Abstract
Purpose
To evaluate induction chemotherapy with docetaxel, cisplatin, and fluorouracil (TPF) followed by surgery and postoperative radiotherapy versus up-front surgery and postoperative radiotherapy in patients with locally advanced resectable oral squamous cell carcinoma (OSCC).
Patients and Methods
A prospective open-label phase III trial was conducted. Eligibility criteria included untreated stage III or IVA locally advanced resectable OSCC. Patients received two cycles of TPF induction chemotherapy (docetaxel 75 mg/m2 on day 1, cisplatin 75 mg/m2 on day 1, and fluorouracil 750 mg/m2 on days 1 to 5) followed by radical surgery and postoperative radiotherapy (54 to 66 Gy) versus up-front radical surgery and postoperative radiotherapy. The primary end point was overall survival (OS). Secondary end points included local control and safety.
Results
Of the 256 patients enrolled onto this trial, 222 completed the full treatment protocol. There were no unexpected toxicities, and induction chemotherapy did not increase perioperative morbidity. The clinical response rate to induction chemotherapy was 80.6%. After a median follow-up of 30 months, there was no significant difference in OS (hazard ratio [HR], 0.977; 95% CI, 0.634 to 1.507; P = .918) or disease-free survival (HR, 0.974; 95% CI, 0.654 to 1.45; P = .897) between patients treated with and without TPF induction. Patients in the induction chemotherapy arm with a clinical response or favorable pathologic response (≤ 10% viable tumor cells) had superior OS and locoregional and distant control.
Conclusion
Our study failed to demonstrate that TPF induction chemotherapy improves survival compared with up-front surgery in patients with resectable stage III or IVA OSCC.
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most common malignant tumor in the oral and maxillofacial region, with approximately 300,000 new cases worldwide each year.1,2 Although progress has been achieved in radical surgical resection with reconstruction and use of postoperative radiotherapy/chemoradiotherapy, the 5-year survival rate has not improved substantially in recent years, remaining at 50% to 60%3,4 and even lower in patients with locally advanced lesions. At present, for patients with resectable locally advanced OSCC, national US and Chinese guidelines5,6 recommend surgical management of the primary tumor and neck followed by postoperative radiotherapy or chemoradiotherapy, depending on the presence of intermediate- or high-risk features. A recent randomized study of surgery preceded or not by induction chemotherapy with cisplatin and fluorouracil (PF) in OSCC failed to demonstrate an improvement in overall survival (OS) with the induction chemotherapy strategy.7 Nonetheless, this study demonstrated that induction chemotherapy reduced the risk of distant metastasis but had no impact on locoregional control, an observation corroborated by meta-analyses8,9 and multi-institutional experience.10 Although these results do not justify the routine use of induction chemotherapy for locally advanced head and neck squamous cell carcinomas (HNSCC), they suggest that this strategy warrants further study.
Novel induction chemotherapy regimens adding docetaxel to PF (TPF) have shown promising results in two recent phase III trials (TAX323 and TAX324) in the setting of nonsurgical treatment of locally advanced HNSCC. In both studies, patients were randomly assigned to receive PF versus TPF before radiotherapy (TAX323) or chemoradiotherapy (TAX324). There was a statistically significant improvement in OS in patients randomly assigned to the TPF arm. As a result, TPF is suggested as the preferred combination chemotherapy regimen when induction treatment is used for nonsurgical management of patients with HNSCC.11–13 However, the potential benefits of TPF induction have recently been questioned; the DeCIDE and PARADIGM trials compared up-front chemoradiotherapy versus TPF induction followed by chemoradiotherapy and failed to demonstrate a significant improvement in OS or disease-free survival (DFS) with TPF.14,15 It is still unknown whether TPF induction improves outcomes when administered before surgery in patients with locally advanced HNSCC, especially OSCC.
We hypothesized that TPF administered before surgery would improve survival in patients with resectable locally advanced OSCC. To test this hypothesis, we conducted a randomized phase III trial of TPF induction chemotherapy followed by surgery and postoperative radiotherapy compared with up-front surgery and postoperative radiotherapy in this patient population.
PATIENTS AND METHODS
Study Design
This was a prospective single-center open-label randomized phase III trial. The study was approved by the Institutional Ethics Committee, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine.
Eligibility Criteria
Patients age 18 to 75 years with histologically confirmed OSCC (originating in oral cavity) were eligible. Patients were required to have a resectable lesion, in the opinion of treating surgeons, with clinical stage III or IVA disease (T1-2N1-2M0 or T3-4N0-2M0 according to Union for International Cancer Control [2002]). Other inclusion criteria included: Karnofsky performance status > 60%, WBC count > 3,000/μL, hemoglobin > 8 g/L, platelet count > 80,000/μL, ALT and AST < 2.5× the upper limit of normal, bilirubin and serum creatinine < 1.5× the upper limit of normal. Patients were excluded if they had distant metastasis or other cancers, had undergone surgery involving primary tumor or lymph nodes (except diagnostic biopsy), had received prior radiotherapy or chemotherapy, had had other malignancies within 5 years, or had creatinine clearance < 30 mL/min.
Randomization
After eligibility was confirmed, patients were randomly allocated to the control arm (surgery followed by postoperative radiotherapy) or experimental arm (TPF induction chemotherapy followed by surgery and postoperative radiotherapy). Randomization without stratification factors was performed using sealed envelopes containing a computer-generated random number code prepared by the Department of Statistics, Shanghai Jiao Tong University School of Medicine. Participants were randomly assigned in blocks of four based on a one-to-one treatment allocation; the block size was known only by the statistician. Treatment allocation was not masked. Treatment started within 10 days after diagnostic biopsy.
Interventions
Induction chemotherapy.
In patients assigned to the experimental arm, the palpable edges of the primary lesion (both longest and shortest axes) were marked before induction chemotherapy by at least four points, which were 0.5 cm away from the lesion; this was done to allow for a reliable clinical assessment of response to treatment. Chemotherapy consisted of docetaxel 75 mg/m2 intravenously on day 1, followed by cisplatin 75 mg/m2 intravenously on day 1, followed by fluorouracil 750 mg/m2 per day as a 120-hour continuous intravenous infusion on days 1 through 5. Induction chemotherapy was administered every 3 weeks for two cycles, unless there was disease progression or unacceptable toxicity. Supportive measures included dexamethasone, antiemetics, and hydration/diuretics; prophylactic antibiotics were administered starting on day 5 of each cycle for 3 days. Primary prophylaxis with recombinant granulocyte colony-stimulating factor was not recommended. Chemotherapy dose reductions were allowed for grade 3 to 4 toxicities occurring after cycle one: 25% and 50% dose reductions of the three chemotherapy agents were suggested for grades 3 and 4 hematologic or GI toxicities, respectively; 25% and 50% cisplatin dose reductions were suggested for grades 3 and 4 renal toxicities, respectively. Surgery was performed at least 2 weeks after completion of induction chemotherapy.
Surgery.
Radical resection of the primary lesion and full neck dissection (functional or radical) with appropriate reconstruction (pedicle or free flap) was performed. The safety margins of the primary lesion were 1.5 cm away from the palpable margins; for patients who received induction chemotherapy, the safety margins were 1.0 cm away from the marks that were placed before induction chemotherapy to ensure the same extent of surgery in both arms. Frozen sections during surgery were performed to confirm adequate margins.
Postoperative radiotherapy.
Radiotherapy was initiated 4 to 6 weeks after surgery. Standard conformal or intensity-modulated radiotherapy was allowed at a dose of 1.8 to 2 Gy per day, 5 days per week, for 6 weeks (54 to 60 Gy in total). In patients with high-risk features, such as positive surgical margins, extracapsular nodal spread, or vascular embolism, a total radiation dose of 66 Gy was recommended. Patients did not receive concurrent chemotherapy with postoperative radiotherapy.
Assessments
A complete medical history was obtained, and tumor assessment was performed at baseline. Clinical tumor response was determined by clinical evaluation and imaging studies (performed at baseline and 2 weeks after cycle two of induction chemotherapy). Responses were characterized according to RECIST (version 1.0).16 In patients receiving induction chemotherapy, pathologic response was assessed by examination of at least 20 slides of the resected specimen. A favorable response was defined as absence of any tumor cells (pathologic complete response) or presence of scattered foci of a few tumor cells (minimal residual disease with < 10% viable tumor cells), as previously described by Licitra et al.7 An unfavorable pathologic response was defined as the presence of ≥ 10% viable tumor cells in the resected specimen. Toxicities were assessed weekly during and after completion of induction chemotherapy and radiotherapy according to Common Terminology Criteria for Adverse Events (version 3.0).
Follow-Up and Outcomes
After completion of radiotherapy, patients were monitored every 3 months during the first 2 years, every 6 months during the subsequent 3 to 5 years, and once per year thereafter until death or data censoring. OS was calculated from the date of random assignment to the date of death. DFS, locoregional recurrence–free survival (LRFS), and distant metastasis–free survival (DMFS) were calculated from the date of random assignment to recurrence, locoregional recurrence, distant metastasis, or death resulting from any cause.
Statistical Considerations
The primary end point was OS. With a sample size of 256 patients, the study had a power of 83%, assuming 5-year survival rates of 55% in the experimental arm and 35% in the control arm,17,18 with a two-sided log-rank test at a level of significance of .05. The recruitment and follow-up periods would both be 3 years, with an estimated rate of early dropout or loss to follow-up of 15%. The sample size was calculated using the software STPLAN. Secondary end points included response to induction chemotherapy, DFS, and toxicity.
For descriptive analysis, categorical data were expressed as number and percentage. The survival analysis was conducted using the Kaplan-Meier method and log-rank test. Hazard ratios (HRs) were calculated using the Cox proportional hazards model. Spearman rank correlation coefficient was calculated to compare the correlation between baseline factors and pathologic and clinical responses. The intention-to-treat principle was applied for efficacy analysis. All treated patients were included in the analysis of adverse events (AEs). All hypothesis-generating tests were two sided, at a significance level of .05.
RESULTS
Patients
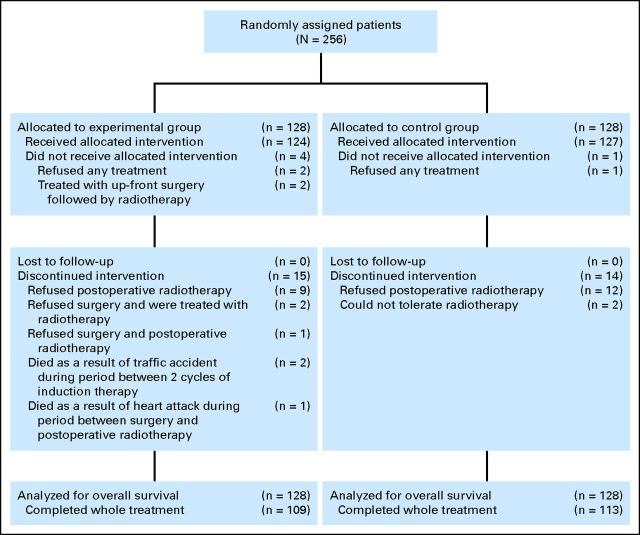
From March 2008 to December 2010, 256 eligible patients were randomly assigned to two study arms (128 patients in each arm). Their baseline demographic and clinical characteristics are summarized in Table 1. After random assignment, five patients withdrew from the trial, three patients died as a result of non–cancer- and non–treatment-related causes, and 26 patients did not complete the full treatment protocol (Fig 1). No patients were lost to follow-up. The median follow-up time was 30 months.
Table 1.
Baseline Patient Demographic and Clinical Characteristics
| Characteristic | Total (N = 256) |
Control Arm (n = 128) |
Experimental Arm (n = 128) |
P* | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Sex | .683 | ||||||
| Male | 179 | 69.9 | 88 | 68.8 | 91 | 71.1 | |
| Female | 77 | 30.1 | 40 | 31.2 | 37 | 28.9 | |
| Age, years | .792 | ||||||
| Median | 55 | 56 | 55 | ||||
| Range | 26-75 | 26-75 | 29-74 | ||||
| < 60 | 168 | 65.6 | 85 | 66.4 | 83 | 64.8 | |
| ≥ 60 | 88 | 34.4 | 43 | 33.6 | 45 | 35.2 | |
| Site | .509 | ||||||
| Tongue | 113 | 44.1 | 60 | 46.9 | 53 | 41.4 | |
| Buccal | 45 | 17.6 | 20 | 15.6 | 25 | 19.5 | |
| Gingiva | 40 | 15.6 | 19 | 14.8 | 21 | 16.4 | |
| Floor of mouth | 30 | 11.7 | 18 | 14.1 | 12 | 9.4 | |
| Palate | 18 | 7.0 | 6 | 4.7 | 12 | 9.4 | |
| Retromolar trigone | 10 | 3.9 | 5 | 3.9 | 5 | 3.9 | |
| T stage | .299 | ||||||
| T1 | 9 | 3.5 | 6 | 4.7 | 3 | 2.3 | |
| T2 | 57 | 22.3 | 27 | 21.1 | 30 | 23.4 | |
| T3 | 149 | 58.2 | 79 | 61.7 | 70 | 54.7 | |
| T4 | 41 | 16.0 | 16 | 12.5 | 25 | 19.5 | |
| N stage | .294 | ||||||
| N0 | 110 | 43.0 | 61 | 47.7 | 49 | 38.3 | |
| N1 | 94 | 36.7 | 42 | 32.8 | 52 | 40.6 | |
| N2 | 52 | 20.3 | 25 | 19.5 | 27 | 21.1 | |
| Disease stage | .223 | ||||||
| III | 177 | 69.1 | 93 | 72.7 | 84 | 65.6 | |
| IVA | 79 | 30.9 | 35 | 27.3 | 44 | 34.4 | |
| Pathologic differentiation | .802 | ||||||
| Well | 80 | 31.2 | 38 | 29.7 | 42 | 32.8 | |
| Moderate | 165 | 64.5 | 85 | 66.4 | 80 | 62.5 | |
| Poor | 11 | 4.3 | 5 | 3.9 | 6 | 4.7 | |
| Smoking status† | .134 | ||||||
| Current/former | 126 | 49.2 | 57 | 44.5 | 69 | 53.9 | |
| Never | 130 | 50.8 | 71 | 55.5 | 59 | 46.1 | |
| Alcohol use‡ | .440 | ||||||
| Positive | 98 | 40.6 | 46 | 39.8 | 52 | 41.4 | |
| Negative | 158 | 59.4 | 82 | 60.2 | 76 | 58.6 | |
P value from χ2 test was reported to compare baseline characteristics between the two treatment arms.
Former/current smokers defined as ≥ one pack-year history of smoking.
Positive alcohol use was defined as current alcohol use of > one drink per day for 1 year (12 oz of beer with 5% alcohol, 5 oz of wine with 12% to 15% alcohol, or 1 oz of liquor with 45% to 60% alcohol). All other patients were classified as negative alcohol users.
Fig 1.
CONSORT diagram.
Treatment Characteristics
In the experimental arm, 1.7% (two of 124) and 98.4% of patients (122 of 124) received one and two cycles of treatment, respectively; chemotherapy dose reductions were implemented in nine patients (7.3%); 91.6% of patients (109 of 119) underwent surgery within 4 weeks after induction chemotherapy (median, 21 days); 96.4% of patients (107 of 111) received radiotherapy no more than 6 weeks after surgery, including 46.8% (52 of 111) within 4 weeks. In the control arm, 97.3% of patients (110 of 113) received radiotherapy no more than 6 weeks after surgery, including 41.6% (47 of 113) within 4 weeks. Appendix Table A1 (online only) summarizes the surgical and radiation treatments.
Pathologic stage and tumor characteristics are listed in Table 2. After pathologic review of the surgical specimen, 15 patients in the control arm who were clinically classified as having N0 disease had pathologic nodal involvement (N+), and two patients who were clinically classified as having N+ disease had pathologic N0 disease; in the experimental arm, seven patients who were clinically N0 before chemotherapy had N+ disease, and 16 patients who were clinically N+ before chemotherapy had pathologic N0 disease.
Table 2.
Description of Pathologic Characteristics of Tumors at Surgical Resection
| Characteristic | Control Arm (n = 127) |
Experimental Arm (n = 121) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Pathologic T stage | ||||
| pT0 | 0 | 0 | 15 | 12.4 |
| pT1 | 7 | 5.5 | 28 | 22.0 |
| pT2 | 29 | 22.8 | 42 | 34.7 |
| pT3 | 64 | 50.4 | 26 | 20.5 |
| pT4 | 27 | 21.3 | 10 | 8.3 |
| Pathologic margins of resection | ||||
| Negative | 127 | 100 | 121 | 100 |
| Positive | 0 | 0 | 0 | 0 |
| Pathologic N stage | ||||
| pN0 | 48 | 37.8 | 55 | 45.5 |
| pN1 | 24 | 18.9 | 17 | 14.0 |
| pN2 | 55 | 43.3 | 49 | 40.5 |
| pN2a | 3 | 2.4 | 3 | 2.5 |
| pN2b | 42 | 30.1 | 35 | 28.9 |
| pN2c | 10 | 7.9 | 11 | 9.1 |
| No. of positive lymph nodes | ||||
| 0 | 48 | 37.8 | 55 | 45.5 |
| 1 | 27 | 21.3 | 20 | 16.5 |
| 2 to 5 | 43 | 33.9 | 38 | 31.4 |
| 6 to 10 | 8 | 6.3 | 7 | 5.8 |
| > 10 | 1 | 0.8 | 1 | 0.8 |
| Extracapsular spread | ||||
| No lymph node involvement | 48 | 37.8 | 55 | 45.5 |
| Yes | 21 | 16.5 | 16 | 13.2 |
| No | 58 | 45.7 | 50 | 41.3 |
Response to TPF Induction Chemotherapy
Responses by RECIST in 124 patients in whom induction chemotherapy was initiated were as follows: 8.1% (10 patients), complete response; 72.6% (90 patients), partial response; 16.9% (21 patients), stable disease; and 0.8%, (one patient), disease progression, totaling a response rate of 80.6%. Two patients (1.6%) died as a result of non–treatment- and non–cancer-related causes during induction chemotherapy and were considered unevaluable for response. A favorable pathologic response was observed in 27.7% of patients (33 of 119) for whom this analysis could be performed, including 16 patients (13.4%) achieving pathologic complete response.
OS and DFS
At the time of data analysis, 82 patients had died (42 and 40 in control and experimental arms, respectively). There was no significant difference in OS between the two arms (HR, 0.977; 95% CI, 0.634 to 1.507; P = .918; Fig 2A). The estimated 2-year OS was 68.2% and 68.8% in the control and experimental arms, respectively. Similarly, there was no significant difference in DFS between the two arms (HR, 0.974; 95% CI, 0.654 to 1.45; P = .897; Fig 2B). The 2-year DFS was 63.6% and 62.2% in the control and experimental arms, respectively.
Fig 2.
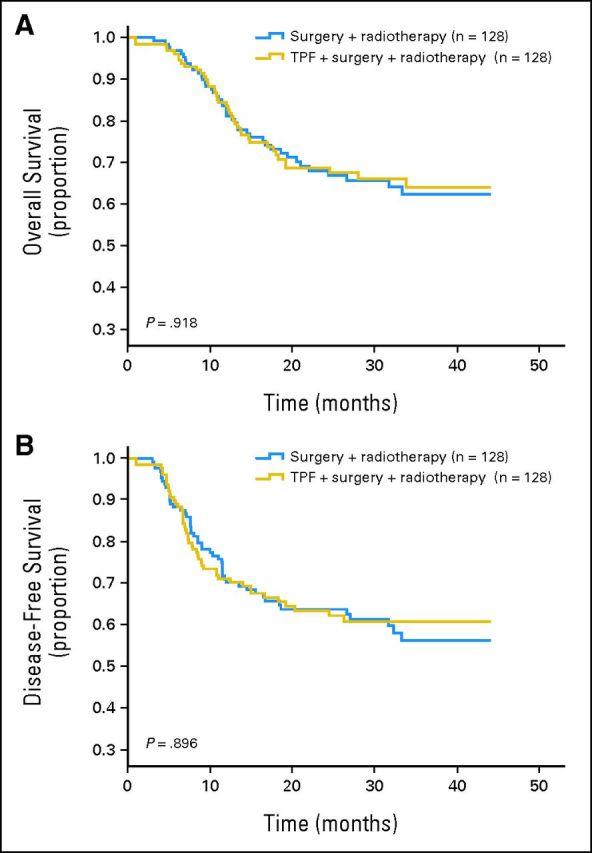
(A) Overall and (B) disease-free survival in the control and experimental arms. TPF, docetaxel, cisplatin, and fluorouracil.
Patterns of Failure and Subgroup Analysis
Locoregional recurrence developed in 30.5% and 31.3% of patients in the control and experimental arms, respectively; there was no difference in LRFS between the two arms (HR, 1.019; 95% CI, 0.618 to 1.524; P = .927; Appendix Fig A1A, online only). There was a trend toward a lower incidence of distant metastasis for patients who received induction chemotherapy compared with up-front surgery (5.5% v 8.7%, respectively), but there was no statistically significant difference in DMFS between the two arms (HR, 0.913; 95% CI, 0.596 to 1.397; P = .674; Appendix Fig A1B, online only).
We performed exploratory subgroup analysis of OS, DFS, LRFS, and DMFS according to baseline characteristics (Fig 3). There was no clear benefit from induction chemotherapy in any of the subgroups, with the exception of patients with cN2 disease, who seemed to have improved OS (HR, 0.418; 95% CI, 0.179 to 0.974; P = .043) and DMFS (HR, 0.418; 95% CI, 0.179 to 0.974; P = .043) when treated with induction chemotherapy.
Fig 3.
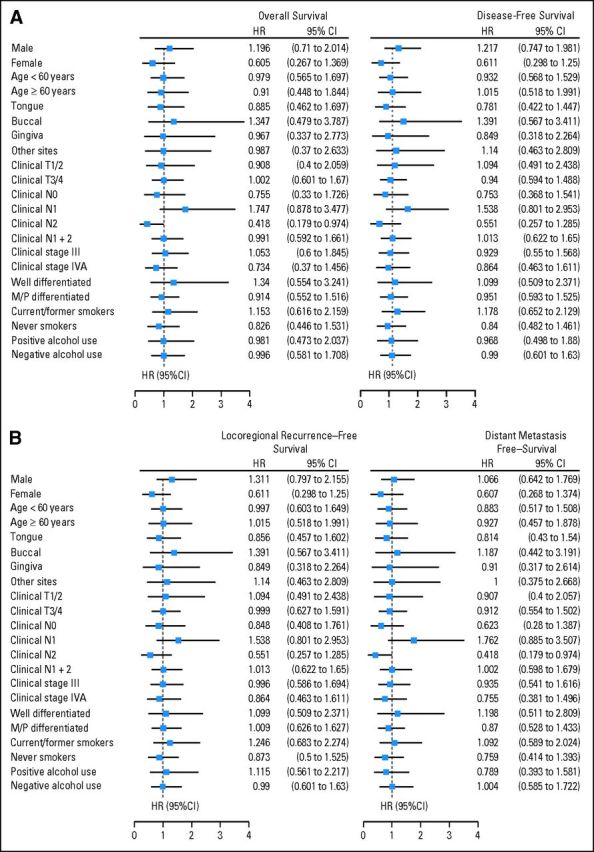
Subgroup analysis of (A) overall and disease-free survival and (B) locoregional recurrence–free and distant metastasis–free survival in the control and experimental arms. HR, hazard ratio; M/P, moderately/poorly.
Correlation Between Response to Induction Chemotherapy and Outcome
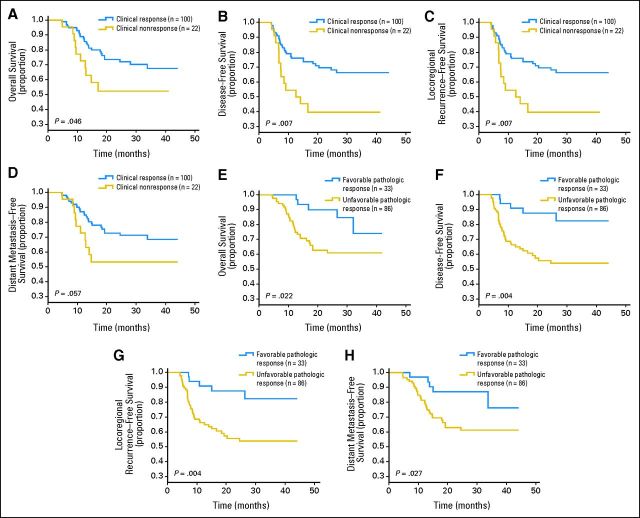
In the experimental arm, there was a significant positive correlation between clinical and pathologic responses (Spearman's ρ correlation coefficient, 0.247; P = .007). A favorable pathologic response or clinical response predicted a better outcome with regard to OS, DFS, LRFS, and DMFS (Appendix Table A2, Appendix Fig A2, online only). On multivariate Cox model analysis (Appendix Table A3, online only), pathologic response was an independent risk factor for OS, DFS, LRFS, and DMFS. However, given the post hoc nature of these analyses, the results should be viewed as exploratory.
AEs
During TPF induction chemotherapy, the most frequent AEs were alopecia (70.5%), nausea and/or vomiting (55.7%), hematologic toxicity (28.7%), altered liver function tests (19.7%), diarrhea (14.8%), and febrile neutropenia (1.6%). No grade 4 AEs occurred. Grade 3 hematologic toxicity occurred in eight patients (6.6%), grade 3 febrile neutropenia in two (1.6%), and grade 3 diarrhea in one (0.8%; Table 3). Induction chemotherapy did not seem to increase postoperative morbidity. Surgical complications included flap failure in four patients (two in each arm), postoperative hematoma in two patients (one in each arm), and chylous fistula after neck dissection in one patient (in the experimental arm). The most common AEs associated with postoperative radiotherapy were oral mucositis (80.4%), dermatitis (66.1%), trismus (63.4%), and dysphagia and odynophagia (54.9%; Table 3), with similar incidence in both arms. No chemotherapy-, surgery-, or radiotherapy-related deaths occurred.
Table 3.
Adverse Events
| Event | Experimental Arm |
Control Arm |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 1 |
Grade 2 |
Grade 3 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Induction chemotherapy | ||||||||||||
| Hematologic toxicity | 18 | 14.8 | 9 | 7.4 | 8 | 6.6 | — | — | — | — | — | — |
| Diarrhea | 11 | 9.0 | 6 | 4.9 | 1 | 0.8 | — | — | — | — | — | — |
| Alopecia | 83 | 68.0 | 3 | 2.5 | 0 | 0 | — | — | — | — | — | — |
| Nausea and/or vomiting | 66 | 54.1 | 2 | 1.6 | 0 | 0 | — | — | — | — | — | — |
| Altered liver function tests | 19 | 15.6 | 5 | 4.1 | 0 | 0 | — | — | — | — | — | — |
| Febrile neutropenia | — | — | — | — | 2 | 1.6 | — | — | — | — | — | — |
| Postoperative radiotherapy | ||||||||||||
| Oral mucositis | 38 | 34.2 | 44 | 39.6 | 7 | 6.3 | 41 | 36.3 | 43 | 38.1 | 7 | 6.2 |
| Trismus | 28 | 25.2 | 35 | 31.5 | 6 | 5.4 | 33 | 29.2 | 34 | 30.1 | 6 | 5.3 |
| Dermatitis | 31 | 27.9 | 41 | 36.9 | 5 | 4.5 | 29 | 25.7 | 38 | 33.6 | 4 | 3.5 |
| Dysphagia and odynophagia | 25 | 22.5 | 29 | 26.1 | 6 | 5.4 | 26 | 23.0 | 31 | 27.4 | 6 | 5.3 |
DISCUSSION
To our knowledge, this was the first randomized phase III study of TPF induction followed by surgery compared with up-front surgery in patients with resectable OSCC. There were no unexpected toxicities, and induction chemotherapy did not increase perioperative morbidity. TPF induction did not improve survival when compared with up-front surgery. Patients who received TPF induction and had a favorable pathologic or clinical response had a decreased risk for death and recurrence.
Our results are in accordance with those of a previous trial evaluating PF as induction chemotherapy before surgery in patients with OSCC, which found no significant impact of PF on survival or locoregional control when compared with up-front surgery.7 However, PF induction was associated with a trend toward a lower incidence of distant metastasis, similar to what was observed in our study with TPF.
The addition of docetaxel to PF induction had been shown to improve survival in the setting of nonsurgical treatment of locally advanced HNSCC (TAX323 and TAX324 trials).11,12 Unfortunately, our study and that by Licitra et al7 failed to support a role for induction chemotherapy with PF or TPF before surgery in OSCC. However, it should be noted that in both TAX323 and TAX324, locoregional recurrence was the most important cause of treatment failure, and the impact of TPF compared with PF on OS was mainly attributed to an improvement in locoregional control. Therefore, in the setting of aggressive treatment to prevent locoregional recurrence, a favorable impact of TPF on OS might not exist.
Our study was not powered to detect improvement in the incidence of distant metastasis, and the analysis of patterns of failure should be viewed as exploratory. However, in our study and in the study by Licitra et al,7 a trend towards a lower incidence of distant metastasis was observed. We also documented a lower risk for death and DMFS in cN2 patients treated with TPF. These results are in accordance with both TAX323 and TAX324 (in which a low incidence of distant metastasis in ranging from 5% to 12.9% was seen with either induction PF or TPF11,12) as well as with the larynx preservation trials conducted by the Veterans Affairs Study Group and European Organisation for Research and Treatment of Cancer,19,20 in which patients who received PF induction experienced a lower incidence of distant failure compared with those who did not receive chemotherapy. Taken together, the aforementioned trials and our study suggest that induction chemotherapy could play a role in improving outcomes in patients at the highest risk of distant failure, and specific clinical trials to address this question are needed, in the settings of both surgical and nonsurgical treatment of locally advanced HNSCC.
A limitation of our study includes the fact that only two doses of induction chemotherapy were administered, as compared with most other trials, which delivered three to four doses of induction chemotherapy to responders.11,12 In contrast, DeCIDE included only two cycles of induction chemotherapy in the experimental arm,14 consistent with the schedule used in our study, illustrating variability in the length of induction treatment used across phase III trials. The rationale for using two cycles of induction chemotherapy in our study was to avoid delays in administering definitive therapy (ie, surgery), toxicity, and noncompliance. However, it is possible that an abbreviated schedule of induction chemotherapy may not be sufficient to improve outcomes (neither DeCIDE nor our study reached their primary end points). The other limitation of our study is the lack of postoperative concomitant chemotherapy in patients with high-risk features, which has been shown to improve locoregional control and survival compared with postoperative radiotherapy alone.21,22 Considering the low rate of severe toxicities in this study, it probably would have been feasible to extend the number of doses of induction chemotherapy in responders and to add postoperative concomitant chemotherapy in the patients with high-risk features; this might have influenced outcomes.
As previously recognized in studies evaluating induction chemotherapy for locally advanced HNSCC, superior outcomes are seen in responders, as assessed by both clinical and pathologic responses.7,23 This was also observed in our study. It is difficult to discern whether survival in the responders improved because of an impact of chemotherapy per se, or whether response to induction chemotherapy might be a marker of more favorable cancer biology. However, these data suggest that pathologic response to induction chemotherapy could be explored as an intermediate end point of efficacy of induction regimens. This end point may be used in future pilot clinical trials of novel induction regimens before launching large randomized phase III studies.
Although our study failed to demonstrate a benefit from induction chemotherapy, it is possible that a subset of patients may indeed have more favorable outcomes when exposed to chemotherapy before surgery. Our post hoc exploratory analysis suggests a survival benefit with TPF induction in cN2 patients, and this may warrant prospective confirmation in future studies. Assessment of molecular markers may also be useful in identifying patients most likely to benefit from induction chemotherapy. For example, p53 gene mutations have been found to be strongly associated with lower response rates to PF induction.24,25 Validation of p53 mutations (as well as other biomarkers such as beta-tubulin and Bcl-xL26–28) as possible prognostic and/or predictive factors of response could ultimately assist in developing personalized induction treatment strategies for resectable OSCC.
In conclusion, our study failed to demonstrate that TPF induction chemotherapy improves survival compared with up-front surgery in patients with resectable stage III or IVA OSCC. If future studies are to be designed for evaluation of induction chemotherapy in this setting, we suggest incorporation of biologic agents to the regimen, coupled with an effort to identify and/or select patients who are more likely to benefit from induction treatment by using either clinical and/or biomarker criteria.
Appendix
Table A1.
Description of Surgery and Postoperative Radiotherapy
| Characteristic | Control Arm (n = 128) |
Experimental Arm (n = 128) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Surgery | ||||
| Local complete resection | 127 | 99.2 | 121 | 94.5 |
| Neck dissection | 127 | 99.2 | 121 | 94.5 |
| Functional | 74 | 57.8 | 67 | 52.3 |
| Radical | 31 | 24.2 | 30 | 23.4 |
| Bilateral | 22 | 17.2 | 24 | 18.8 |
| Reconstruction | 127 | 99.2 | 121 | 94.5 |
| No | 8 | 6.3 | 11 | 8.6 |
| Pedicle flaps | 40 | 31.3 | 46 | 35.9 |
| Free flaps | 79 | 61.7 | 64 | 50.0 |
| No surgery | 1 | 0.8 | 7 | 5.5 |
| Radiotherapy, Gy | ||||
| < 54 | 7 | 5.5 | 7 | 5.5 |
| 54 to 60 | 86 | 67.2 | 91 | 71.1 |
| > 60 | 20 | 15.6 | 13 | 10.2 |
| None | 15 | 11.7 | 17 | 13.3 |
Table A2.
Summary of 2-Year OS, DFS, and Frequency of Locoregional Recurrence and Distant Metastasis According to Clinical and Pathologic Response
| Characteristic | Experimental Arm (%) |
Control Arm (%)Surgery Plus Radiotherapy (n = 128) | |||
|---|---|---|---|---|---|
| CR or PR (n = 100) | SD or PD (n = 22) | Pathologic Response |
|||
| Favorable (n = 33) | Unfavorable (n = 86) | ||||
| OS | 72.5 | 52.4 | 89.9 | 61.8 | 68.2 |
| DFS | 67.9 | 40.1 | 87.6 | 54.0 | 63.6 |
| Locoregional recurrence–free survival | 67.9 | 40.1 | 87.8 | 53.8 | 65.4 |
| Distant metastasis–free survival | 70.9 | 53.1 | 87.3 | 60.9 | 65.4 |
| Locoregional recurrence rate | 26.0 | 59.1 | 15.2 | 37.2 | 30.5 |
| Distant metastasis rate | 6.0 | 4.5 | 6.9 | 5.8 | 8.6 |
Abbreviations: CR, complete response; DFS, disease-free survival; OS, overall survival; PD, progressive disease; PR, partial response; SD, stable disease.
Table A3.
Multivariate Cox Proportional Hazards Models for Predictors of Survival in Experimental Arm
| Variable | HR | 95% CI | df | P |
|---|---|---|---|---|
| OS | ||||
| Pathologic response (favorable v unfavorable) | 0.352 | 0.132 to 0.938 | 1 | .037 |
| Clinical response (response v nonresponse) | 0.648 | 0.297 to 1.413 | 1 | .275 |
| cN stage | 2 | .052 | ||
| cN0 v cN1 + cN2 | 0.361 | 0.157 to 0.830 | 1 | .016 |
| cN2 v cN1 + cN0 | 0.610 | 0.248 to 1.503 | 1 | .283 |
| DFS | ||||
| Pathologic response (favorable v unfavorable) | 0.309 | 0.118 to 0.808 | 1 | .017 |
| Clinical response (response v nonresponse) | 0.529 | 0.262 to 1.067 | 1 | .075 |
| cN stage | 2 | .149 | ||
| cN0 v cN1 + cN2 | 0.500 | 0.238 to 1.050 | 1 | .067 |
| cN2 v cN1 + cN0 | 0.985 | 0.445 to 2.180 | 1 | .970 |
| Locoregional recurrence–free survival | ||||
| Pathologic response (favorable v unfavorable) | 0.309 | 0.118 to 0.808 | 1 | .017 |
| Clinical response (response v nonresponse) | 0.529 | 0.262 to 1.067 | 1 | .075 |
| cN stage | 2 | .149 | ||
| cN0 v cN1 + cN2 | 0.500 | 0.238 to 1.050 | 1 | .067 |
| cN2 v cN1 + cN0 | 0.985 | 0.445 to 2.180 | 1 | .970 |
| Distant metastasis–free survival | ||||
| Pathologic response (favorable v unfavorable) | 0.369 | 0.139 to 0.982 | 1 | .046 |
| Clinical response (response v nonresponse) | 0.662 | 0.304 to 1.442 | 1 | .299 |
| cN stage | 2 | .057 | ||
| cN0 v cN1 + cN2 | 0.368 | 0.160 to 0.845 | 1 | .018 |
| cN2 v cN1 + cN0 | 0.610 | 0.248 to 1.500 | 1 | .282 |
Abbreviations: DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
Fig A1.
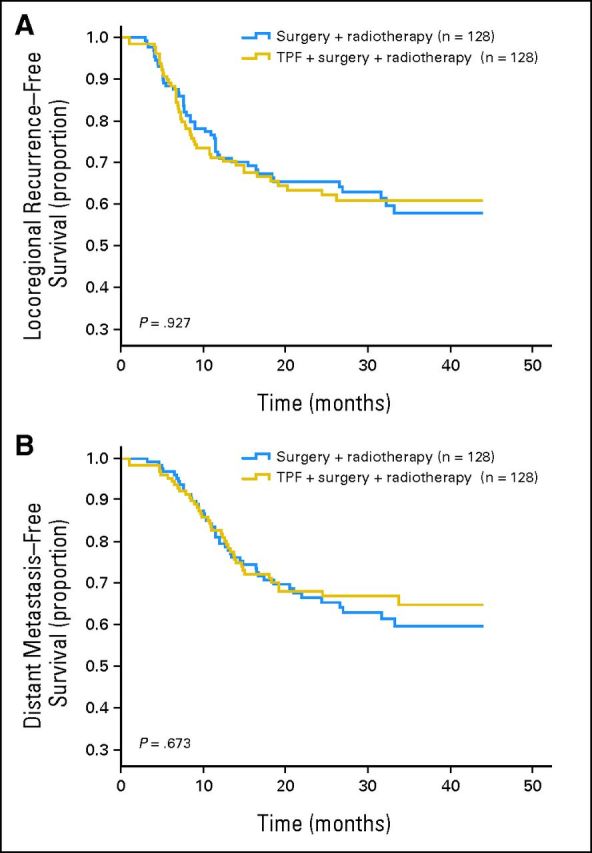
(A) Locoregional recurrence–free and (B) distant metastasis–free survival in the control and experimental arms. TPF, docetaxel, cisplatin, and fluorouracil.
Fig A2.
(A to D) Pathologic and (E to H) clinical response for (A, E) overall, (B, F) disease-free, (C, G) locoregional recurrence–free, and (D, H) distant metastasis–free survival. The landmark point was set at the time of response evaluation.
Footnotes
Supported by Research Grants No. 2007BAI18B03 from the National Key Technology Research and Development Program of China, No. 81272979, 30973344, and 30700953 from the National Natural Science Foundation of China, and No. 10dz1951300 from the Science and Technology Commission of Shanghai Municipality.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01542931.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: William N. William Jr, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Lai-ping Zhong, Chen-ping Zhang, Bo-song Wang, Zhi-yuan Zhang
Collection and assembly of data: Lai-ping Zhong, Chen-ping Zhang, Guo-xin Ren, Wei Guo, Jian Sun, Han-guang Zhu, Wen-yong Tu, Jiang Li, Yi-li Cai, Li-zhen Wang, Xin-dong Fan, Zhong-he Wang, Yong-jie Hu, Tong Ji, Wen-jun Yang, Wei-min Ye, Jun Li, Yue He, Yan-an Wang, Li-qun Xu, Zhi-yuan Zhang
Data analysis and interpretation: Lai-ping Zhong, William N. William Jr, Bo-song Wang, Merrill S. Kies, J. Jack Lee, Jeffrey N. Myers,Zhi-yuan Zhang
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Kademani D: Oral cancer Mayo Clin Proc 82:878–887,2007 [DOI] [PubMed] [Google Scholar]
- 2.Petersen PE: The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of WHO Global Oral Programme Community Dent Oral Epidemiol 31:3–23,2003. suppl 1 [DOI] [PubMed] [Google Scholar]
- 3.Neville BW, Day TA: Oral cancer and precancerous lesions CA Cancer J Clin 52:195–215,2002 [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM Bray F Ferlay J, etal: Global cancer statistics, 2002 CA Cancer J Clin 55:74–108,2005 [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers (version 1.2012) http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. [DOI] [PubMed]
- 6.Division of Oral and Maxillofacial Oncology, Chinese Society of Oral and Maxillofacial Surgery: The protocol of treatment guideline of oral and maxillofacial malignant neoplasms China J Oral Maxillofac Surg 8:98–106,2010 [Google Scholar]
- 7.Licitra L Grandi C Guzzo M, etal: Primary chemotherapy in resectable oral cavity squamous cell cancer: A randomized controlled trial J Clin Oncol 21:327–333,2003 [DOI] [PubMed] [Google Scholar]
- 8.Pignon JP Bourhis J Domenge C, etal: Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data—MACH-NC Collaborative Group: Meta-analysis of chemotherapy on head and neck cancer Lancet 355:949–955,2000 [PubMed] [Google Scholar]
- 9.Pignon JP le Maître A Maillard E, etal: Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients Radiother Oncol 92:4–14,2009 [DOI] [PubMed] [Google Scholar]
- 10.Brockstein B, Haraf DJ, Rademaker AW: Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: A 9-year, 337-patient, multi-institutional experience Ann Oncol 15:1179–1186,2004 [DOI] [PubMed] [Google Scholar]
- 11.Posner RM Hershock MD Blajman RC, etal: Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer N Engl J Med 357:1705–1715,2007 [DOI] [PubMed] [Google Scholar]
- 12.Vermorken BJ Remenar E Herpen VC, etal: Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer N Engl J Med 357:1695–1704,2007 [DOI] [PubMed] [Google Scholar]
- 13.Lorch JH Goloubeva O Haddad RI, etal: Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: Long-term results of the TAX 324 randomised phase 3 trial Lancet Oncol 12:153–159,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen EE Karrison T Kocherginsky M, etal: DeCIDE: A phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol 30:356s,2012suppl 15; abstr 5500 [Google Scholar]
- 15.Haddad RI Rabinowits G Tishler RB, etal: The RARADIGM trial: A phase III study comparing sequential therapy (ST) to concurrent chemoradiotherapy (CRT) in locally advanced head and neck cancer (LAHNC) J Clin Oncol 30:356s,2012suppl 15; abstr 5501 [Google Scholar]
- 16.Therasse P Arbuck SG Eisenhauer EA, etal: New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst 92:205–216,2000 [DOI] [PubMed] [Google Scholar]
- 17.Yang AK Liu TR Chen FJ, etal: Survival analysis of 229 patients with advanced squamous cell carcinoma of the oral tongue [in Chinese] Ai Zheng 27:1315–1320,2008 [PubMed] [Google Scholar]
- 18.Guo ZM Wang YL Zhang Q, etal: Treatment and prognosis of gingival carcinoma: A report of 116 cases [in Chinese] Ai Zheng 27:307–310,2008 [PubMed] [Google Scholar]
- 19.Lefebvre JL Chevalier D Luboinski B, etal: Larynx preservation in pyriform sinus cancer: Preliminary results of a European Organisation for Research and Treatment of Cancer phase III trial—EORTC Head and Neck Cancer Cooperative Group J Natl Cancer Inst 88:890–899,1996 [DOI] [PubMed] [Google Scholar]
- 20.Terrell JE, Fisher SG, Wolf GT: Long-term quality of life after treatment of laryngeal cancer: The Veterans Affairs Laryngeal Cancer Study Group Arch Otolaryngol Head Neck Surg 124:964–971,1998 [DOI] [PubMed] [Google Scholar]
- 21.Cooper JS Pajak TF Forastiere AA, etal: Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350:1937–1944,2004 [DOI] [PubMed] [Google Scholar]
- 22.Bernier J Domenge C Ozsahin M, etal: Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350:1945–1952,2004 [DOI] [PubMed] [Google Scholar]
- 23.Kies MS Boatright DH Li G, etal: Phase II trial of induction chemotherapy followed by surgery for squamous cell carcinoma of the oral tongue in young adults Head Neck 34:1255–1262,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Temam S Flahault A Pédrié S, etal: p53 gene status as a predictor of tumor response to induction chemotherapy of patients with locoregioally advanced squamous cell carcinomas of the head and neck J Clin Oncol 18:385–394,2000 [DOI] [PubMed] [Google Scholar]
- 25.Perrone F Bossi P Cortelazzi B, etal: TP53 mutations and pathologic complete response to neoadjuvant cisplatin and fluorouracil chemotherapy in resected oral cavity squamous cell carcinoma J Clin Oncol 28:761–766,2010 [DOI] [PubMed] [Google Scholar]
- 26.Wu Y Posner MR Schumaker LM, etal: Novel biomarker panel predicts prognosis in human papillomavirus-negative oropharyngeal cancer: An analysis of the TAX 324 trial. Cancer 118:1811–1817,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar B Cordell KG Lee JS, etal: EGFR, p16, HPV titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer J Clin Oncol 26:3128–3137,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cullen KJ Schumaker L Nikitakis N, etal: Beta-tubulin-II expression strongly predicts outcome in patients receiving induction chemotherapy for locally advanced squamous carcinoma of the head and neck: A companion analysis of the TAX 324 trial J Clin Oncol 27:6222–6228,2009 [DOI] [PubMed] [Google Scholar]