ABSTRACT
Objective:
To describe the implementation of a robotic thoracic surgery program at a public tertiary teaching hospital and to analyze its initial results.
Methods:
This was a planned interim analysis of a randomized clinical trial aimed at comparing video-assisted thoracoscopic surgery and robotic surgery in terms of the results obtained after pulmonary lobectomy. The robotic surgery program developed at the Instituto do Câncer do Estado de São Paulo, in the city of São Paulo, Brazil, is a multidisciplinary initiative involving various surgical specialties, as well as anesthesiology, nursing, and clinical engineering teams. In this analysis, we evaluated the patients included in the robotic lobectomy arm of the trial during its first three months (from April to June of 2015).
Results:
Ten patients were included in this analysis. There were eight women and two men. The mean age was 65.1 years. All of the patients presented with peripheral tumors. We performed right upper lobectomy in four patients, right lower lobectomy in four, and left upper lobectomy in two. Surgical time varied considerably (range, 135-435 min). Conversion to open surgery or video-assisted thoracoscopic surgery was not necessary in any of the cases. Intraoperative complications were not found. Only the first patient required postoperative transfer to the ICU. There were no deaths or readmissions within the first 30 days after discharge. The only postoperative complication was chest pain (grade 3), in two patients. Pathological examination revealed complete tumor resection in all cases.
Conclusions:
When there is integration and proper training of all of the teams involved, the implementation of a robotic thoracic surgery program is feasible and can reduce morbidity and mortality.
Keywords: Pneumonectomy, Robotic surgical procedures, Thoracic surgery, Minimally invasive surgical procedures, Lung neoplasms
RESUMO
Objetivo:
Descrever a implantação de um programa de cirurgia torácica robótica em um hospital terciário público universitário e analisar seus resultados iniciais.
Métodos:
Este estudo é uma análise interina planejada de um ensaio clínico aleatorizado cujo objetivo é comparar resultados da lobectomia pulmonar por videotoracoscopia com a robótica. O programa de cirurgia robótica do Instituto do Câncer do Estado de São Paulo, localizado na cidade de São Paulo (SP), foi uma iniciativa multidisciplinar que envolveu diversas especialidades cirúrgicas e equipes de anestesia, enfermagem e engenharia clínica. Nesta análise, avaliamos os pacientes incluídos no braço lobectomia robótica durante os primeiros três meses do estudo (de abril a junho de 2015).
Resultados:
Dez pacientes foram incluídos nesta análise. Eram oito mulheres e dois homens. A média de idade foi de 65,1 anos. Todos apresentavam tumores periféricos. Foram realizadas lobectomia superior direita, em quatro pacientes; lobectomia inferior direita, em quatro; e lobectomia superior esquerda, em dois. Os tempos cirúrgicos variaram bastante (variação, 135-435 min). Não foi necessária a conversão para técnica aberta ou videotoracoscópica em nenhum paciente. Não foram observadas complicações intraoperatórias. Apenas o primeiro paciente foi encaminhado à UTI no pós-operatório. Não houve mortalidade nem reinternações em 30 dias após a alta. A única complicação pós-operatória observada foi dor torácica (grau 3), em dois pacientes. O exame anatomopatológico revelou a ressecção completa do tumor em todos os casos.
Conclusões:
A implantação de um programa de cirurgia torácica robótica, quando há integração e treinamento adequado de todas as equipes envolvidas, é factível e pode reduzir a morbidade e a mortalidade.
INTRODUCTION
Over the past 20 years, minimally invasive surgery has developed rapidly. Beginning in the 1990s, video-assisted technology came to be widely used for surgery, thus playing a decisive role in increasing the prominence of minimally invasive surgery. Video-assisted technology also had an impact on thoracic surgery, pleural procedures and easily performed resections having rapidly come to be performed by means of video-assisted thoracoscopic surgery (VATS) in many countries, including Brazil. 1 , 2
Studies published in the last decade contributed to consolidating the role of VATS in resections that are more complex, such as lobectomy and pneumonectomy. 3 , 4 More recently, robotic surgery has emerged as an alternative to video-assisted surgery, its objective being to increase the amplitude and accuracy of intracavitary maneuvers and movements, as well as to provide better visualization via three-dimensional imaging. Studies have shown that robotics can be applied to thoracic surgery, being particularly useful for mediastinal tumors and anatomic lung resections, such as pulmonary lobectomy. 5 - 8
The real role of robotics in thoracic surgery has yet to be defined. Although large case series have shown good results regarding intraoperative morbidity, intraoperative mortality, and length of hospital stay, 9 retrospective studies involving databases have raised questions regarding the costs and complications of the new method. 10 The results of an analysis of a US hospital database including 15,502 patients undergoing lung resection via VATS or robot-assisted thoracic surgery showed that the latter had significantly higher costs and longer operative times. 10
In this setting of uncertainty, the implementation of a robotic surgery program is particularly challenging, and, in addition to risk minimization, attention should be given to structural issues and costs. The objectives of the present study were to describe the implementation of a robotic thoracic surgery program at the University of São Paulo School of Medicine Hospital das Clínicas Instituto do Câncer do Estado de São Paulo (ICESP, São Paulo State Cancer Institute), in the city of São Paulo, Brazil, and to analyze its initial results.
METHODS
This was a planned interim analysis of a randomized clinical trial that is currently under way at our institution and that is aimed at comparing VATS and robotic surgery in terms of the results obtained after pulmonary lobectomy. In this analysis, we evaluated the patients included in the robotic lobectomy arm of the trial during its first three months (from April to June of 2015), i.e., after the surgical team had been certified (in March of 2015). All of the patients who were included in the study gave written informed consent, and the study was approved by the local research ethics committee.
In addition to the presence of primary lung cancer or lung metastasis and written informed consent, the criteria for inclusion in the randomized clinical trial were as follows, having been evaluated during the clinical staging phase:
eligibility for the treatment of lung cancer or lung metastasis by pulmonary lobectomy
presence of a tumor of less than 5 cm in diameter at its widest point
absence of hilar or mediastinal lymphadenopathy
absence of tumor invasion of the chest wall, the mediastinum, or another lung lobe
absence of tumor invasion of a main bronchus or a lobar bronchus less than 1 cm from the secondary carina
Clinical and anesthetic evaluation results showing that the patient was able to undergo the proposed procedure
The exclusion criteria were as follows:
having previously undergone a thoracic surgical procedure in the hemithorax that was to be operated on
being unable to remain on single-lung ventilation during the procedure
In the present analysis, the following variables were evaluated: operative time; length of hospital stay; complications; patient-reported pain; extent of lymph node dissection; and number of lymph nodes removed during lymph node dissection. Intraoperative and postoperative complications (up to postoperative day 30) were recorded and classified on the basis of the Common Terminology Criteria for Adverse Events, version 4.0. 11 The magnitude of the systemic inflammatory response was assessed by measuring serum creatine phosphokinase and C-reactive protein levels on postoperative day 2. Pain was assessed by a visual analog pain scale (a Likert scale)-which was administered in the morning on postoperative days 1, 2, and 3 and during follow-up visits on postoperative days 15 and 30-and by the duration of use and dose of opioids. In the early postoperative period, opioids were administered at fixed times; subsequently, they were administered as needed. The date of opioid discontinuation was defined as the day on which patients received their last dose of opioids. The extent of lymph node dissection was determined by counting the resected lymph nodes. The resected lymph nodes were counted by using a procedure that has been standardized by the ICESP Department of Anatomic Pathology and that is consistent with the current literature.
The robotic surgery program developed at the ICESP is a multidisciplinary initiative involving various surgical specialties, as well as anesthesiology, nursing, and clinical engineering teams. All involved received specific training in operating the robot. The thoracic surgery team training consisted of an online course on how the robotic surgical system works; 20 hours of virtual reality simulation in order to familiarize participants with the movements of the robot; and lobectomy simulation in animal models. The process of certification lasted 2 days, having taken place in a specialized center abroad and having involved animal models and human cadavers. After certification, we participated as observers in various procedures performed at centers of excellence in robotic surgery. Before the first procedure, simulations were performed with the participation of the entire multidisciplinary team.
All surgical procedures were performed with the use of selective intubation and an epidural catheter for postoperative analgesia. We used a slightly modified version of a robotic lobectomy technique that was originally described by Dylewski et al. 12 Patients are placed in the lateral decubitus position with pads under their axillae, the robot being placed over their heads. A total of four ports are used: three for the robotic arms and one for the assistant surgeon (Figure 1). The first incision is made in the 6th intercostal space at the anterior axillary line. After insertion of a 5-mm trocar, carbon dioxide insufflation is initiated. With the aid of a 5-mm endoscope, the locations of the remaining ports are determined. Initially, the diaphragm insertion on the chest wall at the level of the 10th intercostal space is identified, and a 12-mm trocar is inserted at that site, the trocar being used by the assistant surgeon for exposure, aspiration, stapling, introduction/removal of materials (such as gauze), and removal of specimens for pathological examination. Subsequently, two other ports are placed in the 7th or 8th intercostal space at the midaxillary and posterior axillary lines, respectively. The robotic camera is introduced through the midaxillary line trocar, and the robotic graspers are introduced through the remaining two ports. It is extremely important that these ports are caudal to the oblique fissure.
Figure 1. Port placement. In A, a patient in the right lateral decubitus position. Ribs 9 through 12 are marked on the chest wall. The arrows indicate the ports for arms 1 and 2, as well as the camera port and the assistant port. In B, trocars placed in the aforementioned ports. In C, intraoperative appearance. Note the instruments placed in each port.
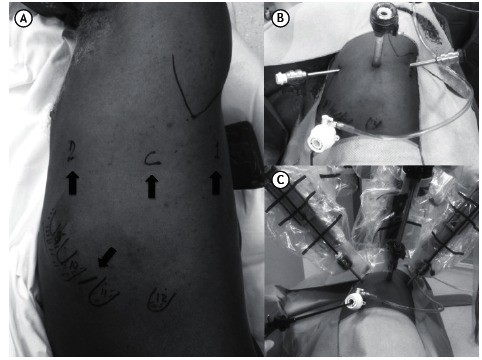
The surgical procedure was systematized in order to minimize intraoperative lung manipulation. In all cases, the first step was to section the pulmonary ligament. Next, in a posterior and superior direction, paraesophageal and subcarinal lymph nodes were dissected. Right interlobar lymph nodes or those located between the pulmonary artery and the left main bronchus were then resected. In cases of right or left lower lobectomy, the oblique fissure is divided and the pulmonary artery is exposed and sectioned with a stapler. In cases of right or left upper lobectomy, dissection is performed cranially, the right main bronchus, the right pulmonary artery, and left pulmonary artery branches being sectioned. Pulmonary artery branches and, subsequently, the pulmonary vein are then divided, the bronchus and oblique fissure remaining to be divided last. In cases of right or left lower lobectomy, the bronchus and pulmonary vein are dissected after the pulmonary artery has been sectioned, being stapled sequentially. The procedure is completed with dissection of right paratracheal lymph nodes and left para-aortic lymph nodes. A 28-Fr chest tube is then introduced through the lower port.
Two aspects of our robotic surgical technique are noteworthy. First, the robotic ports are closed in order to allow the use of carbon dioxide, which, in addition to increasing the workspace by lowering the diaphragm and reducing visual interference from the "smoke" from cauterization, facilitates dissection of hilar structures and the oblique fissure. Second, removal of the surgical specimen is an important step in our robotic surgical technique. To that end, the lower port is used as originally described by Dylewski et al. 12 Given that the lower port is located at the transition between the diaphragm and the chest wall and below the 10th rib, the resected lobe can be removed without the limitation imposed by the ribs, although the same is not true for the remaining ports. Larger specimens can be removed this way as well, resulting in less pain.
In the postoperative period, patients are habitually transferred to the hospital ward. Elderly patients with multiple comorbidities or patients with intraoperative complications are admitted to the ICU. Postoperative analgesia includes oral dipyrone every 6 h and patient-controlled epidural anesthesia (local anesthetics and opioids), which is discontinued immediately after chest tube removal. Anti-inflammatory drugs and oral opioids are administered as needed.
The data in the present study were prospectively collected and stored with the aid of specific software. Continuous variables are expressed as mean and standard deviation or as median and interquartile range. Categorical variables are expressed as absolute numbers and proportions.
RESULTS
During the first three months of our program, ten patients underwent robot-assisted pulmonary lobectomy for the treatment of lung cancer. Patient demographic data are detailed in Table 1. During the same period, seven patients were randomly allocated to the VATS arm of our comparative study, but they were not included in the present interim analysis.
Table 1. Characteristics of the patients studied.a .
| Variable | (N = 10) |
|---|---|
| Gender | |
| Male | 2 (20) |
| Female | 8 (80) |
| Age, yearsb | 64 (55-80) |
| Comorbidities | |
| Systemic arterial hypertension | 7 (70) |
| Diabetes mellitus | 1 (10) |
| COPD | 2 (20) |
| BMIc, kg/m2 | 27.8 ± 4.5 |
| Affected lobe | |
| LUL | 2 (20) |
| RUL | 4 (40) |
| RLL | 4 (40) |
| Clinical stage (TNM staging system) | |
| T1aN0M0 | 3 (30) |
| T1bN0M0 | 4 (40) |
| T2aN0M0 | 1 (10) |
| T2bN0M0 | 1 (10) |
| T1bN0M1b | 1 (10) |
| Tumor diameter*, cmb,d | 2.5 (1.2-4.7) |
LUL: left upper lobe; RUL: right upper lobe; RLL: right lower lobe; and TNM: tumor-node-metastasis. aValues expressed as n (%), except where otherwise indicated. bValues expressed as median (interquartile range).cValue expressed as mean ± SD. dTumor diameter measured at its widest point on CT scans of the chest (lung window).
All patients had peripheral tumors. In nine, lung adenocarcinoma was found to be the most common histological type. Of those nine patients, four had acinar predominant adenocarcinoma, four had lepidic predominant adenocarcinoma, and one had papillary predominant adenocarcinoma. One patient was diagnosed with large cell carcinoma. Of the ten patients included in the present analysis, four underwent mediastinoscopy: two did because of suspicion of mediastinal lymph node involvement; one did because of the presence of a tumor > 3 cm in size; and one did because of a history of surgery for brain metastasis. Pathological examination showed no hilar or mediastinal lymph node involvement in any of the patients.
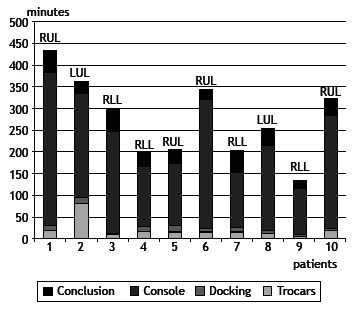
Operative times varied considerably across patients and are detailed in Figure 2. It is of note that, in the cases of patients 2 and 6, intraoperative complications significantly prolonged operative times. In the case of patient 2, the patient had received a stab wound, which required chest tube drainage at the time. The patient did not disclose that information during the process of selection and randomization. However, in the intraoperative period, a large quantity of pleuropulmonary adhesions were identified, most of which were lysed during the VATS procedure performed prior to robot-assisted surgery. In the case of patient 6, the selective tube was displaced during the procedure and compromised the surgical field, given that the operated lung inflated. An attempt was made to reposition the tube with the patient in the lateral decubitus position; however, after several unsuccessful attempts, the robot was disconnected and the tube was repositioned with the patient in the supine position. After the tube was confirmed to be in the correct position, the robot was reconnected and the surgical procedure was completed as originally planned.
Figure 2. Operative times for all patients undergoing robotic lobectomy, in chronological order (mean, 277.3 min). Trocars: time elapsed between skin incision placement and robot docking. It includes port placement, video-assisted thoracoscopic surgery for cavity inspection, and trocar placement. Docking: time elapsed between the positioning of the robotic arms and the beginning of robotic surgery. It includes connecting the robotic arms to the trocars and positioning the robotic instruments (camera and forceps). Console: duration of intracavitary manipulation of instruments using the robotic arms. Conclusion: it includes undocking of the robotic arms, removal of the surgical specimen, and closure of the incisions. LUL: left upper lobe; RUL: right upper lobe; and RLL: right lower lobe.
There were no intraoperative complications, and the mean quantity of bleeding was 49.1 ± 35.7 mL. None of the patients required blood transfusion. Only the first patient required postoperative transfer to the ICU, because of prolonged operative time; the remaining patients were taken to the recovery room and, subsequently, to a ward bed. Data regarding duration of chest tube drainage, length of hospital stay, postoperative pain, and markers of systemic inflammation are summarized in Table 2. There were no deaths or readmissions within the first 30 days after discharge. The only postoperative complication was chest pain (grade 3), which was observed in two patients and prolonged their hospital stay by 1 and 2 days. For pain control, patients received additional doses of intravenous morphine.
Table 2. Robotic lobectomy results in the patients studied.a .
| Variable | (N = 10) |
|---|---|
| Duration of chest tube drainage, h | |
| ≤ 24 | 2 (20) |
| 24-48 | 6 (60) |
| > 48b | 2 (20) |
| Length of hospital stay, h | |
| ≤ 48 | 6 (60) |
| 48-72 | 2 (20) |
| > 72c | 2 (20) |
| Paind,e | |
| Postoperative day 1 | 2.75 ± 2.50 |
| Postoperative day 2 | 0.87 ± 1.80 |
Values expressed as n (%), except where otherwise indicated. bChest tube removed on postoperative days 3 and 5, respectively. cDischarge on postoperative days 4 and 6, respectively. dVisual analog pain scale (Likert scale). eValue expressed as mean ± SD.
Pathological examination revealed complete tumor resection in all cases. The mean number of resected lymph nodes was 9.5 ± 3.5. The number of resected lymph nodes increased with experience; in the last two patients, 12 lymph nodes (7 mediastinal lymph nodes and 5 hilar lymph nodes) and 15 lymph nodes (9 mediastinal lymph nodes and 6 hilar lymph nodes) were resected, whereas, in the first two patients, 5 lymph nodes (2 mediastinal lymph nodes and 3 hilar lymph nodes) and 7 lymph nodes (4 mediastinal lymph nodes and 3 hilar lymph nodes) were resected.
DISCUSSION
The present study showed that, when there is an institutional program and proper training of the multidisciplinary team, robotic thoracic surgery can be implemented with satisfactory results from the very beginning. Although operative times were long, particularly in the first patients, there were no significant complications, and 80% of the patients were discharged within the first 72 h after surgery. Two patients had pain that was more severe, and this prolonged their hospitalizations by 1 and 2 days; however, mean daily pain scores were low, especially in comparison with the results obtained on postoperative day 2.
The results of the present study are very encouraging, especially if we take into account that they refer to our initial experience. Morbidity was found to be very low (two cases of grade 3 pain); this finding is consistent with those of large studies, such as those conducted by Nasir et al. 9 and Melfi et al., 13 who reported mortality rates of less than 0.5% and complication rates of 27% and 33%, respectively. The length of hospital stay in our patients constitutes further evidence that they responded well to robotic surgery. Our finding that 80% of our patients were discharged within the first 72 h after surgery is consistent with those of other studies, in which the length of hospital stay ranged from 2 days to 4 days. 9 , 10 , 13
The fact that operative times were long during our initial experience with robotic surgery is a cause for concern. Our mean operative time was 277.3 min, slightly longer than that for the first 60 procedures performed by Melfi et al., 13 i.e., 237 min. However, results from a large multihospital database in the USA show a mean operative time of 269 min for robotic lobectomy. 10 In addition, despite the small number of patients, our operative times were found to decrease with increasing experience, a finding that is consistent with those of other studies. 13
The technique used in order to perform a robotic lobectomy has changed over time. Initially, the procedure was divided into the robotic dissection phase, in which the robot is used in order to dissect the vessels and bronchi, and the VATS lobectomy phase, in which the robot is removed and the surgeon returns to the operating table in order to staple the vessels and remove the surgical specimen; the procedure involved incisions that resembled those used for VATS. 5 Subsequently, a total endoscopic robotic video-assisted approach involving three robotic arms and the use of carbon dioxide in order to increase the surgical field was developed. 12 Finally, completely portal robotic lobectomy with four arms emerged, providing surgeons with greater autonomy, given that the fourth arm allows surgeons to retract the lung for themselves. 6 Although the use of a total endoscopic robotic video-assisted approach is clearly advantageous, no differences have been reported between the three-arm approach and the four-arm approach. We chose the former because it is easier to learn and less costly, given that fewer forceps are used. Given that the assistant surgeon can easily retract the lung, we believe that the advantage of using a fourth robotic arm is relative.
The advantages of robotic surgery over conventional surgery have been demonstrated by Cerfolio et al., 6 who, in a comparative study including propensity score analysis, found a lower rate of postoperative complications (27% vs. 38%) and a shorter hospital stay (median length of hospital stay, 2 days vs. 4 days) in patients undergoing robotic surgery. Similar results were reported in another study, in which data from a large US database were analyzed. 14 The authors of the aforementioned study found significant reductions in mortality, length of hospital stay, and overall complication rates in the group of patients undergoing robotic surgery. 14
The advantages of robotic lobectomy over VATS lobectomy are less clear. Studies in which large databases and propensity scores were used in order to compare the two techniques showed no differences regarding morbidity, mortality, or length of hospital stay. 10 , 14 Randomized studies are needed in order to confirm the aforementioned findings and compare the two methods in terms of long-term survival. Although VATS is an excellent procedure in experienced hands, the learning curve for it is steep because of limitations inherent to the use of new instruments and approaches. The da Vinci robotic surgical system was primarily developed to overcome such limitations. 15 The learning curve for robotic lobectomy when performed by surgeons who are experienced in video-assisted thoracic surgery has been estimated at 18 ± 3 cases. 16
We believe that robotic surgery was successfully implemented at our institution because of institutional investment and the intensive training of all teams involved; therefore, we believe that our results are generalizable to specialized tertiary institutions adopting the same policy. The main limitation of the present study is the number of patients, which is insufficient to ensure the safety of the method in Brazil. Therefore, more cases are needed in order to confirm the low rate of complications observed in the present study.
In conclusion, robotic thoracic surgery can be safely implemented in a tertiary hospital provided that all teams involved participate in the process. Our initial results with robotic lobectomy are very encouraging, and we hope to publish definitive comparative data on robotic lobectomy and VATS lobectomy at our institution.
ACKNOWLEDGMENTS
We would like to thank Dr. Ricardo Abdalla for his commitment, expertise, and dedication to tutoring us in all of the cases reported in the present study. In addition, we would like to thank Professor Ivan Ceconello for his unconditional support and for making it possible to implement a robotic surgery program at the ICESP. Finally, we would like to thank Evelise Zaidan and the ICESP Clinical Research Center for their operational support and the collection of the study data.
Footnotes
Study carried out in the Disciplina de Cirurgia Torácica and at the Instituto do Câncer do Estado de São Paulo - ICESP - Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil.
Financial support: None.
REFERENCES
- 1.Terra RM, Waisberg DR, Almeida JJ, Devido MS, Pego-Fernandes PM, Jatene FB. Does videothoracoscopy improve clinical outcomes when implemented as part of a pleural empyema treatment algorithm. Clinics (Sao Paulo) 2012;67(6):557–564. doi: 10.6061/clinics/2012(06)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirino LM, Milanez de Campos JR, Fernandez A, Samano MN, Fernandez PP, Filomeno LT. Diagnosis and treatment of mediastinal tumors by thoracoscopy. Chest. 2000;117(6):1787–1792. doi: 10.1378/chest.117.6.1787. [DOI] [PubMed] [Google Scholar]
- 3.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy experience with 1,100 cases. Ann Thorac Surg. 2006;81(2):421–425. doi: 10.1016/j.athoracsur.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 4.Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, Rizk NP. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138(1):11–18. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Gharagozloo F, Margolis M, Tempesta B, Strother E, Najam F. Robot-assisted lobectomy for early-stage lung cancer report of 100 consecutive cases. Ann Thorac Surg. 2009;88(2):380–384. doi: 10.1016/j.athoracsur.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 6.Cerfolio RJ, Bryant AS, Skylizard L, Minninch DJ. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg. 2011;142(4):740–746. doi: 10.1016/j.jtcvs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Louie BE, Farivar AS, Aye RW, Vallières E. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg. 2012;93(5):1598–1604. doi: 10.1016/j.athoracsur.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 8.Park BJ, Melfi F, Mussi A, Maisonneuve P, Spaggiari L, Da Silva RK. Robotic lobectomy for non-small cell lung cancer (NSCLC) long-term oncologic results. J Thorac Cardiovasc Surg. 2012;143(2):383–389. doi: 10.1016/j.jtcvs.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Nasir BS, Bryant AS, Minnich DJ, Wei B, Cerfolio RJ. Performing robotic lobectomy and segmentectomy cost, profitability, and outcomes. Ann Thorac Surg. 2014;98(1):203–208. doi: 10.1016/j.athoracsur.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 10.Swanson SJ, Miller DL, McKenna RJ, Jr, Howington J, Marshall MB, Yoo AC. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection results from a multihospital database (Premier) J Thorac Cardiovasc Surg. 2014;147(3):929–937. doi: 10.1016/j.jtcvs.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Bethesda: National Institutes of Health; 2014. 196p. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf [Google Scholar]
- 12.Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg. 2011;23(1):36–42. doi: 10.1053/j.semtcvs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Melfi FM, Fanucchi O, Davini F, Romano G, Lucchi M, Dini P. Robotic lobectomy for lung cancer evolution in technique and technology. Eur J Cardiothorac Surg. 2014;46(4):626–630. doi: 10.1093/ejcts/ezu079. [DOI] [PubMed] [Google Scholar]
- 14.Kent M, Wang T, Whyte R, Curran T, Flores R, Gangadharan S. Open, video-assisted thoracic surgery, and robotic lobectomy review of a national database. Ann Thorac Surg. 2014;97(1):236–242. doi: 10.1016/j.athoracsur.2013.07.117. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Asaf BB, Cerfolio RJ, Sood J, Kumar R. Robotic lobectomy The first Indian report. J Minim Access Surg. 2015;11(1):94–98. doi: 10.4103/0972-9941.147758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer M, Gharagozloo F, Tempesta B, Margolis M, Strother E, Christenson D. The learning curve of robotic lobectomy. Int J Med Robot. 2012;8(4):448–452. doi: 10.1002/rcs.1455. [DOI] [PubMed] [Google Scholar]


