Abstract
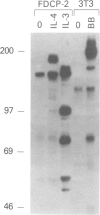
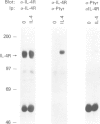
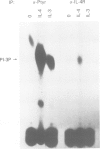
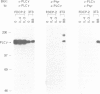
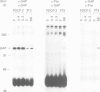
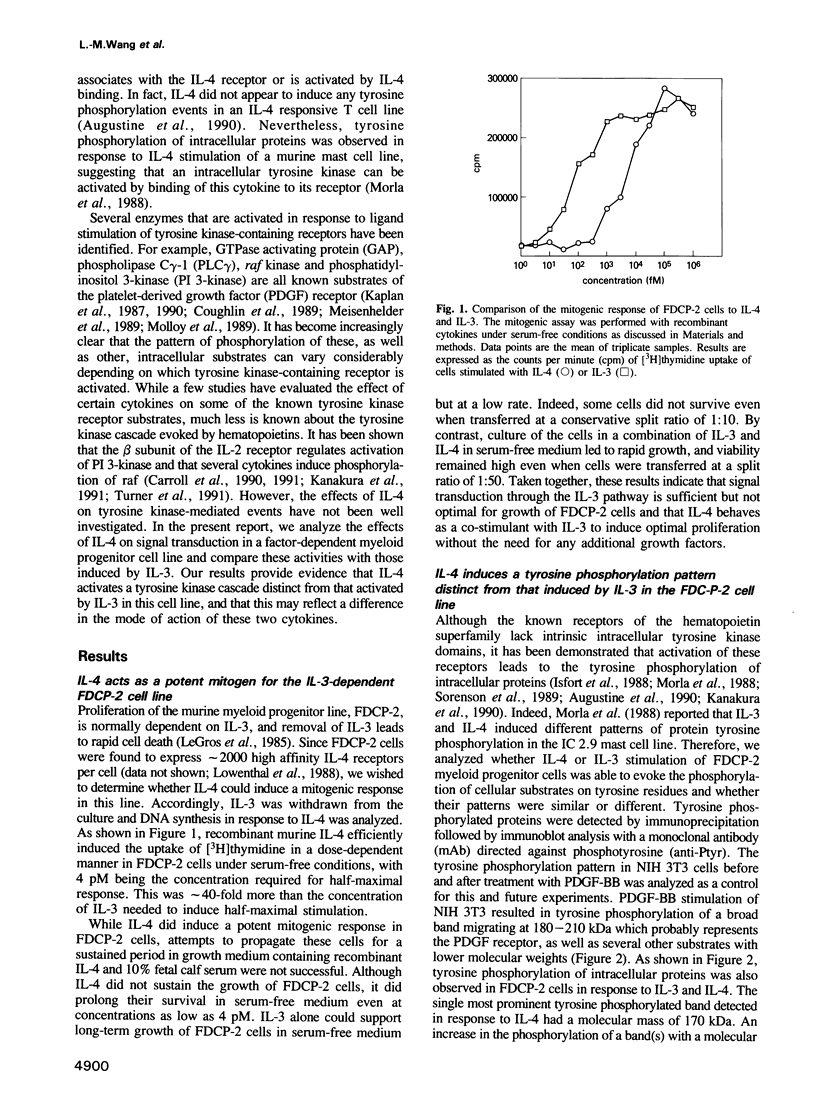
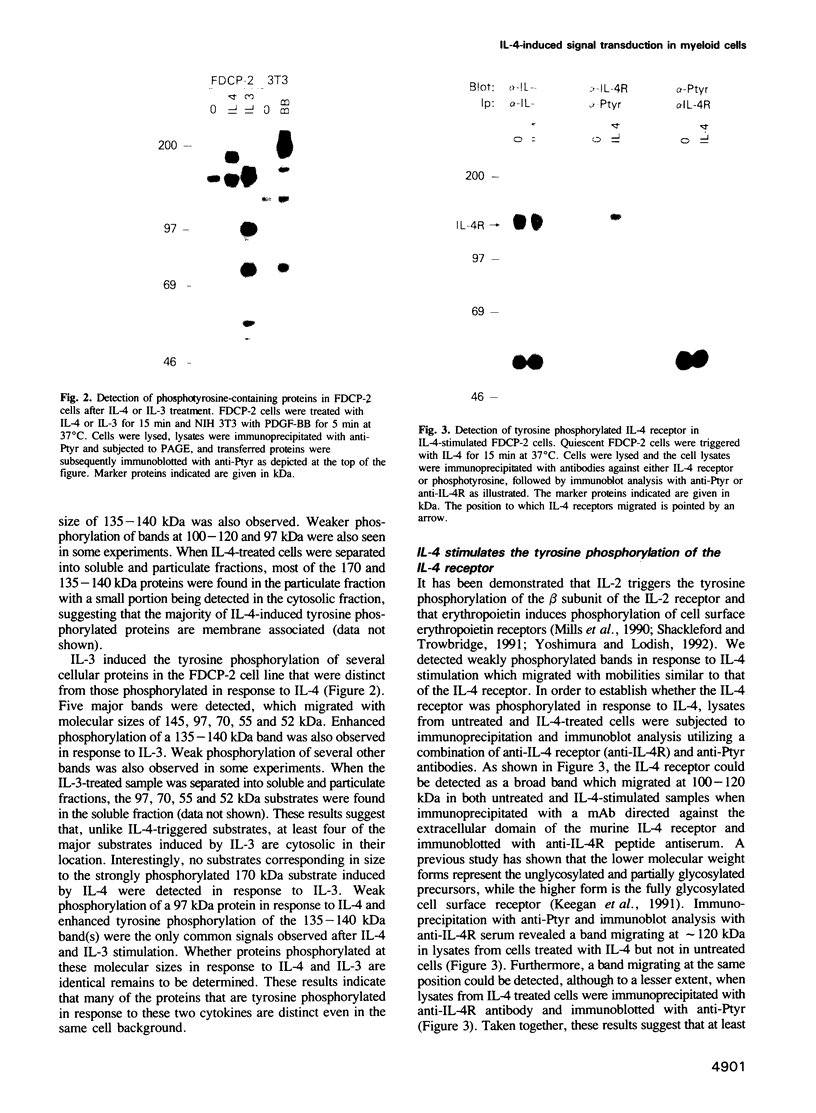
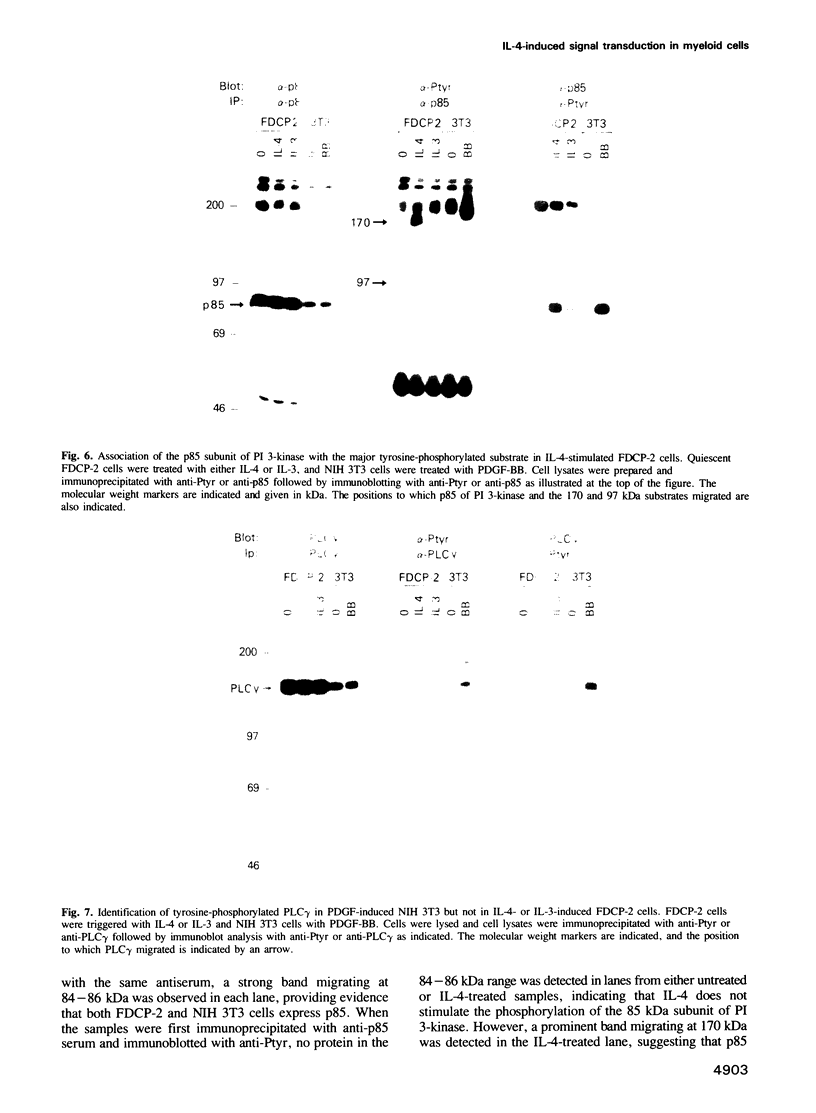
Interleukin-4 (IL-4) was shown to induce a potent mitogenic response in the IL-3-dependent myeloid progenitor cell line, FDCP-2. Although IL-4 could not sustain long-term growth of FDCP-2 cells, it enhanced their growth in serum-free medium containing IL-3. IL-4 triggered prominent tyrosine phosphorylation of a substrate(s) migrating at 170 kDa and less striking phosphorylation of several other proteins, including the IL-4 receptor. By contrast, IL-3 induced distinct tyrosine phosphorylation of proteins migrating at 145, 97, 70, 55 and 52 kDa in the same cell line. IL-4 treatment of FDCP-2 cells caused a dramatically strong association of phosphatidylinositol 3-kinase (PI 3-kinase) both with the 170 kDa tyrosine phosphorylated substrate and with the IL-4 receptor itself. By contrast, IL-3 triggered only weak association of PI 3-kinase activity with the 97 kDa substrate. While IL-4 did not affect cellular raf, IL-3 stimulation did induce a shift in its mobility presumably due to serine/threonine phosphorylation. Taken together, our results indicate that IL-4 and IL-3 activate distinct phosphorylation cascades in the same cell background; this may reflect a difference in the biological function of these two cytokines.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine J. A., Schlager J. W., Abraham R. T. Differential effects of interleukin-2 and interleukin-4 on protein tyrosine phosphorylation in factor-dependent murine T cells. Biochim Biophys Acta. 1990 May 2;1052(2):313–322. doi: 10.1016/0167-4889(90)90227-5. [DOI] [PubMed] [Google Scholar]
- Augustine J. A., Sutor S. L., Abraham R. T. Interleukin 2- and polyomavirus middle T antigen-induced modification of phosphatidylinositol 3-kinase activity in activated T lymphocytes. Mol Cell Biol. 1991 Sep;11(9):4431–4440. doi: 10.1128/mcb.11.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carroll M. P., Clark-Lewis I., Rapp U. R., May W. S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990 Nov 15;265(32):19812–19817. [PubMed] [Google Scholar]
- Carroll M. P., Spivak J. L., McMahon M., Weich N., Rapp U. R., May W. S. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991 Aug 15;266(23):14964–14969. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Conrad D. H., Waldschmidt T. J., Lee W. T., Rao M., Keegan A. D., Noelle R. J., Lynch R. G., Kehry M. R. Effect of B cell stimulatory factor-1 (interleukin 4) on Fc epsilon and Fc gamma receptor expression on murine B lymphocytes and B cell lines. J Immunol. 1987 Oct 1;139(7):2290–2296. [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio V., Welham M. J., Abraham S., Dryden P., Schrader J. W. p21ras activation via hemopoietin receptors and c-kit requires tyrosine kinase activity but not tyrosine phosphorylation of p21ras GTPase-activating protein. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1587–1591. doi: 10.1073/pnas.89.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J. A., Kaplan D. R., Kavanaugh W. M., Turck C. W., Williams L. T. A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol Cell Biol. 1991 Feb;11(2):1125–1132. doi: 10.1128/mcb.11.2.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizzi J. P., Zuber C. E., Harada N., Gorman D. M., Djossou O., Kastelein R., Banchereau J., Howard M., Miyajima A. Molecular cloning of a cDNA encoding the human interleukin 4 receptor. Int Immunol. 1990;2(7):669–675. doi: 10.1093/intimm/2.7.669. [DOI] [PubMed] [Google Scholar]
- Grabstein K., Eisenman J., Mochizuki D., Shanebeck K., Conlon P., Hopp T., March C., Gillis S. Purification to homogeneity of B cell stimulating factor. A molecule that stimulates proliferation of multiple lymphokine-dependent cell lines. J Exp Med. 1986 Jun 1;163(6):1405–1414. doi: 10.1084/jem.163.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Lacal P. M., Robbins K. C. Thrombin-dependent association of phosphatidylinositol-3 kinase with p60c-src and p59fyn in human platelets. Mol Cell Biol. 1990 Jul;10(7):3806–3809. doi: 10.1128/mcb.10.7.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Brock M., Taylor C. R., Lyons B. IL-4 regulates differentiation and proliferation of human precursor B cells. J Immunol. 1988 Aug 15;141(4):1185–1190. [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Hu P., Margolis B., Skolnik E. Y., Lammers R., Ullrich A., Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol. 1992 Mar;12(3):981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak S. A., Gollnick S. O., Conrad D. H., Kehry M. R. Murine B-cell stimulatory factor 1 (interleukin 4) increases expression of the Fc receptor for IgE on mouse B cells. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4606–4610. doi: 10.1073/pnas.84.13.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzerda R. L., March C. J., Mosley B., Lyman S. D., Vanden Bos T., Gimpel S. D., Din W. S., Grabstein K. H., Widmer M. B., Park L. S. Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfamily. J Exp Med. 1990 Mar 1;171(3):861–873. doi: 10.1084/jem.171.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R., Huhn R. D., Frackelton A. R., Jr, Ihle J. N. Stimulation of factor-dependent myeloid cell lines with interleukin 3 induces tyrosine phosphorylation of several cellular substrates. J Biol Chem. 1988 Dec 15;263(35):19203–19209. [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Wood K. W., Mamon H. J., Okuda K., Roberts T. M., Griffin J. D. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce rapid phosphorylation and activation of the proto-oncogene Raf-1 in a human factor-dependent myeloid cell line. Blood. 1991 Jan 15;77(2):243–248. [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Keegan A. D., Beckmann M. P., Park L. S., Paul W. E. The IL-4 receptor: biochemical characterization of IL-4-binding molecules in a T cell line expressing large numbers of receptors. J Immunol. 1991 Apr 1;146(7):2272–2279. [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Klippel A., Escobedo J. A., Fantl W. J., Williams L. T. The C-terminal SH2 domain of p85 accounts for the high affinity and specificity of the binding of phosphatidylinositol 3-kinase to phosphorylated platelet-derived growth factor beta receptor. Mol Cell Biol. 1992 Apr;12(4):1451–1459. doi: 10.1128/mcb.12.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros G. S., Gillis S., Watson J. D. Induction of IL 2 responsiveness in a murine IL 3-dependent cell line. J Immunol. 1985 Dec;135(6):4009–4014. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Castle B. E., Christiansen J., Schreurs J., Rennick D., Arai N., Hoy P., Takebe Y., Howard M. Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J Immunol. 1988 Jan 15;140(2):456–464. [PubMed] [Google Scholar]
- McInnes A., Rennick D. M. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988 Feb 1;167(2):598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mills G. B., May C., McGill M., Fung M., Baker M., Sutherland R., Greene W. C. Interleukin 2-induced tyrosine phosphorylation. Interleukin 2 receptor beta is tyrosine phosphorylated. J Biol Chem. 1990 Feb 25;265(6):3561–3567. [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Haldar S., Prystowsky M. B., Lammie P. Lymphokine regulation of inflammatory processes: interleukin-4 stimulates fibroblast proliferation. Clin Immunol Immunopathol. 1988 Nov;49(2):292–298. doi: 10.1016/0090-1229(88)90119-5. [DOI] [PubMed] [Google Scholar]
- Moran M. F., Koch C. A., Anderson D., Ellis C., England L., Martin G. S., Pawson T. Src homology region 2 domains direct protein-protein interactions in signal transduction. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8622–8626. doi: 10.1073/pnas.87.21.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A. O., Schreurs J., Miyajima A., Wang J. Y. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Bond M. W., Coffman R. L., Ohara J., Paul W. E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991 Apr;6(4):495–500. [PubMed] [Google Scholar]
- Reedijk M., Liu X. Q., Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990 Nov;10(11):5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Ligand-stimulated tyrosine phosphorylation of the IL-2 receptor beta chain and receptor-associated proteins. Cell Regul. 1991 Jan;2(1):73–85. doi: 10.1091/mbc.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S. E., Chatterjee S., Chaudhuri M., White M. F. YMXM motifs of IRS-1 define substrate specificity of the insulin receptor kinase. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2027–2031. doi: 10.1073/pnas.89.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Margolis B., Mohammadi M., Lowenstein E., Fischer R., Drepps A., Ullrich A., Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991 Apr 5;65(1):83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. B cell stimulatory factor-1 (interleukin 4) prepares resting murine B cells to secrete IgG1 upon subsequent stimulation with bacterial lipopolysaccharide. J Immunol. 1987 Jul 1;139(1):10–17. [PubMed] [Google Scholar]
- Sorensen P. H., Mui A. L., Murthy S. C., Krystal G. Interleukin-3, GM-CSF, and TPA induce distinct phosphorylation events in an interleukin 3-dependent multipotential cell line. Blood. 1989 Feb;73(2):406–418. [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Turner B., Rapp U., App H., Greene M., Dobashi K., Reed J. Interleukin 2 induces tyrosine phosphorylation and activation of p72-74 Raf-1 kinase in a T-cell line. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1227–1231. doi: 10.1073/pnas.88.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Stegmann E. W., Dull T. J., Ullrich A., Kahn C. R. Characterization of an endogenous substrate of the insulin receptor in cultured cells. J Biol Chem. 1987 Jul 15;262(20):9769–9777. [PubMed] [Google Scholar]
- Widmer M. B., Acres R. B., Sassenfeld H. M., Grabstein K. H. Regulation of cytolytic cell populations from human peripheral blood by B cell stimulatory factor 1 (interleukin 4). J Exp Med. 1987 Nov 1;166(5):1447–1455. doi: 10.1084/jem.166.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Lodish H. F. In vitro phosphorylation of the erythropoietin receptor and an associated protein, pp130. Mol Cell Biol. 1992 Feb;12(2):706–715. doi: 10.1128/mcb.12.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Ransom J., Frank G., Fischer M., Howard M. Interleukin 4 is a growth factor for activated thymocytes: possible role in T-cell ontogeny. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3856–3860. doi: 10.1073/pnas.84.11.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]