Abstract
Objective(s):
Crocus sativus (saffron) has been widely used in traditional medicine. It has also been found to possess many beneficial properties in modern medicine. The most important ingredients of saffron are crocin, crocetin, safranal, and picrocrocin. This study evaluated the protective effects of crocin against the inflammation, oxidative stress, and functional disturbances of the kidney induced by renal ischemia/reperfusion (I/R).
Materials and Methods:
Different doses of crocin (0, 100, 200, and 400 mg/kg) were administered intraperitoneally 30 min before I/R. The rats of the sham group were also injected with normal saline before the sham surgery. For induction of I/R, both renal artery and vein clamped for 30 min, bilaterally. The I/R-induced renal injuries were assessed by measuring leukocyte infiltration, intercellular adhesion molecule-1 (ICAM-1) and tumor necrosis factor-alpha (TNF-α) mRNA expression levels, malondialdehyde (MDA) and ferric reducing/antioxidant power (FRAP) levels in the kidney tissue, and plasma creatinine and urea-nitrogen concentrations.
Results:
Except for the tissue level of FRAP which decreased, all other measured parameters increased following I/R induction. Pretreatment with all doses of crocin significantly reduced the severity of these disturbances (P<0.05 to P<0.001). In fact, while there was no significant differences between MDA and FRAP levels, plasma creatinine and urea-nitrogen concentrations of the crocin-treated animals and the sham group, crocin administration reduced leukocyte infiltration and ICAM-1 and TNF-α mRNA expression levels in a dose-dependent manner.
Conclusion:
The present study clearly demonstrated the anti-inflammatory, antioxidant, and protective effects of crocin, a main constituent of saffron, against renal damages resulted from I/R in rats.
Keywords: Crocin, ICAM-1, Inflammation, Ischemia-reperfusion Leukocyte infiltration, TNF-α
Introduction
Acute kidney injury (AKI) is a significant clinical issue with a high prevalence and no definitive treatment. One of the most common causes of AKI is renal ischemia/reperfusion (I/R) resulting in injury to the vascular endothelium, interstitial inflammation, and tubular damages (1). Vascular endothelial injury reduces renal blood flow during the reperfusion period by causing vasoconstriction and increasing adhesive molecules, e.g. intercellular adhesion molecule-1 (ICAM-1), and thus the adhesion of leukocytes, platelets, and red blood cells. Interstitial inflammation can also cause several damages including the activation of the complement system and the production of proinflammatory factors such as tumor necrosis factor-alpha (TNF-α). It can also promote the infiltration of leukocytes into the renal interstitium which, in turn, increases the risk of vascular and tubular damages by increasing the production of reactive oxygen species (ROS) and proxynitrite. The resulting tubular damage can be observed in the form of tubular necrosis or apoptosis (1, 2).
Saffron (Crocus sativus L. from the Iridaceae family) is widely cultivated in countries such as Iran, India, and Greece (3). Chemical studies have identified crocin, crocetin, safranal, and picrocrocin as the most important ingredients of saffron (4). Numerous studies on the pharmacological effects of crocin have confirmed analgesic (5), anti-atherosclerotic (6), anti-platelet aggregation (7), anti-tumor, anti-cancer (8), and antioxidant properties (9, 10). Crocin has also been found to have protective effects against gentamicin-induced nephrotoxicity (11). According to Nam et al. (12), crocin represses microglial activation and reduces inflammation-induced neurotoxicity in rats.
Our previous study highlighted the antioxidant, anti-inflammatory, and protective effects of the hydro-ethanolic extract of saffron in I/R-induced AKI (13). Since saffron contains various compounds, its anti-inflammatory properties cannot be exactly attributed to a particular component. Therefore, this study aimed to evaluate the anti-inflammatory effects of crocin, as a main component of saffron, and its protective role in AKI induced by I/R in rats.
Materials and Methods
The study protocol, designed according to the National Guidelines for Laboratory Animal Welfare and the European Economic Community Guidelines for the care and use of laboratory animals (EEC Directive of 1986; 86/609/EEC), was approved by the Ethics Committee of Kermanshah University of Medical Sciences (approval number: KUMS.REC.1394. 85). The smallest possible number of animals was recruited and the depth of anesthesia was constantly monitored during the surgery to prevent any response to painful stimuli. Moreover, the well-being of the animals was assessed during the entire study period and rats showing unexpected suffering or severe and enduring signs of distress and pain, including reduced mobility, inactivity, or abnormal posture, were euthanized. Euthanasia was conducted through deep anesthesia with diethyl ether.
Male Wistar rats (250-300 g) were housed three per cage at a controlled temperature (23±2 °C) and a 12 hr light/dark cycle. They were given ad libitum access to food (pellets) and water. Based on procedures explained in similar previous studies (10), the animals received intraperitoneal (IP) injections of 0.5 ml of normal saline (sham group, n=7) or various concentrations (0, 100, 200, or 400 mg/kg) of crocin (Sigma, USA) in normal saline (n=7 each). After 30 min, the rats underwent 30 min of bilateral renal ischemia and 24 hr of reperfusion as described below. Sham surgery was performed in the sham group. All the animals received 100 international units (IU) of IP heparin half an hr before the surgery.
Surgery
The animals were first anesthetized with diethyl ether alone. A longitudinal incision was then made in the linea alba and the clamping of the bilateral renal arteries and veins was simultaneously performed (Roboz Surgical Instruments, Gaithersburg, MD, USA). The clamps were maintained for half an hr and then removed to ensure reperfusion. A rectal probe (BAT-12 Microprobe Thermometer, Physitemp Instruments, Inc., USA) was used to measure the animal’s body temperature during the surgery and body temperatures below or above 37±1 °C were not allowed. Blood samples were obtained from the abdominal aorta after the reperfusion period and the plasma levels of creatinine and urea-nitrogen were measured. Deep anesthesia was then induced and the animals were sacrificed. In the next stage, the animals’ kidneys were removed. In order to determine oxidative stress, malondialdehyde (MDA) and ferric reducing/antioxidant power (FRAP) levels in the right kidneys were measured. The left kidneys were decapsulated on dry ice and halved lengthwise. Hematoxylin and eosin (H & E) staining was performed on one half and leukocyte infiltration was evaluated through light microscopy. For this purpose, the preserved left kidneys were embedded in paraffin, and 5-μm-thick sections were obtained using a microtome. Sections were subjected to staining with H & E. The reverse transcription polymerase chain reaction (RT-PCR) method was applied to determine TNF-α and ICAM-1 mRNA expression levels in the cortex of the other half.
Inflammation assessment
The rate of leukocyte infiltration into the renal interstitium, as well as TNF-α and ICAM-1 mRNA expression levels were measured for the assessment of inflammation. To determine the amount of leukocyte infiltration, the number of leukocytes in 20 microscopic fields (0.14 mm2 each) was counted, averaged, and used to estimate the number of leukocytes per square millimeters (14).
TNF-α and ICAM-1 expression levels in the kidney were assessed by RT-PCR using β-actin as a control. The sequences of PCR primers used for TNF-α, ICAM-1, and β-actin are shown in Table 1. The PCR products were run on 1% agarose gel and semiquantification analysis of mRNA expression was accomplished by obtaining the ratio of the band density of the gene product of each group to that of sham.
Table 1.
Primer sequences used to amplify mRNAs encoding proinflammatory cytokines
| Primer | Sequence | Annealing t °C | Product size (bp) | |
|---|---|---|---|---|
| TNF-α | F | ATGAGCACTGAAAGCATGAT | 60 | 550 |
| R | CTCTTGATGGCAGAGAGGAG | |||
| ICAM-1 | F | CAGCAGACCACTGTGCTTTGA | 61 | 450 |
| R | GTCGAGCTTCAGGACCCTAGT | |||
| β-Actin | F | GCCATGTACGTAGCCATCCA | 56 | 375 |
| R | GAACCGCTCATTGCCGATAG | |||
TNF-α: Tumor necrosis factor-alpha; ICAM-1: Intercellular adhesion molecule-1
Assessment of oxidative stress
In order to evaluate oxidative stress, the level of MDA, the final product of lipid peroxidation, was measured in the kidney tissue by colorimetric assay method as described previously (15). The FRAP levels in the kidney tissue were also measured using Benzie & Strain method (16). This method is based on the ability of plasma or tissue extract to reduce Fe3+ (ferric) to Fe2+ (ferro) in the presence of 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ). At low pH, the reduction of Fe3+-TPTZ complex to ferrous (Fe2+) creates a blue complex with maximum absorbance at the wavelength of 593 nm (13, 17, 18). All the chemicals were purchased from Sigma (USA).
Statistical analysis
SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The values of all measured variables were presented as mean±standard error (SE). One-way analysis of variance (ANOVA) and Duncan’s post-hoc test were applied for comparison of the measured parameters. The least significant difference (LSD) test was used to determine the exact P-values in cases with significant differences. P<0.05 was considered as the level of significance.
Results
Leukocytes infiltration rate
Following renal I/R, lymphocytes were the most leukocytes that significantly infiltrated from renal vessels according to light microscopy and mainly accumulated in the cortical interstitium. The number of lymphocytes in the interstitial space of the kidney was very low in the sham group (about 1-4/mm2) which significantly increased up to about 36 times after I/R induction (P<0.001; Figures 1A, B and 2). Pretreatment with crocin (100 or 200 mg/kg) resulted in a significant reduction in lymphocyte infiltration (P<0.01). As shown in Figures 1C, D and 2, lymphocytes accumulation in the interstitium of the kidney was 3.1 and 3.6 times lower in I/R + C(100) and I/R + C(200) groups than the I/R group, respectively. Crocin treatment at 400 mg/kg reduced lymphocyte infiltration (P<0.001), in such a way that the numbers of infiltrated lymphocytes in the I/R + C(400) group were not significantly different from those in the sham group (Figures 1E and 2).
Figure 1.
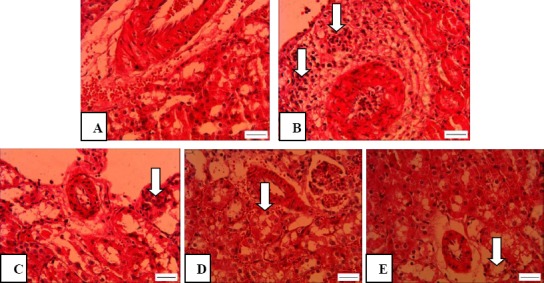
Light microscopic images of renal cortex representing leukocyte infiltration in rats which underwent sham surgery (A) and those which underwent renal ischemia/reperfusion (I/R) while pretreated with saline (B) or 100, 200, and 400 mg crocin (C-E). Magnification: 400X; Scale bar: 100 μm
Figure 2.
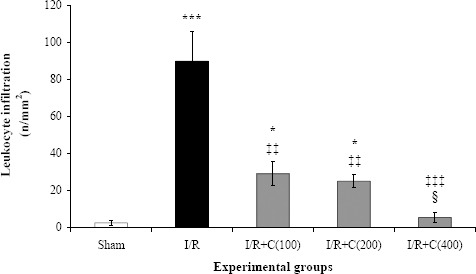
Leukocyte infiltration (mean±SE per square millimeters) at the end of the reperfusion period in rats which underwent renal ischemia/reperfusion (I/R) while pretreated with different doses (0, 100, 200, or 400 mg/kg) of crocin compared to the sham group
* P<0.05; ***P<0.001 in comparison with the sham group
‡‡ P<0.01; ‡‡‡ P<0.001 in comparison with the I/R group
¦ P<0.05 in comparison with the I/R + C(100) and I/R + C(200) groups
RT-PCR assay for mRNA expression
The kidney cortices of all groups were examined to determine the TNF-α and ICAM-1 mRNA expression levels. Figure 3A and 3B illustrate the obtained agarose gel images and the quantified relative band intensities, respectively. After 30 min of bilateral renal ischemia and 24 hr of reperfusion, the TNF-α and ICAM-1 mRNA expression levels in the I/R group increased significantly compared to the sham group (P<0.01 and P<0.001, respectively). Moreover, dose-dependently significant reductions in the expression of both genes were seen following crocin administration. TNF-α and ICAM-1 mRNA levels in the rats which received 400 mg/kg crocin were decreased significantly in comparison with the I/R group. Furthermore, the expression levels for both genes in the I/R+ C(400) group were significantly different from those in the I/R + C(100) and I/R + C(200) groups.
Figure 3.
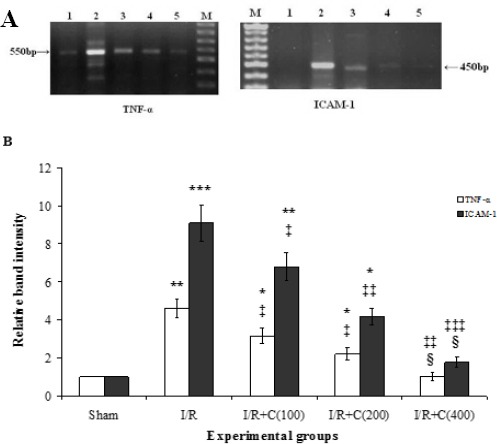
Representative images of semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) of mRNA encoding tumor necrosis factor-alpha (TNF-α) and intercellular adhesion molecule-1 (ICAM-1) (A) in the renal cortex of the sham group (lane 1) and rats which underwent ischemia/reperfusion after being pretreated with crocin at 0, 100, 200, and 400 mg/kg (lanes 2-5, respectively). Lane M is a 50-bp RNA size marker. Densitometric quantification of relative band intensities from RT-PCR assays for TNF-α and ICAM-1 (B)
*P<0.05; **P<0.01; ***P<0.001 in comparison with the sham group
‡P<0.05; ‡‡P<0.01; ‡‡‡P<0.001 in comparison with the I/R group.
¦ P<0.05 in comparison with their own I/R + C(100) and I/R + C(200) groups
Renal function
Bilateral renal I/R led to a significant increase in plasma creatinine concentration (P<0.001; Figure 4A). Crocin administration led to a significant decrease in plasma creatinine concentration (P<0.001). However, there was no significant difference between creatinine concentrations in the I/R + C groups and the sham group. It is noteworthy that a crocin concentration as low as 100 mg/kg was sufficient to reduce the creatinine concentration in the I/R group to the normal value (the level in the sham group). No significant difference was observed between groups receiving different doses of crocin.
Figure 4.
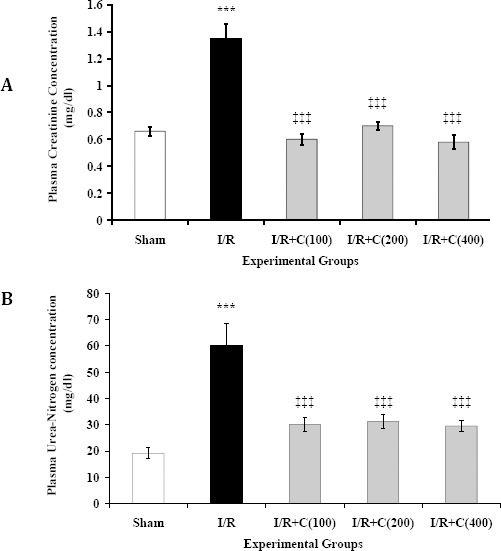
Plasma creatinine (A) and urea-nitrogen (B) concentrations at the end of reperfusion period in rats which underwent renal ischemia/reperfusion and treated with crocin at zero (I/R), 100, 200, or 400 mg/kg (I/R + C) compared to the sham group. Data is presented as mean ± SE (n = 7), *** P<0.001 in comparison with the sham group. ‡‡‡ P<0.001 in comparison with the I/R group
Plasma urea-nitrogen concentrations in the sham and I/R groups were 19.2±2.1 and 60±8.6 mg/dl, respectively (P<0.001; Figure 4B). Pretreatment with crocin could significantly decrease plasma urea-nitrogen concentrations compared to the I/R group (P<0.001). In fact, urea-nitrogen levels in the sham and I/R + C groups were not significantly different. Moreover, the groups pretreated with different dosages of crocin did not have significantly different plasma urea-nitrogen concentrations.
Oxidative stress
As seen in Figure 5A, the MDA level in the kidneys of the I/R group was about 70% higher than that of the sham group (P<0.001). Crocin administration at 100 or 400 mg/kg could significantly reduce the MDA level in the kidneys of the I/R group (P<0.001). However, these levels were still significantly higher compared to the sham group (P<0.01). Interestingly, administration of 200 mg/kg of crocin could more effectively decrease the MDA level compared to its other two doses.
Figure 5.

Tissue malondialdehyde (MDA) and ferric reducing/antioxidant power (FRAP) levels per g kidney weight at the end of the reperfusion period in rats which underwent renal ischemia/reperfusion (I/R) and those treated with zero (I/R), 100, 200, or 400 mg/kg of crocin (I/R+C) compared to the sham group. Data are presented as mean±SE (n=7)
*P<0.05; **P<0.01; ***P<0.001 in comparison with the sham group. ‡‡P<0.01; ‡‡‡P<0.001 in comparison with the I/R group
Moreover, while inducing I/R significantly decreased the kidney FRAP level compared to the sham group (P<0.001; Figure 5B), crocin had significant dose-dependent effects on increasing the FRAP levels in the kidneys of the I/R+C groups (P<0.01 for 100 mg/kg and P<0.001 for 200 and 400 mg/kg). Nevertheless, the FRAP levels of crocin-treated groups were not significantly different.
Discussion
This study assessed the anti-inflammatory, antioxidant, and protective effects of crocin on renal functional disturbances resulted from renal I/R in rats. According to the obtained findings, I/R significantly increased lymphocyte infiltration as well as TNF-α and ICAM-1 mRNA expression. Previous studies have also reported that increased levels of adhesive molecules, e.g. ICAM-1, P-selectin, and E-selectin, in the endothelium of blood vessels within one hr after renal ischemia (19, 20). Higher synthesis of proinflammatory cytokines such as TNF-α, interleukin-1 (IL-1), and IL-6 has also been confirmed in AKI (21). Inflammatory responses seem to have a major role in tissue damage development. TNF-α, for instance, increases the risk of failure in the kidney function by exerting direct negative effects including vasoconstriction, blood flow restriction, and leukocyte infiltration (22). It not only reduces renal blood flow and glomerular filtration rate (GFR), but also increases glomerular permeability to albumin and enhances ICAM-1 production and cell infiltration (22). Kelly et al. (20) found increased ICAM-1 expression in endothelial cells within the first hr after renal I/R. Such an increment facilitated the endothelial binding of leukocytes and enhanced their migration into the interstitium. Following ischemia, some cytokines, such as IL-6 and IL-18, are produced by proximal tubule epithelial cells. Then they move into the interstitium and activate the leukocytes which are moved out of the blood vessels. These activated leukocytes may induce tubular and vascular injury by producing oxygen free radicals and vasoconstrictors. Previous researches have, in fact, indicated that inhibiting elevations in ICAM-1 levels would prevent the renal damages caused by I/R (20, 23).
In this study, lymphocytes were the most type of leukocytes that infiltrated into the interstitium after renal I/R according to light microscopic study. Similar findings have also been reported by previous study (13). The antioxidant effects of crocin might have been responsible for the reduction in lymphocyte activation and interstitial infiltration in the I/R + C groups. It is suggested that anti-inflammatory effects of crocin in rat brain microglial cells as the main cause of reduced TNF-α, IL-1β, and ROS levels (12). Crocin also reduces nuclear factor-kappaB (NF-κB) activation in glial cells cultures (11) and inhibits TNF-α-induced neuronal death in vitro (24).
The present study also showed that 24 hr reperfusion following 30 min of ischemia led to a significant increase in plasma creatinine and urea-nitrogen concentrations in comparison with the sham group. Interestingly, all three doses of crocin were able to reduce the concentration of both plasma creatinine and urea-nitrogen and this effect was not dose-dependent. Considering the inverse relationship between plasma creatinine concentration and GFR, the mentioned increase in plasma creatinine levels was probably caused by the reduction in GFR (25). Numerous studies have identified sharp reductions in renal creatinine clearance, as an indicator of GFR, after I/R (26, 27) and justified this reduction by decreased renal blood flow, increased pressure in Bowman’s space, and back leak of GFR (1, 2). The injury to proximal tubule cells and the thick ascending limb of loop of Henle might also increase the tubular flow in macula densa and activate the tubuloglomerular feedback by reducing tubular reabsorption. These changes will, in turn, decrease renal blood flow and GFR. The disturbance of the balance between vasoconstrictors (such as adenosine and endothelin) and vasodilators (such as nitric oxide and prostaglandins) is one of the main causes of decreased renal blood flow following I/R (1). It is found that crocin increased blood flow in the retina and choroid (28). A similar process seems to occur in case of renal blood flow and GFR.
It has also been shown that crocin decreases methyl methanesulfonate-induced DNA damage, cell necrosis, and exfoliation into the tubular lumen (29). Thus, crocin may increase the GFR and adjust plasma creatinine and urea-nitrogen concentrations by increasing renal blood flow, reducing cell injuries, and decreasing the pressure in the Bowman’s space. Part of the protective effects of crocin may also come from the reduction in TNF-α production resulting in improved blood flow and GFR. TNF-α has a decreasing effect on renal blood flow and GFR (22).
This study also showed that owing to its known antioxidant effects, crocin decreased lipid peroxidation (indicated by decreased MDA) and enhanced the antioxidant capacity (indicated increased FRAP) in the kidney tissue. These findings are consistent with a large body of studies highlighting the antioxidant properties of crocin in different tissues. Crocin has been found to decrease MDA and glutathione peroxidase levels and thus reduce the oxidative stress induced by cisplatin (30). Apparently, due to its antioxidant effects, crocin inhibits ROS production which, in turn, decreases MDA levels and increases FRAP in the I/R + C groups.
This study confirmed the anti-inflammatory, antioxidant, and protective effects of various doses (100, 200, and 400 mg/kg) of crocin in renal I/R. These doses were 20 times higher than the effective doses of saffron in our previous study (13). Therefore, it seems that crocin is not the only factor in preventing these disturbances and other compounds may also be involved in the beneficial effects of saffron. Nevertheless, the effects of crocin were somewhat more specific than those of saffron.
Conclusion
In summary, crocin is capable of reducing inflammation and oxidative stress induced by bilateral 30-min renal ischemia and 24 hr reperfusion in rats. Moreover, owing to its antioxidant effects, crocin can prevent the renal dysfunction resulted from I/R by inhibiting the activation of pro-inflammatory factors and increasing blood flow.
Acknowledgment
The results described in this paper were part of student thesis (Z. Mohamadi Yarijani) for MSc degree in physiology. The authors would like to thank the Vice-Chancellery for Research affairs of Kermanshah University of Medical Sciences for financial support (grant no. 91077).
Conflict of interest
The authors declare no competing financial interest.
References
- 1.Clarkson M, Friedewald J, Eustace J, Rabb H. Brenner & Rector’s The Kidney. 8th ed. Philadelphia: WB Saunders; 2008. Acute kidney injury; pp. 943–986. [Google Scholar]
- 2.Kribben A, Edelstein CL, Schrier RW. Pathophysiology of acute renal failure. J Nephrol. 1999;12:S142–S151. [PubMed] [Google Scholar]
- 3.Hosseinzadeh H, Modaghegh MH, Saffari Z, Crocus sativus L. (Saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rios J, Recio M, Giner R, Manez S. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–193. [Google Scholar]
- 5.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee IA, Lee JH, Baek NI, Kim DH. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull. 2005;28:2106–2110. doi: 10.1248/bpb.28.2106. [DOI] [PubMed] [Google Scholar]
- 7.Liakopoulou-Kyriakides M, Skubas AI. Characterization of the platelet aggregation inducer and inhibitor isolated from Crocus sativus. Biochem Int. 1990;22:103–110. [PubMed] [Google Scholar]
- 8.Garc-Olmo DC, Riese HH, Escribano J, Ontanon J, Fernandez JA, Atiénzar M, et al. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): an experimental study in the rat. Nutr Cancer. 1999;35:120–126. doi: 10.1207/S15327914NC352_4. [DOI] [PubMed] [Google Scholar]
- 9.Asdaq SM, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. 2010;162:358–372. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 10.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharm Sci. 2005;8:387–393. [PubMed] [Google Scholar]
- 11.Mohamadi Yarijani Z, Najafi H, Madani SH. Protective effect of crocin on gentamicin-induced nephrotoxicity in rats. Iran J Basic Med Sci. 2016;19:337–343. [PMC free article] [PubMed] [Google Scholar]
- 12.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoudzadeh L, Najafi H, Changizi-Ashtiyani S, Mohamadi yarijani Z. Anti-inflammatory and protective effects of saffron extract in ischaemia-reperfusion induced acute kidney injury. Nephrology. 2016 doi: 10.1111/nep.12849. doi:10.1111/nep.12849. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 17.Changizi Ashtiyani S, Najafi H, Jalalvandi S, Hosseinei F. Protective effects of rosa canina L fruit extracts on renal disturbances induced by reperfusion injury in rats. Iran J Kidney Dis. 2013;7:290–298. [PubMed] [Google Scholar]
- 18.Najafi H, Firouzifar MR, Shafaat O, Changizi Ashtiyani S, Hosseini N. Protective effects of tribulus terrestris L extract against acute kidney injury induced by reperfusion injury in rats. Iran J Kidney Dis. 2014;8:292–298. [PubMed] [Google Scholar]
- 19.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly K, Williams WW, Colvin RB, Meehan SM, Springer TA, Gutiérrez-Ramos JC, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnahoo KK, Shames BD, Harken AH, Meldrum DR. The role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- 23.Kelly K, Williams WW, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci USA. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soeda S, Ochiai T, Paopong L, Tanaka H, Shoyama Y, Shimeno H. Crocin suppresses tumor necrosis factor-α-induced cell death of neuronally differentiated PC-12 cells. Life Sci. 2001;69:2887–2898. doi: 10.1016/s0024-3205(01)01357-1. [DOI] [PubMed] [Google Scholar]
- 25.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Chien CC, Grigoryev DN, Gandolfo MT, Colvin RB, Rabb H. Effect of T cells on vascular permeability in early ischemic acute kidney injury in mice. Microvasc Res. 2009;77:340–347. doi: 10.1016/j.mvr.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Rabb H, Haq M, Shull GE, Soleimani M. A possible molecular basis of natriuresis during ischemic-reperfusion injury in the kidney. J Am Soc Nephrol. 1998;9:605–613. doi: 10.1681/ASN.V94605. [DOI] [PubMed] [Google Scholar]
- 28.Xuan B, Zhou YH, Li N, Min ZD, Chiou GC. Effects of crocin analogs on ocular blood flow and retinal function. J Ocul Pharmacol Ther. 1999;15:143–152. doi: 10.1089/jop.1999.15.143. [DOI] [PubMed] [Google Scholar]
- 29.Hosseinzadeh H, Abootorabi A, Sadeghnia HR. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol. 2008;27:657–664. doi: 10.1089/dna.2008.0767. [DOI] [PubMed] [Google Scholar]
- 30.Naghizadeh B, Mansouri SM, Mashhadian NV. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food Chem Toxicol. 2010;48:2650–2655. doi: 10.1016/j.fct.2010.06.035. [DOI] [PubMed] [Google Scholar]


