3). Briefly, 7-hydroxy-coumarin (1M), trans-trans farnesyl bromide (1.5 M), and DBU (1, 8-diazabicyclo [5.4.0] undec-7-ene) (2 M) were dissolved in acetone and the mixture was stirred at room temperature for 24 hr. Then, the mixture was concentrated under reduced pressure and the concen-trate was run on a silica gel column using petroleum ether: ethyl acetate, (9:1 v/v) as a solvent system. The pooled fraction containing umbelliprenin produced white crystals with a 70% yield after solvent removal (mp=57.5-59.1°C). Umbelliprenin was dissolved in liquid paraffin just before the injections using bath sonicator (Sonorex digitec, Germany) for 30 min, at 20°C. All materials used in this study were pyrogen and endotoxin free.
In vivo study
C57BL/6 inbred female mice (6–8 weeks) were obtained from Pasteur Institute, Tehran, Iran. The mice were housed in Shiraz University of Medical Sciences’ animal house. All mice were housed and fed laboratory animal chow and tap water ad libitum according to standard animal care procedure. All animal experimental procedures were approved by the ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran.
C57BL/6 mice with an average weight of 20±2 g were divided into three groups of six mice per group. Treatments were scheduled based on intraperitonea-lly (IP) injection of paraffin as solvent (Group 1), (2.5 mg/200 µl) umbelliprenin/paraffin as test (Group 2), and PBS as untreated control (Group 3) every other day for 8 days.
Umbelliprenin LD50 determination was done using C57BL/6 inbred female mice in our previous study (8, 15). Totally, 20 mg of umbelliprenin was used for each mouse. Mice were weighed every 3 days. Five days after the last injection, the mice were sacrificed and samples of spleen, lung and liver tissues were collected.
Cytokine detection
The spleens of mice were removed and splenocytes were isolated under sterile conditions by Ficoll- Hypaque (Lymphoprep, Norway) centrifugation technique (400 g, 30 min, 20°C). Then, the mononuclear layer was collected and washed twice (1500 rpm, 10 min at RT) with RPMI-1640 medium. The total amount of 6×105 cells/well in CM10 was added to 96-well culture plates. Cells were cultured in the presence of Concanavalin A (Con A) (3 µg/ml) compared with PBS treated groups. All experiments were done in triplicate. After 72 hr, culture supernatants were harvested and frozen at -20°C. Blood from each group was collected a day before sacrifice. IL-10, IFN-γ, and IL-4 production in the culture supernatants and also in the sera of each group were determined using ELISA kit (Mabtech, Sweden) according to the manufacturer’s instructions. The absorptions were read at 450 nm with a microplate reader Gen5 (BioTek, USA).
H&E staining
Lungs and livers were collected, washed in PBS for blood removing and fixed in formalin 10% for further histological tests. Paraffin-embedded sections were stained using the hematoxylin and eosin method (16).
Statistical analysis
All values given represent mean±standard error of the mean (SEM). Statistical tests including Mann-Whitney U tests and Kruskal-Wallis test followed by Dunn’s multiple comparisons test were performed using SPSS and GraphPad Prism software packages. A P-value of less than 0.05 was considered significant.
Results
Mice weight
Mice weights were evaluated every 3 days. There was no significant difference in weight gains between any groups (Figure 1).
Figure 1.
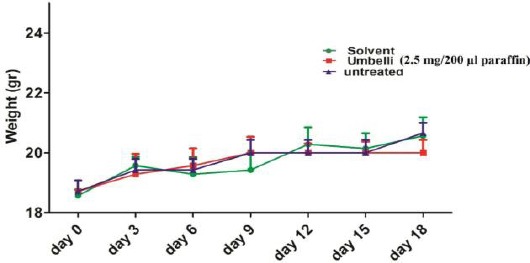
Changes in mice weight during the study. Weights of the animals were measured every three days (n=6 mice/group). The weight gain was not significantly changed in (G1) solvent, (G2) umbelliprenin (20 mg/mice), and (G3) untreated groups
Cytokine production in splenocytes culture
Con A stimulated splenocytes
In the presence of Con A, IFN-γ concentration in the umbelliprenin treated group (G2) was lower than in the untreated group (G3) (1.7 fold, P=0.1), and higher than in the solvent group (G1) (2 fold, P=0.1) (Figure 2A). The IL-4 level in umbelliprenin treated mice (G2) was decreased (1.3 fold, P=0.1) in comparison with untreated mice (G3) (Figure 2B). The IL-10 level was increased in the umbelliprenin treated group (G2) compared with the solvent (G1) (2.6 fold, P=0.1) and untreated groups (G3) (2.2 fold, P=0.1), respectively (Figure 2C). However, IL-10 concentration in the umbelliprenin treated group (G2) in the presence of Con A was increased (5 fold, P=0.05) compared with unstimulated G2 splenocytes.
Figure 2.
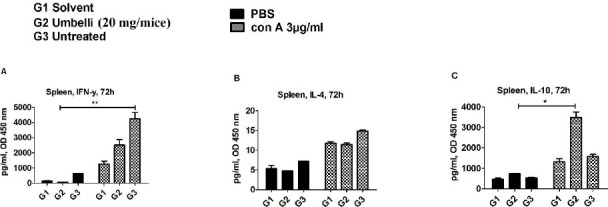
Effect of umbelliprenin on the cytokine levels of splenocytes’ culture media.
(A) IFN-γ, (B) IL-4, and (C) IL-10 secretion were shown in the culture supernatants of splenocytes in (G1) solvent, (G2) umbelliprenin (20 mg/mice), and (G3) untreated groups. All cells were cultured in the presence of Con A (3 µg/ml) compared with PBS treated groups. The results were represented as mean±SEM of two independent experiments done in triplicate. Significance levels were shown as * P<0.05
Unstimulated splenocytes
In PBS-treated splenocytes, IFN-γ concentration in the umbelliprenin group (G2) was lower than in the untreated (G3) (8.4 fold, P=0.1), and solvent groups (G1) (1.8 fold, P=0.1), respectively (Figure 2A). The IL-4 level in umbelliprenin treated mice (G2) was decreased (1.5 fold, P=0.1) in comparison with untreated mice (G3) (Figure 2B). IL-10 level was increased in umbelliprenin the treated group (G2) compared with the solvent (G1) (1.6 fold, P=0.1) and untreated groups (G3) (1.3 fold, P=0.1), respectively (Figure 2C).
Cytokine production in sera
Umbelliprenin could induce the sera production of IFN-γ (3.1 fold, P=0.027) and IL-4 (28 fold, P=0.027) significantly in comparison with the untreated group (Figure 3A and B). Sera IL-10 is not significantly (P>0.05) changed in umbelliprenin treated mice compared with other groups (Figure 3C).
Figure 3.
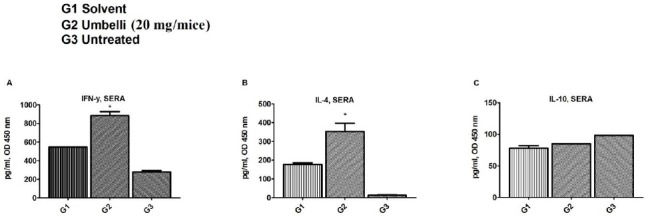
Effect of umbelliprenin on the sera cytokine levels of mice.
(A) IFN-γ, (B) IL-4, and (C) IL-10 levels were shown in the sera of (G1) solvent, (G2) umbelliprenin (20 mg/mice), and (G3) untreated groups. The results were represented as mean±SEM of two independent experiments done in triplicate. Significance levels were shown as * P<0.05
H&E results
Figure 4 shows lung sections of different groups of mice in our experiment. Interstitial pneumonitis and pulmonary hemorrhage are detectable in lung tissues of solvent and umbelliprenin treated groups (Figures 4A and B). Figure 4C shows normal lung tissue section of mice. Also, liver fatty change and inflammation were seen in the solvent treated and in umbelliprenin treated mice (Figure 4D and E). Normal liver histology was revealed in the untreated group (Figure 4F).
Figure 4.
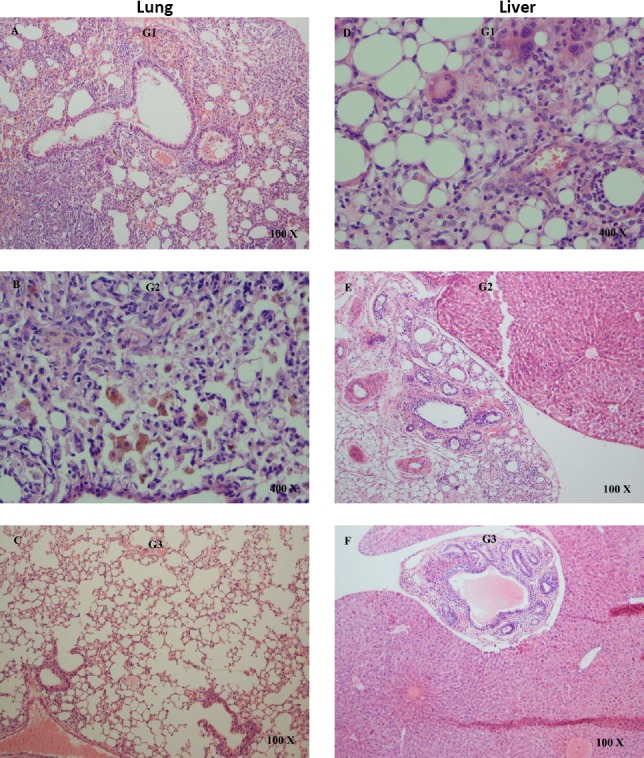
Consumption of umbelliprenin effect on tissue histology by using the hematoxylin and eosin stain (H&E). Lung tissue; (A) and (B) show interstitial pneumonitis and pulmonary hemorrhage in lung tissues of solvent and umbelliprenin treated mice, respectively, (C) normal lung tissue. Liver tissue; (D) liver fatty change and inflammation in the solvent treated mice, (E) fat accumulation in umbelliprenin treated group, and (F) normal liver tissue. G1: solvent; G2: umbelliprenin (20 mg/mice); G3: untreated
Discussion
Previously, the effects of umbelliprenin on some immune responses were assessed in mice tumor model (8). Here, the predominance of Th1 and Th2 responses were also investigated in umbelliprenin treated C57BL/6 mice. The effect of umbelliprenin on the adaptive immune response in mice was assessed using IFN-γ as Th1 cells response, IL-4 as Th2 cells hallmark, and IL-10 as an immune modulator cytokine. The main sources of IFN-γ are NK, Th1, and
NKT cells (17). IFN-γ serves to activate many genes and plays important roles in both innate and adaptive immunity. It regulates Th1 cell differentiation, function, and homeostasis but inhibits Th2 cell development (17). IFN-γ has a role in the production of chemokines and recruitment of specific effector cells to the site of inflammation (18).
IL-4 is the signature cytokine of the Th2 subset. IL-4 has an effect on diverse populations of cells such as B cells and type 2 macrophages. Depending on the target cell, IL-4 can induce pro-inflammatory, regulatory or suppressive responses. IL-4 induces Th2 cell development, which also produces IL-10 (19, 20). IL-10 is an anti-inflammatory cytokine. Most cell types can produce IL-10 more or less but the main source of this cytokine is Foxp3+ T-reg cells (21).
This investigation showed that umbelliprenin could significantly increase the secretion of IFN-γ and IL-4 in sera and IL-10 in splenocytes cultures of mice. Here, the comparison of IFN-γ/IL-10 in the spleen showed lower ratio (0.7) in umbelliprenin treated splenocytes than in solvent (0.97) and untreated (2.6) groups exposed to Con A. However, in sera, the umbelliprenin treated group showed higher ratio (10.3) of IFN-γ/IL-10 than solvent (6.9) and untreated (2.8) groups. This controversy in the production of systemic IFN-γ and splenocytes cultures may come from persistent inflammation in spleen and production of higher IL-10 as an immune regulatory cytokine. Recent studies have shown that IFN-γ producer Th1 clones, could gradually switch to IL-10 producing T cells in response to a persistent inflammation and antigen presentation (22, 23).
Also, the comparison of IFN-γ/IL-4 in the spleen of mice showed a lower ratio (218) in umbelliprenin treated splenocytes than the untreated (286) group exposed to Con A. Moreover, the comparison of IFN-γ/IL-4 in the sera of mice showed a lower ratio (2.5) in umbelliprenin treated mice than in solvent (3) and the untreated (22) groups. Thus, the predominance of Th2 and some immune regulatory responses in the spleen region can be concluded. An investigation on a similar compound, auraptene, has shown some inhibitory effects on in vitro T cell responses isolated from C57BL/6 mice (13). Auraptene could decrease IFN-γ, IL-2, and IL-4 secretion from activated T cell.γ They concluded that auraptene exhibits anti-inflammatory properties via inhibiting T cell proliferation and some inflammatory cytokines (13). However, we observed the proliferative effects of umbelliprenin on both human peripheral blood mononuclear cell (PBMNCs)(6) and mice splenocytes at lower concentrations (20 and 10 mM) (8). Thus, these two compounds could show different properties based on their concentrations (14).
A question which should be discussed was why anti-inflammatory responses of umbelliprenin are induced. To explore this, H&E staining was performed on lung and liver tissues of each group. Interstitial pneumonitis was seen in lung sections of both umbelliprenin and solvent treated groups. Also, the liver sections showed fatty change and accumulation of inflammatory cells in both solvent and umbelliprenin treated groups. These inflammatory responses could not be detected in lungs and livers of the untreated group. A hypothesis is that several injections of paraffin may stimulate inflammatory responses in liver tissues. Although umbelliprenin could induce anti-inflammatory responses, the presence of paraffin as solvent may be responsible for these inflammatory reactions. Another hypothesis may come from precise attention on cytokine profile of Th2 and Th1 in this study. Here we showed the excess of IL-4/IFN-γ ratio in umbelliprenin treated mice both in sera and spleen culture. Previous investigations have shown the IL-4 role in pathogenesis of drug-induced liver injury (DILI) using an animal model (20). DILI results from two mechanisms: direct toxic agents and their metabolites effects, or drug hapten altered native protein induced immunemediated toxicity (20). It has been shown that IL-4 induces immunemediated T cell hepatitis (24). Then its role can be modulated by the induction of IL-10. IL-4 can reduce the production of IFN-γ and increase IL-10 secretion via Th2 and T-reg cells (20). More consideration of cytokine assay showed that umbelliprenin could induce the sera production of IL-4 (28 fold) significantly in comparison with the untreated group. It can be concluded that IL-4 as the main regulator of DILI has been induced by umbelliprenin as an orchestration of all other events.
Focus on H&E staining in lung tissues of mice showed similar findings in agreement with cytokine assay. Repeated intraperitoneal injections may stimulate inflammatory responses, while decrease in IFN-γ/IL-10 and IFN-γ/IL-4 ratios may be implicated in anti-inflammatory and immune regulatory responses in the umbelliprenin treated group. Although, use of paraffin as a solvent and/or high concentration of umbelliprenin may induce mice liver injury and accumulation of inflammatory cells in the lung and liver, here we showed the positive impact of umbelliprenin on anti-inflammatory cytokine profile which can also be supported by previous findings (9, 25, 26). To exclude unwanted effects of solvents like paraffin, umbelliprenin-coated Fe3O4 magnetite nanoparticles (MNPs) may be recommended in further in vivo investigations (27).
Conclusion
Here, immunologic findings of umbelliprenin were shown in C57/BL6 mice. The predominant response to umbelliprenin treated mice was Th2; however, we could not exclude other immune modulatory effects. Umbelliprenin can induce a higher fold of IL-4 than IFN-γ in comparison with the untreated group. However, the selection of other solvents for further in vivo studies is recommended.
Acknowledgment
We would like to thank Dr Tanideh, Dr M Vahedi, and Mr Koohi for their technical support in the animal lab. The results presented in this paper were parts of a student thesis. The present study was funded by grants from Shiraz University of Medical Sciences, Shiraz, Iran (no. 89-5291) and Shiraz Cancer Research Center, Shiraz, Iran (no. ICR-87-503).
References
- 1.Iranshahi M, Shahverdi AR, Mirjani R, Amin G, Shafiee A. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z Naturforsch C. 2004;59:506–508. doi: 10.1515/znc-2004-7-809. [DOI] [PubMed] [Google Scholar]
- 2.Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, et al. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry. 2007;68:554–561. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Askari M, Sahebkar A, Iranshahi M. Synthesis and purification of 7-prenyloxycoumarins and herniarin as bioactive natural coumarins. Iran J Basic Med Sci. 2009;12:63–69. [Google Scholar]
- 4.Barthomeuf C, Lim S, Iranshahi M, Chollet P. Umbelliprenin from Ferula szowitsiana inhibits the growth of human M4Beu metastatic pigmented malignant melanoma cells through cell-cycle arrest in G1 and induction of caspase-dependent apoptosis. Phytomedicine. 2008;15:103–111. doi: 10.1016/j.phymed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Ziai SA, Gholami O, Iranshahi M, Zamani AH, Jeddi-Tehrani M. Umbelliprenin induces apoptosis in CLL cell lines. Iran J Pharm Res. 2012;11:653–659. [PMC free article] [PubMed] [Google Scholar]
- 6.Khaghanzadeh N, Mojtahedi Z, Ramezani M, Erfani N, Ghaderi A. Umbelliprenin is cytotoxic against QU-DB large cell lung cancer cell line but anti-proliferative against A549 adenocarcinoma cells. DARU. 2012;20:69. doi: 10.1186/2008-2231-20-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iranshahi M, Sahebkar A, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin in vivo. Eur J Cancer Prev. 2009;18:412–415. doi: 10.1097/CEJ.0b013e32832c389e. [DOI] [PubMed] [Google Scholar]
- 8.Khaghanzadeh N, Samiei A, Ramezani M, Mojtahedi Z, Hosseinzadeh M, Ghaderi A. Umbelliprenin induced production of IFN-gamma and TNF-alpha, and reduced IL-10, IL-4, Foxp3 and TGF-beta in a mouse model of lung cancer. Immunopharmcol Immunotoxicol. 2014;36:25–32. doi: 10.3109/08923973.2013.863912. [DOI] [PubMed] [Google Scholar]
- 9.Iranshahi M, Askari M, Sahebkar A, Hadjipavlou-Litina D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU. 2009;17:99–103. [Google Scholar]
- 10.Shahverdi AR, Saadat F, Khorramizadeh MR, Iranshahi M, Khoshayand MR. Two matrix metalloproteinases inhibitors from Ferula persica var. persica. Phytomedicine. 2006;13:712–717. doi: 10.1016/j.phymed.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Bayrami G, Boskabady MH, Iranshahi M, Gholamnezhad Z. Relaxant effects of asafoetida extract and its constituent umbelliprenin on guinea-pig tracheal smooth muscle. Chin J Integr Med. 2013 doi: 10.1007/s11655-013-1550-3. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Sugiura H, Inaba R, Nishikawa A, Murakami A, Koshimizu K, et al. Immunomodulatory action of citrus auraptene on macrophage functions and cytokine production of lymphocytes in female BALB/c mice. Carcinogenesis. 1999;20:1471–1476. doi: 10.1093/carcin/20.8.1471. [DOI] [PubMed] [Google Scholar]
- 13.Niu X, Huang Z, Zhang L, Ren X, Wang J. Auraptene has the inhibitory property on murine T lymphocyte activation. Eur J Pharmacol. 2015;750:8–13. doi: 10.1016/j.ejphar.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Gholami O, Shamsara J. Comparison of the cytotoxic effects of umbelliprenin and auraptene. Int J Pharm Pharm Sci. 2016;8:1–4. [Google Scholar]
- 15.Akhila JS, Shyamjith Deepa, Alwar MC. Acute toxicity studies and determination of median lethal dose. Curr Sci. 2007;93:917–920. [Google Scholar]
- 16.Taboas JO, Ceremsak RJ. A rapid hematoxylin and eosin stain. Tech Bull Regist Med Technol. 1967;37:119–120. [PubMed] [Google Scholar]
- 17.Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 6th ed. Saunders: an imprint of Elsevier Inc; 2007. Cytokines; pp. 267–302. [Google Scholar]
- 18.Zaidi MR, Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res. 2011;17:6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho MY, Tang SJ, Sun KH, Yang W. Immunotherapy for lung cancers. J Biomed Biotechnol. 2011;2011:250860. doi: 10.1155/2011/250860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Njoku DB. Suppressive and pro-inflammatory roles for IL-4 in the pathogenesis of experimental drug-induced liver injury: a review. Expert Opin Drug Metab Toxicol. 2010;6:519–531. doi: 10.1517/17425251003601979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierson W, Liston A. A new role for interleukin-10 in immune regulation. Immunol Cell Biol. 2010;88:769–770. doi: 10.1038/icb.2010.105. [DOI] [PubMed] [Google Scholar]
- 22.Han S, Asoyan A, Rabenstein H, Nakano N, Obst R. Role of antigen persistence and dose for CD4+T-cell exhaustion and recovery. Proc Natl Acad Sci U S A. 2010;107:20453–20458. doi: 10.1073/pnas.1008437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cope A, Le Friec G, Cardone J, Kemper C. The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol. 2011;32:278–286. doi: 10.1016/j.it.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 25.Shakeri A, Iranshahy M, Iranshahi M. Biological properties and molecular targets of umbelliprenin--a mini-review. J Asian Nat Prod Res. 2014;16:884–889. doi: 10.1080/10286020.2014.917630. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Li X, Cao L, Zhang L, Shen L, Zhu J, et al. Sesquiterpene coumarins from seeds of Ferula sinkiangensis. Fitoterapia. 2015;103:222–226. doi: 10.1016/j.fitote.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Khorramizadeh MR, Esmail-Nazari Z, Zarei-Ghaane Z, Shakibaie M, Mollazadeh-Moghaddam K, Iranshahi M, et al. Umbelliprenin-coated Fe3O4 magnetite nanoparticles: Antiproliferation evaluation on human Fibrosarcoma cell line (HT-1080) Materials Sci Eng C Mater Biol Appl. 2010;30:1038–1042. [Google Scholar]


