Abstract
Purpose
Tivozanib is a potent and selective tyrosine kinase inhibitor of vascular endothelial growth factor receptor 1 (VEGFR1), -2, and -3. This phase III trial compared tivozanib with sorafenib as initial targeted therapy in patients with metastatic renal cell carcinoma (RCC).
Patients and Methods
Patients with metastatic RCC, with a clear cell component, prior nephrectomy, measurable disease, and 0 or 1 prior therapies for metastatic RCC were randomly assigned to tivozanib or sorafenib. Prior VEGF-targeted therapy and mammalian target of rapamycin inhibitor were not permitted. The primary end point was progression-free survival (PFS) by independent review.
Results
A total of 517 patients were randomly assigned to tivozanib (n = 260) or sorafenib (n = 257). PFS was longer with tivozanib than with sorafenib in the overall population (median, 11.9 v 9.1 months; hazard ratio [HR], 0.797; 95% CI, 0.639 to 0.993; P = .042). One hundred fifty-six patients (61%) who progressed on sorafenib crossed over to receive tivozanib. The final overall survival (OS) analysis showed a trend toward longer survival on the sorafenib arm than on the tivozanib arm (median, 29.3 v 28.8 months; HR, 1.245; 95% CI, 0.954 to 1.624; P = .105). Adverse events (AEs) more common with tivozanib than with sorafenib were hypertension (44% v 34%) and dysphonia (21% v 5%). AEs more common with sorafenib than with tivozanib were hand-foot skin reaction (54% v 14%) and diarrhea (33% v 23%).
Conclusion
Tivozanib demonstrated improved PFS, but not OS, and a differentiated safety profile, compared with sorafenib, as initial targeted therapy for metastatic RCC.
INTRODUCTION
Renal cell carcinoma (RCC) with clear cell histology is characterized by overexpression of vascular endothelial growth factor (VEGF) and an increase in tumor angiogenesis.1 VEGF-targeted antiangiogenic agents have proven antitumor effects in RCC.2–7
Sorafenib and sunitinib were the first tyrosine kinase inhibitors (TKIs) to gain regulatory approval, and they established a prominent role in RCC treatment. Each showed antitumor activity in phase III trials by prolonging progression-free survival (PFS) compared with interferon alfa or placebo.2,5,6 Both drugs are characterized by a broad spectrum of tyrosine kinase inhibition in addition to VEGF receptor (VEGFR) kinases,8,9 which are believed to be the primary target for RCC response.2,5,6,10 Adverse events (AEs) such as skin rash, hand-foot skin reaction, and myelosuppression associated with these two multitargeted agents may result from inhibition of these other kinases, such as c-KIT and FLT3.11 Therefore, a more potent, highly selective inhibitor of VEGFR may improve efficacy and tolerability, and thus meet an unmet need for efficacious agents with differentiated safety profiles.
Tivozanib hydrochloride (tivozanib) is a potent and selective VEGFR TKI with a relatively long half-life (approximately 4 days).12–14 Tivozanib inhibits phosphorylation of VEGFR1, -2, and -3 at picomolar concentrations and inhibits other kinases such as c-KIT and platelet-derived growth factor receptor beta at 10× higher concentrations, suggesting the potency and specificity of tivozanib.14 A phase I study determined the maximum-tolerated dose of oral tivozanib to be 1.5 mg per day.12 A phase II study was conducted in 272 patients with metastatic clear cell and other histologic subtypes of RCC. The median PFS was 11.7 months in all patients and 14.8 months in the subgroup of 176 patients with clear cell RCC and prior nephrectomy.15 Hypertension (45%) was the predominant treatment-related AE, with low rates of diarrhea (12%), fatigue (8%), and hand-foot skin reaction (4%).15 These data provided the rationale for this phase III trial comparing tivozanib with sorafenib as first-line targeted therapy for patients with metastatic RCC.
PATIENTS AND METHODS
Patients
Eligibility criteria included written informed consent; age ≥ 18 years; prior nephrectomy; histologically confirmed RCC with a clear cell component and recurrence or metastases; measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) criteria; Eastern Cooperative Oncology Group performance status (ECOG PS) 0 to 1; and adequate hematologic, renal, and hepatic function. Patients could be treatment-naive or could have received one or fewer prior systemic treatments (immunotherapy, chemotherapy, or hormonal therapy) for metastatic RCC. Prior systemic therapy given as an adjuvant following nephrectomy was counted as a prior therapy if recurrence was detected within 6 months of completing treatment. Prior VEGF-targeted therapies or mammalian target of rapamycin–targeted therapy were not permitted.
Patients were excluded for significant cardiovascular disease, including uncontrolled hypertension, myocardial infarction, or thromboembolic disorders, within 6 months of study entry. Uncontrolled hypertension was defined as blood pressure > 150/100 mmHg (while taking two or more antihypertensive medications) documented on two consecutive measurements taken > 24 hours apart. Patients with brain metastases were allowed if the metastases were stable for at least 3 months following prior treatment. The study was approved by an institutional review board or ethics committee at each center and was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. An independent data monitoring committee monitored the study.
Study Design
This was an open-label, randomized phase III trial. Patients were randomly assigned 1:1 to either tivozanib or sorafenib as their initial targeted therapy. Random assignment of patients was stratified by geographic region, number of prior treatments for metastatic disease, and number of metastatic sites/organs involved.
Study drugs were provided by the sponsor. Tivozanib was administered orally at 1.5 mg once per day every day for 3 weeks followed by 1 week off (one cycle is 3 weeks on, 1 week off). Sorafenib was administered orally at a dose of 400 mg (two 200-mg tablets) twice per day continuously (one cycle is 4 weeks on). Patients continued to receive the study drug until disease progression, unacceptable toxicity, death, or for some other reason for discontinuing the study drug. Hypertension for tivozanib16 or skin toxicity for sorafenib17 was managed according to specific guidelines. For other AEs, tivozanib dose reduction to 1.0 mg per day and sorafenib dose reductions to 400 mg once daily and then to 400 mg every other day were allowed for patients with grade ≥ 3 drug-related AEs.
Patients randomly assigned to sorafenib who had RECIST-defined progressive disease (PD) per investigator assessment were given the option to cross over to receive tivozanib in a separate protocol (NCT01076010). All patients were followed for collection of subsequent cancer therapy information and overall survival (OS).
End Points and Assessments
The primary end point was PFS, defined as the time interval between the date of random assignment and the date of disease progression or death. Patients without disease progression were censored at the last tumor assessment documenting the absence of disease progression. Death was considered an event only if it occurred within 140 days of the last tumor assessment documenting the absence of PD on the basis of US Food and Drug Administration guidance regarding the censoring rule for PFS18 (see Methods in the Appendix, online only).
Secondary end points included OS, objective response rate (ORR; complete response plus partial response), safety and tolerability, kidney-specific symptoms, and health-related quality of life (HRQoL).
Tumor assessments made by using computed tomography scan or magnetic resonance imaging were performed at baseline, week 8, and once every 8 weeks thereafter until PD. All imaging scans were evaluated by independent radiology review blinded to study treatment. Tumor response was evaluated according to RECIST version 1.0.
Patients with radiologic evidence of PD, as assessed by the investigator, had confirmation by blinded independent radiology review within 48 hours. This independent review to confirm investigator-called PD was a separate process from the third-party review of response performed by the core imaging laboratory to assess the primary end point. Confirmation of PD was not required if significant clinical deterioration, appearance of new lesions, or > 50% increase in measurable disease per RECIST was noted by the investigator.
Safety was evaluated by AEs, vital signs, physical examinations, ECOG PS, ECG, laboratory values, and concomitant medications. AEs were collected throughout the patients' participation, including a period of 30 days after the last dose of study drug. AEs were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events version 3.0.
HRQoL was assessed with the Functional Assessment of Cancer Therapy-General (FACT-G),19 FACT Kidney Symptom Index–Disease-Related Symptoms (FKSI-DRS),20 and EuroQol-5D (EQ-5D)21 questionnaires. These questionnaires were administered on day 1 of each cycle and on discontinuation from the study drug.
Statistical Methods and Analysis
Target enrollment was 500 patients (250 patients per arm) to observe 310 events (progression or death) yielding 90% power to detect a difference (P < .05) between treatment arms with respect to PFS, assuming the median PFS for patients receiving sorafenib and tivozanib was 6.7 months and 9.7 months, respectively (a projected increase of 3 months or 44.8%). The final PFS analysis was to be performed after 310 events occurred. Final OS analysis was to be performed after completion of follow-up for all patients, or after all patients in the follow-up had been on study for at least 2 years. Assuming the median OS for patients receiving sorafenib and tivozanib was 18 months and 24 months, respectively, approximately 300 events would be observed by the time of the final OS analysis, yielding 70% power to detect a difference (P < .05) between the treatment arms with respect to OS.
Efficacy end points were analyzed in the intent-to-treat (ITT) population, which comprised all randomly assigned patients. Safety analyses were performed in the safety population, which included all randomly assigned patients receiving at least one dose of study drug.
PFS between treatment arms was compared on the basis of independent radiology review assessment by using a stratified log-rank test; stratification factors were the number of prior treatments (0 or 1) and the number of metastatic sites/organs involved (1 or ≥ 2). The distribution of the PFS was estimated by using the Kaplan-Meier method. The hazard ratio (HR) and its 95% CI were determined by using the Cox proportional hazards model. PFS was also compared between treatment arms in predefined subgroup analyses on the basis of baseline characteristics, including ECOG PS, prior treatment for metastatic disease, and Memorial Sloan-Kettering Cancer Center risk group.22 ORR was compared between treatment arms by using the Cochran-Mantel-Haenszel statistics, stratified as for the primary PFS analysis. Repeated-measures mixed-effects (RMME) models were fitted to test for HRQoL differences between treatment arms.23,24 All P values were two-tailed. Statistical analyses were performed by using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patients
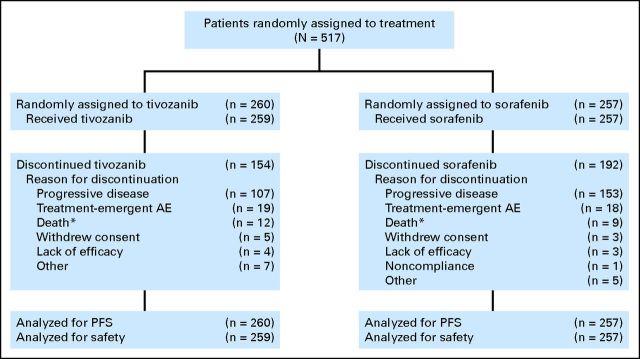
Between February and August 2010, 517 patients were randomly assigned at 76 centers across 15 countries. Most patients (457 [88%]) were enrolled in Central or Eastern Europe. Five hundred sixteen patients received treatment: 259 received tivozanib, and 257 received sorafenib. One patient was randomly assigned to tivozanib but was not dosed (Fig 1). At the data cutoff (December 15, 2011), 59% of patients in the tivozanib arm and 75% in the sorafenib arm had discontinued the study treatment, most often because of PD (Fig 1).
Fig 1.
CONSORT diagram based on data cutoff date of December 15, 2011. AE, adverse event; PFS, progression-free survival. (*) Includes patient deaths that occurred on study drug any time after first dose but before patient being discontinued from study drug for any reason.
Baseline characteristics were well balanced between the two arms (Table 1), except for ECOG PS. More patients had a favorable ECOG PS of 0 in the sorafenib arm compared with the tivozanib arm (139 [54%] v 116 [45%], respectively; Fisher's exact test P = .035). Seventy percent of patients had received no prior systemic treatment for metastatic disease. For the remaining 30% of previously treated patients, the predominant therapy (> 90%) was interferon alfa. Fewer than 10% of patients had received prior adjuvant systemic therapy.
Table 1.
Baseline Demographics and Clinical Characteristics
| Characteristic | Tivozanib (n = 260) |
Sorafenib (n = 257) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 59 | 59 | ||
| Range | 23-83 | 23-85 | ||
| Sex | ||||
| Male | 185 | 71 | 189 | 74 |
| Female | 75 | 29 | 68 | 26 |
| Race/ethnicity | ||||
| White | 249 | 96 | 249 | 97 |
| Asian | 10 | 4 | 8 | 3 |
| Black | 1 | <1 | 0 | 0 |
| Time from diagnosis to study entry, years* | ||||
| < 1 | 109 | 42 | 105 | 41 |
| > 1 | 137 | 53 | 137 | 53 |
| Most common sites of metastasis | ||||
| Lung | 212 | 82 | 204 | 79 |
| Lymph nodes | 182 | 70 | 166 | 65 |
| Adrenal gland | 78 | 30 | 57 | 22 |
| Liver | 67 | 26 | 49 | 19 |
| Bone | 61 | 23 | 52 | 20 |
| No. of organs involved | ||||
| 1 | 76 | 29 | 88 | 34 |
| 2 | 99 | 38 | 106 | 41 |
| > 2 | 85 | 33 | 63 | 25 |
| ECOG PS | ||||
| 0 | 116 | 45 | 139 | 54 |
| 1 | 144 | 55 | 118 | 46 |
| MSKCC prognostic group | ||||
| Favorable | 70 | 27 | 87 | 34 |
| Intermediate | 173 | 67 | 160 | 62 |
| Poor | 17 | 7 | 10 | 4 |
| Prior systemic therapy for metastatic RCC† | ||||
| 0 | 181 | 70 | 181 | 70 |
| 1 | 78 | 30 | 76 | 30 |
| Prior systemic therapy by setting‡ | ||||
| Metastatic | 49 | 19 | 55 | 21§ |
| Adjuvant | 23 | 9 | 22 | 9§ |
| Other | 13 | 5 | 9 | 4 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; MSKCC, Memorial Sloan-Kettering Cancer Center; RCC, renal cell carcinoma.
Time since initial diagnosis to study entry was not calculated for patients with partial dates.
Recorded based on the stratification factor and number of prior treatments at random assignment.
Recorded based on the setting of prior therapy. Metastatic setting refers to patients who received systemic therapy when they had overt metastatic disease. Adjuvant setting refers to patients who received systemic therapy in the adjuvant setting, but recurrence was detected within 6 months of completion of adjuvant treatment, in which case it was counted as a prior systemic therapy for metastatic RCC. “Other” refers to patients who received prior therapy, but the setting was not available or other than metastatic or adjuvant.
Three patients in the sorafenib group who received prior adjuvant therapy also received one prior therapy for metastatic disease.
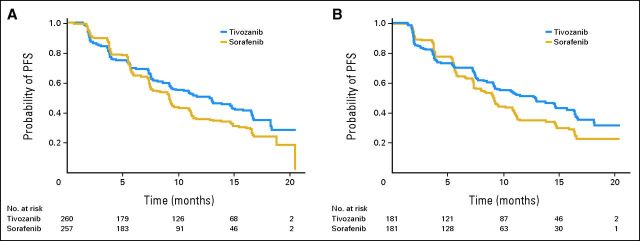
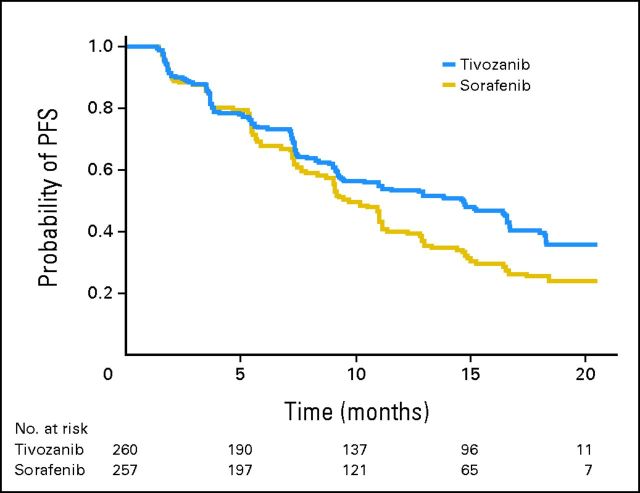
PFS
Tivozanib prolonged PFS compared with sorafenib (Table 2 and Fig 2A). Among the overall ITT population, 153 patients (58.8%) progressed or died while taking tivozanib versus 168 (65.4%) taking sorafenib (data cutoff was December 15, 2011). Median PFS, based on independent radiology review, was 11.9 months for tivozanib and 9.1 months for sorafenib (HR, 0.797; 95% CI, 0.639 to 0.993; P = .042).
Table 2.
Summary of Efficacy Measures in Intent-to-Treat Population
| Efficacy Measure | Independent Radiology Review |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tivozanib (n = 260) |
Sorafenib (n = 257) |
HR for Progression or Death | 95% CI | P | |||||
| No. | % | 95% CI | No. | % | 95% CI | ||||
| PFS | |||||||||
| Overall estimated median PFS, months | 11.9 | 9.3 to 14.7 | 9.1 | 7.3 to 9.5 | 0.797 | 0.639 to 0.993 | .042 | ||
| Stratified estimated median PFS, months | |||||||||
| Prior treatment | |||||||||
| No prior treatment | 12.7 | 9.1 to 15.0 | 9.1 | 7.3 to 10.8 | 0.756 | 0.580 to 0.985 | .037 | ||
| Prior systemic therapy for metastatic RCC | 11.9 | 8.0 to 16.6 | 9.1 | 7.2 to 11.1 | 0.877 | 0.587 to 1.309 | .520 | ||
| ECOG PS | |||||||||
| 0 | 14.8 | 11.3 to N/A | 9.1 | 7.5 to 11.0 | 0.617 | 0.442 to 0.860 | .004 | ||
| 1 | 9.1 | 7.5 to 12.9 | 9.0 | 7.2 to 10.9 | 0.920 | 0.680 to 1.245 | .588 | ||
| MSKCC prognostic group | |||||||||
| Favorable | 16.7 | 14.7 to N/A | 10.8 | 9.0 to 16.5 | 0.590 | 0.378 to 0.921 | .018 | ||
| Intermediate | 9.4 | 8.2 to 13.0 | 7.4 | 7.1 to 9.2 | 0.786 | 0.601 to 1.028 | .076 | ||
| Poor* | 3.7 | 1.9 to 7.4 | 10.9 | 5.3 to 11.0 | 1.361 | 0.546 to 3.393 | .504 | ||
| Tumor response | |||||||||
| Best observed RECIST response | |||||||||
| Complete response | 3 | 1.2 | 2 | 0.8 | — | — | |||
| Partial response | 83 | 31.9 | 58 | 22.6 | — | — | |||
| Stable disease | 134 | 51.5 | 168 | 65.4 | — | — | |||
| Progressive disease | 34 | 13.1 | 19 | 7.4 | — | — | |||
| Not evaluable | 6 | 2.3 | 10 | 3.9 | — | — | |||
| Objective response rate | 86 | 33.1 | 27.4 to 39.2 | 60 | 23.3 | 18.3 to 29.0 | — | .014 | |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; MSKCC, Memorial Sloan-Kettering Cancer Center; N/A, not achieved; PFS, progression-free survival; RCC, renal cell carcinoma.
Based on 17 patients given tivozanib and 10 patients given sorafenib, corresponding to 5% of patients enrolled onto the trial.
Fig 2.
Kaplan-Meier plot of progression-free survival (PFS) as determined by independent radiology review. (A) Overall intent-to-treat population; (B) no prior treatment.
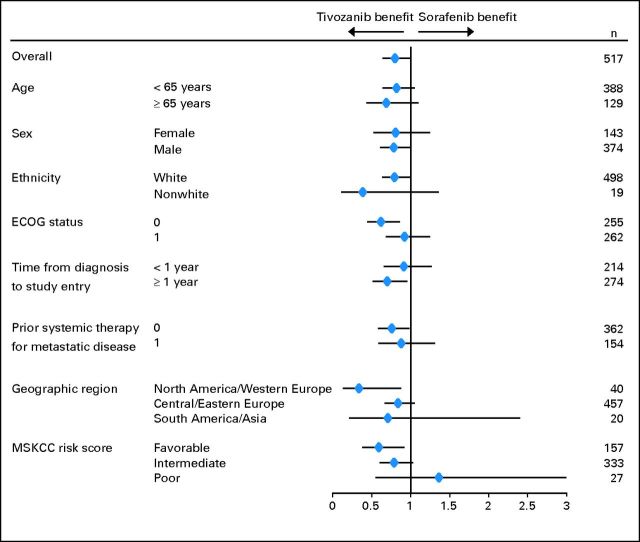
Prespecified PFS subgroup analyses based on baseline characteristics showed a consistent advantage with tivozanib treatment (Fig 3). In the subgroup of patients who were treatment naive for metastatic disease (n = 181 for each arm), median PFS was 12.7 months for tivozanib and 9.1 months for sorafenib (Table 2, Fig 2B; HR, 0.756; 95% CI, 0.580 to 0.985; P = .037).
Fig 3.
Forest plot: subgroup hazard ratios for progression-free survival and their 95% CIs. ECOG status, Eastern Cooperative Oncology Group performance score; MSKCC, Memorial Sloan-Kettering Cancer Center.
A sensitivity analysis of PFS in the ITT population, per investigator assessment, was consistent with the primary PFS result; median PFS was 14.7 months for tivozanib and 9.6 months for sorafenib (Appendix Fig A1, online only; HR, 0.722; 95% CI, 0.580 to 0.899; P = .003).
Baseline ECOG PS favored the sorafenib arm, but its impact on the PFS analysis compared with tivozanib was small. When adjustment was made for baseline imbalances in ECOG PS, the PFS superiority for tivozanib over sorafenib was modestly strengthened (adjusted v unadjusted unstratified HR, 0.765 v 0.785 [ITT population by independent review]).
ORR
The confirmed ORR for tivozanib, based on blinded independent radiology review of tumor response, was 33.1% (95% CI, 27.4% to 39.2%) versus 23.3% (95% CI, 18.3% to 29.0%) for sorafenib (Table 2; P = .014). The ORR for tivozanib, based on investigator assessment, was 35.4% (95% CI, 29.6% to 41.5%) versus 30.7% (95% CI, 25.2% to 36.8%) for sorafenib (P = .260).
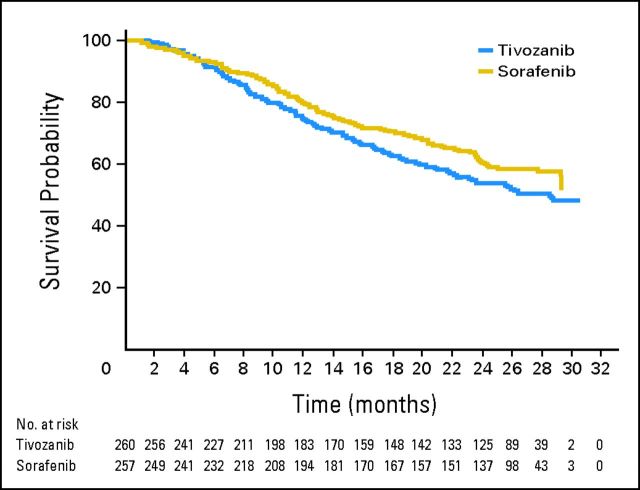
OS
A total of 219 deaths (42% of patients) had occurred at the protocol-specified final OS analysis in the ITT population (data cutoff was August 27, 2012), with 118 deaths in the tivozanib arm and 101 in the sorafenib arm. The final OS analysis showed a trend toward longer survival on the sorafenib arm than on the tivozanib arm (median, 29.3 v 28.8 months; HR, 1.245; 95% CI, 0.954 to 1.624; P = .105; Fig 4). A greater proportion of patients in the sorafenib arm received a next-line targeted therapy for RCC (63% in the sorafenib arm v 13% in the tivozanib arm; Table 3). Almost all of the patients in the sorafenib arm who received a next-line targeted agent (156 of 162) received tivozanib (Table 3) in the separate companion protocol that allowed patients on the sorafenib arm to cross over to tivozanib at progression on the phase III trial. Compared with patients from Central/Eastern Europe, patients from North America/Western Europe on the tivozanib arm received more next-line therapy, including next-line targeted therapy (Table 3). A trend toward longer OS in the tivozanib arm (HR, 0.503; 95% CI, 0.174 to 1.451; P = .195) was observed in the stratum of patients from North America/Western Europe (n = 40).
Fig 4.
Overall survival of the intent-to-treat population.
Table 3.
Summary of Next-Line Therapy in the Overall Population and by Region
| Category | Overall Population |
Central/Eastern Europe |
North America/Western Europe |
Rest of World |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tivozanib (n = 260) |
Sorafenib (n = 257) |
Tivozanib (n = 229) |
Sorafenib (n = 228) |
Tivozanib (n = 22) |
Sorafenib (n = 18) |
Tivozanib (n = 9) |
Sorafenib (n = 11) |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Patients who discontinued assigned therapy* | 190 | 73† | 226 | 88 | 165 | 72 | 198 | 87 | 16 | 73† | 17 | 94 | 9 | 100 | 11 | 100 |
| Patients with next-line therapy | 68 | 26 | 168 | 65 | 52 | 23 | 145 | 64 | 13 | 59 | 14 | 78 | 3 | 33 | 9 | 82 |
| Patients with next-line targeted therapy | 34 | 13 | 162 | 63 | 26 | 11 | 139 | 61 | 7 | 32 | 14 | 78 | 1 | 11 | 9 | 82 |
| VEGFR inhibitor | 18 | 7 | 158 | 61 | 13 | 6 | 138 | 61 | 5 | 23 | 11 | 61 | 0 | 9 | 82 | |
| Tivozanib | 0 | 156 | 61 | 0 | 138 | 61 | 0 | 10 | 56 | 0 | 8 | 73 | ||||
| mTOR inhibitor | 16 | 6 | 4 | 2 | 13 | 6 | 1 | < 1 | 2 | 9 | 3 | 17 | 1 | 11 | 0 | |
| Cytokines | 14 | 5 | 3 | 1 | 14 | 6 | 3 | 1 | 0 | 0 | 0 | 0 | ||||
| Radiotherapy | 10 | 4 | 2 | 1 | 4 | 2 | 2 | 1 | 5 | 23 | 0 | 1 | 11 | 0 | ||
| Other | 10 | 4 | 1 | < 1 | 8 | 3 | 1 | < 1 | 1 | 5 | 0 | 1 | 11 | 0 | ||
Abbreviations: mTOR, mammalian target of rapamycin; VEGFR, vascular endothelial growth factor receptor.
On or before August 27, 2012.
One patient erroneously appears as “discontinued” but was ongoing as of August 27, 2012.
Safety
Patients received tivozanib for a median duration of 12.0 months and sorafenib for 9.5 months at the data cutoff of June 1, 2012. Most patients (484 [94%]) experienced at least one treatment-emergent AE: 235 (91%) in the tivozanib arm versus 249 (97%) in the sorafenib arm. The most common AEs in both arms are listed in Table 4. Grade ≥ 3 AEs were reported in 338 patients (66%) overall: 159 (61%) in the tivozanib arm versus 179 (70%) in the sorafenib arm. AEs more common with tivozanib compared with sorafenib included hypertension and dysphonia. AEs more common with sorafenib compared with tivozanib included hand-foot syndrome and diarrhea. Common laboratory abnormalities are also listed in Table 4.
Table 4.
Common Treatment-Emergent Adverse Events (≥ 10% in either treatment arm) and Selected Clinical Laboratory Abnormalities
| Variable | Tivozanib (n = 259) |
Sorafenib (n = 257) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All |
Grade 3 |
Grade 4 |
All |
Grade 3 |
Grade 4 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Adverse event | ||||||||||||
| Hypertension* | 115 | 44 | 66 | 25 | 4 | 2 | 88 | 34 | 45 | 18 | 1 | < 1 |
| Diarrhea | 59 | 23 | 6 | 2 | 0 | 84 | 33 | 17 | 7 | 0 | ||
| Dysphonia | 55 | 21 | 0 | 0 | 12 | 5 | 0 | 0.0 | 0 | |||
| Fatigue | 50 | 19 | 14 | 5 | 0 | 41 | 16 | 9 | 4 | 0 | ||
| Weight decreased | 47 | 18 | 7 | 3 | 0 | 53 | 21 | 9 | 4 | 0 | ||
| Asthenia | 40 | 15 | 10 | 4 | 1 | < 1 | 43 | 17 | 7 | 3 | 0 | |
| Palmar-plantar erythrodysesthesia | 36 | 14 | 5 | 2 | 0 | 139 | 54 | 43 | 17 | 0 | ||
| Back pain | 35 | 14 | 8 | 3 | 0 | 21 | 8 | 5 | 2 | 0 | ||
| Nausea | 31 | 12 | 1 | < 1 | 0 | 19 | 7 | 1 | < 1 | 0 | ||
| Stomatitis | 29 | 11 | 1 | < 1 | 0 | 23 | 9 | 2 | 1 | 0 | ||
| Dyspnea | 29 | 11 | 4 | 2 | 0 | 22 | 9 | 5 | 2 | 0 | ||
| Decreased appetite | 27 | 10 | 1 | < 1 | 0 | 24 | 9 | 2 | 1 | 0 | ||
| Alopecia | 6 | 2 | 0 | 0 | 55 | 21 | 0 | 0 | ||||
| Clinical chemistry† | ||||||||||||
| Increased ALT | 73 | 28 | 2 | 1 | 0 | 88 | 34 | 7 | 3 | 2 | 1 | |
| Increased AST | 97 | 37 | 5 | 2 | 0 | 130 | 51 | 8 | 3 | 2 | 1 | |
| Increased amylase | 104 | 40 | 9 | 4 | 3 | 1 | 135 | 53 | 15 | 6 | 2 | 1 |
| Increased lipase | 119 | 46 | 23 | 9 | 6 | 2 | 164 | 64 | 52 | 20 | 11 | 4 |
| Hypophosphatemia | 76 | 29 | 11 | 4 | 0 | 182 | 71 | 67 | 26 | 0 | ||
| Proteinuria | 186 | 72 | 8 | 3 | 0 | 187 | 73 | 7 | 3 | 0 | ||
| Hematology | ||||||||||||
| Low hemoglobin | 105 | 41 | 5 | 2 | 4 | 2 | 125 | 49 | 7 | 3 | 1 | < 1 |
| Neutropenia | 28 | 11 | 5 | 2 | 1 | < 1 | 27 | 11 | 3 | 1 | 2 | 1 |
| Thrombocytopenia | 47 | 18 | 0 | 1 | < 1 | 31 | 12 | 0 | 0 | |||
Hypertension event includes hypertension and hypertensive crisis.
In addition to laboratory abnormalities noted in the table, 78 (30%) patients in the tivozanib arm and 18 (7%) in the sorafenib arm had normal thyroid-stimulating hormone levels prior to dosing that increased to > 10 μIU/mL after treatment. Nonetheless, few of these patients had low T3 (tivozanib v sorafenib: 23 patients [9%] v five patients [2%]) or low T4 (five [2%] v two [1%]) on or after the date that the increases in thyroid-stimulating hormone were observed.
Ten patients (4%) in the tivozanib arm and 14 (5%) in the sorafenib arm discontinued therapy due to treatment-related AEs. More patients in the sorafenib arm had treatment interruptions and dose reductions due to AEs than those in the tivozanib arm: treatment interruptions due to AEs occurred in 92 patients (36%) treated with sorafenib versus 50 (19%) treated with tivozanib; dose reductions due to AEs occurred in 111 patients (43%) treated with sorafenib versus 37 (14%) treated with tivozanib (Fisher's exact test P < .001 for both). Dose reductions were most commonly due to hand-foot syndrome (tivozanib v sorafenib, 2% v 18%), diarrhea (1% v 5%), and hypertension (2% v 4%). Mean relative dose intensities of tivozanib and sorafenib were 94% and 80%, respectively.
On the basis of the assessment of the deaths that occurred within 30 days of the last dose of study drug, eight in the tivozanib arm and two in the sorafenib arm were due to PD, whereas 13 in the tivozanib arm and 12 in the sorafenib arm were related to other causes. In the tivozanib arm, two deaths resulted from myocardial infarction, two from cardiac failure, and one each from hypertension, dyspnea, cerebrovascular accident, aortic aneurysm rupture, arteriosclerosis coronary artery, cardiac arrest, apnea, pulmonary embolism, and death not otherwise specified. In the sorafenib arm, three deaths were due to cerebrovascular accident, one each to cardiac failure, arteriosclerosis coronary artery, coronary artery insufficiency, hemorrhage, pleural effusion, jaundice, acute respiratory distress syndrome, and pulmonary embolism. One patient in the sorafenib arm had two AEs with an outcome of death within 30 days of last dose: pulmonary embolism and acute cardiac failure.
HRQoL
HRQoL questionnaires were completed by > 99% of patients in both arms at baseline. Completion rates decreased over time, in line with study dropout, falling below 50% after cycle 13. As a result, only data from the first 12 months (cycle 13) were considered in the RMME model analysis.
Baseline HRQoL scores were well balanced between the two arms. The RMME model analysis showed no statistical difference between the two arms in score changes from baseline (data cutoff on December 15, 2011; Appendix Table A1, online only). HRQoL was maintained at a level comparable to the baseline level during the first 12 months of treatment in both arms (ie, decrease in scores did not exceed the pre-established criteria for clinically meaningful changes).20,25,26
DISCUSSION
Tivozanib was associated with a significant improvement in PFS compared with sorafenib when administered to patients with metastatic RCC as initial targeted therapy. Preplanned subgroup analyses of patients who were treatment naive for metastatic RCC also showed higher PFS for those being given tivozanib than those being given sorafenib.
Inhibiting the VEGF pathway has been proposed as the primary mechanism for tumor inhibition for clear cell RCC.27 Tivozanib is characterized from preclinical data as a highly potent, selective inhibitor of VEGFR, with less inhibition of other kinases, including c-KIT and platelet-derived growth factor receptor beta,12–14 and a long half-life compared with sorafenib.9,17 AEs observed for tivozanib in this study had a similar spectrum to those reported previously15 and were consistent with those of a selective inhibitor of the VEGF pathway. Hypertension was the predominant AE for tivozanib. In this phase III trial, hypertension occurred in nearly half the patients. However, in most instances, it was controlled with medication, with 2% of patients requiring dose reduction and < 1% of patients requiring dose discontinuation for hypertension. Several other studies have suggested that development of hypertension in the setting of VEGF-targeted therapy is associated with improved efficacy and may indicate on-target activity.28–31 Other AEs in this study, such as hand-foot syndrome and diarrhea, were more common with sorafenib than with tivozanib. One retrospective study suggested that patients find hand-foot syndrome and diarrhea to be among the most burdensome AEs associated with TKIs.32
Compared with historical results from the pivotal phase III trial of sorafenib in metastatic RCC2 and a randomized phase II trial of sorafenib as first-line therapy for metastatic RCC,33 patients in the sorafenib arm of this study had a longer PFS than anticipated. However, the PFS and ORR (23.3%) for the sorafenib-treated patients in this study are in line with those in a recent randomized phase II trial with sorafenib as the control arm (PFS, 9.0 months; ORR, 24%)34 and an expanded-access study of sorafenib (PFS, 8.3 months)35 in a treatment-naive metastatic RCC population.
Sorafenib was selected as the active comparator in this trial on the basis of regulatory approval and its widespread use for advanced RCC. Since 2005, seven new targeted agents have gained regulatory approval in the United States for metastatic/advanced RCC indications (sorafenib, sunitinib, pazopanib, bevacizumab [plus interferon], axitinib, temsirolimus, and everolimus). Most of these agents were compared with either interferon alfa or placebo in randomized phase III trials.2,4–7,36,37 This study represents one of only three phase III trials with the use of an active, targeted therapy comparator38,39 evaluating efficacy and tolerability of first-line targeted treatment in metastatic RCC.
OS in this study was confounded by differential use of next-line targeted cancer therapies, because the one-way cross-over design allowed patients who had progressed on sorafenib to switch to tivozanib. An alternative hypothesis to explain the trend toward longer OS on the sorafenib arm is that sorafenib is more effective than tivozanib for improving OS, notwithstanding the antitumor activity of next-line tivozanib in the patients in the sorafenib arm for whom treatment failed. On the basis of the trial extension design and data, our hypothesis is that the trend toward longer OS in the sorafenib arm is related to the greater proportion of patients in the sorafenib arm who received next-line targeted RCC treatment (63% v 13% in the tivozanib arm). At the time of the final OS analysis for this study, 156 patients (61%) randomly assigned to sorafenib had crossed over to tivozanib in the companion trial. The efficacy results for this cohort of patients in the extension study have been reported: the ORR was 13%, and the median PFS was 8.4 months.40 The predominant enrollment in Central/Eastern Europe appears to be the main contributor to the imbalance in next-line targeted therapy between the two arms. Patients treated in this region on the tivozanib arm of our study were observed to receive less next-line targeted therapy as part of the standard of care (Table 3). The OS result is consistent with other observations that two consecutive targeted agents are associated with a longer OS than treatment with only one line of targeted therapy.41,42
In conclusion, tivozanib improved PFS compared with sorafenib in patients with metastatic RCC. Although tivozanib was characterized by higher rates of hypertension and dysphonia, it was generally well tolerated and had lower rates of certain AEs, including hand-foot skin reaction and diarrhea, and it required fewer dose reductions and interruptions compared with sorafenib. Further study of tivozanib is warranted to provide additional insights into the utility of tivozanib for the treatment of patients with metastatic RCC.
Acknowledgment
We thank the investigators, their study coordinators, nurses, and the patients and their families involved in this study. We thank Pankaj Bhargava, MD, for contribution to study design; Jim Kostka, MS, Ronny Oren, MMHS, and Madhavi Kamma, MS, for clinical project management; and independent data monitoring committee members Alain Ravaud, MD, Walter Stadler, MD, FACP, and KyungMann Kim, PhD, for monitoring the conduct of the study.
Appendix
Methods
Censoring rule for progression-free survival.
On the basis of US Food and Drug Administration guidance regarding the censoring rule for progression-free survival,18 death or progression after more than one (ie, at least two) missed tumor assessments should be censored at the date of last radiologic assessment of measured lesions. Because tumor assessments were performed every 8 weeks until disease progression in this trial, this guidance was interpreted as death should not be considered an event if it occurred after two to three missed tumor assessments or > 140 days after the last tumor assessment documenting the absence of progressive disease.
Table A1.
Health-Related Quality of Life Assessment of Patients Treated With Tivozanib or Sorafenib
| FACT-G |
FKSI-DRS |
EQ-5D |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tivozanib (n = 257) | Sorafenib (n = 248) | P* | Tivozanib (n = 256) | Sorafenib (n = 248) | P* | Tivozanib (n = 256) | Sorafenib (n = 250) | P* | |
| Baseline | |||||||||
| Mean | 77.01 | 77.27 | 29.16 | 29.35 | 0.73 | 0.73 | |||
| SD | 14.98 | 15.94 | 4.77 | 5.10 | 0.25 | 0.26 | |||
| Change from baseline | .805 | .965 | .391 | ||||||
| LS mean change† | −2.83 | −3.10 | −0.94 | −0.93 | −0.05 | −0.06 | |||
| SE | 1.04 | 1.02 | 0.33 | 0.34 | 0.02 | 0.02 | |||
NOTE. Health-related quality of life analysis included patients with baseline and one or more postbaseline evaluable forms.
Abbreviations: EQ-5D, EuroQol-5D; FACT-G, Functional Assessment of Cancer Therapy-General; FKSI-DRS, FACT Kidney Symptom Index–Disease-Related Symptoms; LS, least square; MSKCC, Memorial Sloan-Kettering Cancer Center; SD, standard deviation.
P value is for the overall differences between the two treatment arms.
The least-square means for each treatment arm were estimated by using data from the first 12 months (cycle 13) of assessments by repeated-measures mixed-effects models controlling for treatment, assessment time, treatment-by-time interaction, baseline score, age, ECOG performance status, geographic region, number of metastatic sites, number of prior treatments, MSKCC prognostic factor status, time from diagnosis to study entry and any dose reduction during the study. Negative differences from baseline indicate worsened quality of life or more symptoms.
Fig A1.
Kaplan-Meier plot of progression-free survival (PFS) as determined by investigator assessment in intent-to-treat population.
Footnotes
See accompanying editorial on page 3746
Supported by AVEO Oncology and Astellas (parties to a collaboration agreement for the codevelopment of tivozanib). Editorial assistance was funded by AVEO Oncology and Astellas and was provided by Jared Wels, PhD, and Jinling Wu, MD, PhD, Chameleon Communications International.
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01030783.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Andrew Krivoshik, Astellas Pharma (C); Andrew Strahs, AVEO Pharmaceuticals (C); Brooke Esteves, AVEO Pharmaceuticals (C); Anna Berkenblit, AVEO Pharmaceuticals (C) Consultant or Advisory Role: Robert J. Motzer, Pfizer (C), AVEO Pharmaceuticals (C), Genentech (C); Dmitry Nosov, AVEO Pharmaceuticals (U), Astellas Pharma (U); Timothy Eisen, AVEO Pharmaceuticals (U), Astellas Pharma (U); Cezary Szczylik, Bayer Pharmaceuticals (C), Astellas Pharma (C), Pfizer (C); David Cella, AVEO Pharmaceuticals (C), Astellas Pharma (C); Thomas E. Hutson, Pfizer (C), AVEO Pharmaceuticals (C), Novartis (C), GlaxoSmithKline (C) Stock Ownership: Dmitry Nosov, AstraZeneca; Timothy Eisen, AstraZeneca; Andrew Strahs, AVEO Pharmaceuticals; Brooke Esteves, AVEO Pharmaceuticals; Anna Berkenblit, AVEO Pharmaceuticals Honoraria: Timothy Eisen, Astellas Pharma; Cora N. Sternberg, Astellas Pharma; Cezary Szczylik, Bayer Pharmaceuticals, Astellas Pharma, Pfizer; Thomas E. Hutson, Pfizer, AVEO Pharmaceuticals, Novartis, GlaxoSmithKline Research Funding: Robert J. Motzer, AVEO Pharmaceuticals, Pfizer, GlaxoSmithKline; Dmitry Nosov, Bayer Pharmaceuticals, Pfizer, GlaxoSmithKline; Timothy Eisen, Bayer Pharmaceuticals, Pfizer, GlaxoSmithKline; Vladimir Lesovoy, Pfizer; Anna Alyasova, AVEO Pharmaceuticals, Astellas Pharma; David Cella, AVEO Pharmaceuticals; Thomas E. Hutson, Pfizer, AVEO Pharmaceuticals, Novartis, GlaxoSmithKline Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Robert J. Motzer, Cora N. Sternberg, Brooke Esteves
Administrative support: Igor Bondarenko
Provision of study materials or patients: Robert J. Motzer, Dmitry Nosov, Timothy Eisen, Igor Bondarenko, Oleg Lipatov, Cora N. Sternberg, Thomas E. Hutson
Collection and assembly of data: Robert J. Motzer, Igor Bondarenko, Vladimir Lesovoy, Oleg Lipatov, Piotr Tomczak, Oleksiy Lyulko, Anna Alyasova, Mihai Harza, Mikhail Kogan, Boris Y. Alekseev, Cora N. Sternberg, Brooke Esteves, Anna Berkenblit, Thomas E. Hutson
Data analysis and interpretation: Robert J. Motzer, Dmitry Nosov, Timothy Eisen, Piotr Tomczak, Oleksiy Lyulko, Cora N. Sternberg, Cezary Szczylik, David Cella, Cristina Ivanescu, Andrew Krivoshik, Andrew Strahs, Brooke Esteves, Anna Berkenblit, Thomas E. Hutson
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Costa LJ, Drabkin HA: Renal cell carcinoma: New developments in molecular biology and potential for targeted therapies Oncologist 12:1404–1415,2007 [DOI] [PubMed] [Google Scholar]
- 2.Escudier B Eisen T Stadler WM, etal: Sorafenib in advanced clear-cell renal-cell carcinoma N Engl J Med 356:125–134,2007 [DOI] [PubMed] [Google Scholar]
- 3.Rini BI Escudier B Tomczak P, etal: Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial Lancet 378:1931–1939,2011 [DOI] [PubMed] [Google Scholar]
- 4.Rini BI Halabi S Rosenberg JE, etal: Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206 J Clin Oncol 28:2137–2143,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ Hutson TE Tomczak P, etal: Sunitinib versus interferon alfa in metastatic renal-cell carcinoma N Engl J Med 356:115–124,2007 [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ Hutson TE Tomczak P, etal: Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma J Clin Oncol 27:3584–3590,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sternberg CN Davis ID Mardiak J, etal: Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial J Clin Oncol 28:1061–1068,2010 [DOI] [PubMed] [Google Scholar]
- 8.Mendel DB Laird AD Xin X, etal: In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship Clin Cancer Res 9:327–337,2003 [PubMed] [Google Scholar]
- 9.Wilhelm SM Carter C Tang L, etal: BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis Cancer Res 64:7099–7109,2004 [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ Hutson TE Olsen MR, etal: Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma J Clin Oncol 30:1371–1377,2012 [DOI] [PubMed] [Google Scholar]
- 11.Kumar R Crouthamel MC Rominger DH, etal: Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors Br J Cancer 101:1717–1723,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskens FA de Jonge MJ Bhargava P, etal: Biologic and clinical activity of tivozanib (AV-951, KRN-951), a selective inhibitor of VEGF receptor-1, -2, and -3 tyrosine kinases, in a 4-week-on, 2-week-off schedule in patients with advanced solid tumors Clin Cancer Res 17:7156–7163,2011 [DOI] [PubMed] [Google Scholar]
- 13.Cotreau MM: Comparison of the pharmacokinetics of tivozanib in oncology subjects compared with healthy volunteers Clin Pharmacol Ther 89:S49,2011suppl 1 abstr PII-36 [Google Scholar]
- 14.Nakamura K Taguchi E Miura T, etal: KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties Cancer Res 66:9134–9142,2006 [DOI] [PubMed] [Google Scholar]
- 15.Nosov DA Esteves B Lipatov ON, etal: Antitumor activity and safety of tivozanib (AV-951) in a Phase II randomized discontinuation trial in patients with renal cell carcinoma J Clin Oncol 30:1678–1685,2012 [DOI] [PubMed] [Google Scholar]
- 16.Esteves B Regan EM Fischer PM, etal: Management of hypertension during tivozanib therapy: Results from a phase 2 randomized discontinuation trial Oncol Nurs Forum 38:E153,2011abstr 1052744 [Google Scholar]
- 17.Bayer HealthCare Pharmaceuticals. Nexavar (sorafenib) full prescribing information. 2012. Dec,
- 18.US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Clinical trial endpoints for the approval of cancer drugs and biologics. 2007. May, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071590.pdf.
- 19.Webster K, Cella D, Yost K: The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation Health Qual Life Outcomes 1:79,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cella D Yount S Brucker PS, etal: Development and validation of a scale to measure disease-related symptoms of kidney cancer Value Health 10:285–293,2007 [DOI] [PubMed] [Google Scholar]
- 21.EuroQol: A new facility for the measurement of health-related quality of life—The EuroQol Group Health Policy 16:199–208,1990[No authors listed] [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ Mazumdar M Bacik J, etal: Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma J Clin Oncol 17:2530–2540,1999 [DOI] [PubMed] [Google Scholar]
- 23.Fitzmaurice GM, Laird NM, Ware JH: Applied Longitudinal Analysis 2004Hoboken, NJ: Wiley-Interscience [Google Scholar]
- 24.Singer JD, Willett JB: Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence 2003New York, NY: Oxford University Press [Google Scholar]
- 25.Pickard AS, Neary MP, Cella D: Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer Health Qual Life Outcomes 5:70,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yost KJ, Eton DT: Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience Eval Health Prof 28:172–191,2005 [DOI] [PubMed] [Google Scholar]
- 27.Rini BI: Metastatic renal cell carcinoma: Many treatment options, one patient J Clin Oncol 27:3225–3234,2009 [DOI] [PubMed] [Google Scholar]
- 28.Schneider BP Wang M Radovich M, etal: Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100 J Clin Oncol 26:4672–4678,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahlberg SE Sandler AB Brahmer JR, etal: Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599 J Clin Oncol 28:949–954,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rini BI Cohen DP Lu DR, etal: Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib J Natl Cancer Inst 103:763–773,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin R Seymour L Ding K, etal: Hypertension (HTN) in National Cancer Institute of Canada Clinical Trials Group study BR.24: A randomized, double-blind phase II trial of carboplatin (C) and paclitaxel (P) with either daily oral cediranib (CED), an inhibitor of vascular endothelial growth factor receptors, or placebo, in patients with advanced non-small cell lung cancer J Clin Oncol 27:152s,2009suppl abstr 3527 [Google Scholar]
- 32.Wong MKK Mohamed AF Hauber AB, etal: Selecting renal cell carcinoma therapy: Ranking of patient perspective on toxicities J Clin Oncol 30:303s,2012suppl abstr 4608 [Google Scholar]
- 33.Escudier B Szczylik C Hutson TE, etal: Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma J Clin Oncol 27:1280–1289,2009 [DOI] [PubMed] [Google Scholar]
- 34.Rini BI Szczylik C Tannir NM, etal: AMG 386 in combination with sorafenib in patients (pts) with metastatic renal cell cancer (mRCC): A randomized, double-blind, placebo-controlled, phase II study Cancer 118:6152–6161,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stadler WM Figlin RA McDermott DF, etal: Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America Cancer 116:1272–1280,2010 [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ Escudier B Oudard S, etal: Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial Lancet 372:449–456,2008 [DOI] [PubMed] [Google Scholar]
- 37.Hudes G Carducci M Tomczak P, etal: Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma N Engl J Med 356:2271–2281,2007 [DOI] [PubMed] [Google Scholar]
- 38.Hutson T Escudier B Esteban E, etal: Temsirolimus vs sorafenib as second line therapy in metastatic renal cell carcinoma: Results from the INTORSECT trial Ann Oncol 23:ixe14,2012suppl 9 abstr LBA22_PR [Google Scholar]
- 39.Motzer R Hutson TE Reeves J, etal: Randomized, open-label, phase III trial of pazopanib versus sunitinib in first-line treatment of patients with metastatic renal cell carcinoma (MRCC): Results of the COMPARZ trial Ann Oncol 23,2012suppl 9 abstr LBA8 PR [Google Scholar]
- 40.Motzer RJ Nosov D Tomczak P, etal: Efficacy and safety data from patients with advanced renal cell cancer treated with tivozanib hydrochloride after progression on sorafenib J Clin Oncol 31,2013suppl abstr 364 [Google Scholar]
- 41.Harrison MR George DJ Walker MS, etal: Beyond metastatic renal cell carcinoma (mRCC) clinical trials: Targeted therapy use and overall survival in community oncology clinics J Clin Oncol 30,2012suppl abstr e1506123169502 [Google Scholar]
- 42.Xie W Choueiri TK Lee J-L, etal: Characteristics of long-term and short-term survivors of metastatic renal cell carcinoma (mRCC) treated with targeted therapy: Results from the International mRCC Database Consortium J Clin Oncol 30:286s,2012suppl abstr 4538 [DOI] [PubMed] [Google Scholar]